Abstract
Background
Heart failure (HF) with improved ejection fraction (EF, HFimpEF) is a distinct HF subtype, characterized by left ventricular (LV) reverse remodeling and myocardial functional recovery. Multiple cardiometabolic factors are implicated in this process. Epicardial adipose tissue (EAT), emerging as an endocrine and paracrine organ, contributes to the onset and progression of HF. However, the relation between EAT and the incidence of HFimpEF is still unclear.
Methods
A total of 203 hospitalized HF patients with reduced EF (HFrEF, LVEF ≤ 40%) who underwent coronary CT angiography (CCTA) during index hospitalization were consecutively enrolled between November 2011 and December 2022. Routine follow-up and repeat echocardiograms were performed. The incidence of HFimpEF was defined as (1) an absolute LVEF improvement ≥ 10% and (2) a second LVEF > 40% (at least 3 months apart). EAT volume and density were semiautomatically quantified on non-enhanced series of CCTA scans.
Results
During a median follow-up of 8.6 (4.9 ~ 13.3) months, 104 (51.2%) patients developed HFimpEF. Compared with HFrEF patients, HFimpEF patients had lower EAT volume (115.36 [IQR 87.08 ~ 154.78] mL vs. 169.67 [IQR 137.22 ~ 218.89] mL, P < 0.001) and higher EAT density (-74.92 ± 6.84 HU vs. -78.76 ± 6.28 HU, P < 0.001). Multivariate analysis showed lower EAT volume (OR: 0.885 [95%CI 0.822 ~ 0.947]) and higher density (OR: 1.845 [95%CI 1.023 ~ 3.437]) were both independently associated with the incidence of HFimpEF. Subgroup analysis revealed that the association between EAT properties and HFimpEF was not modified by HF etiology.
Conclusions
This study reveals that lower EAT volume and higher EAT density are associated with development of HFimpEF. Therapies targeted at reducing EAT quantity and improving its quality might provide favorable effects on myocardial recovery in HF patients.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Attributed to the development of guideline-based medical and device therapies, a substantial portion of heart failure (HF) patients with reduced ejection fraction (EF, HFrEF) have experienced partial or complete myocardial recovery [1, 2]. According to the 2022 AHA/ACC/HFSA Guideline for the Management of HF, patients with previous HFrEF whose left ventricular EF (LVEF) develops to > 40% are now termed as “HF with improved EF” (HFimpEF) [3]. Existing evidence shows that HFimpEF is a distinct HF phenotype underlined by coordinated changes in gene transcription, adaptation of cellular and metabolic processes, and alteration of extracellular matrix composition, thereby leading to improved cardiomyocyte contractility and left ventricular (LV) geometric restoration [4,5,6]. Development of HFimpEF is generally considered as a surrogate endpoint representing a better cardiovascular prognosis. However, the predictors for HFimpEF are still being explored.
Epicardial adipose tissue (EAT), a layer of adipose tissue located between the myocardium and visceral pericardium, has been widely recognized as an endocrine and paracrine organ [7, 8]. Due to its unobstructed proximity and shared microcirculation, EAT has a direct crosstalk with adjacent myocardium. Physiologically, EAT serves as a heart protector by mechanical, thermogenic, metabolic, and paracrine mechanisms [9, 10]. Under certain pathological conditions, EAT tends to switch to a pro-inflammatory, immune-responsive, and pro-fibrosis state, which is also reflected by geometric and compositional changes of EAT from a macro perspective [7, 11, 12]. Current data demonstrate that EAT is closely implicated in the development of various cardiovascular diseases including coronary heart disease, atrial fibrillation and HF [13, 14]. Especially, the crucial role of EAT in HF has been verified by a number of studies, mainly focusing on HF with preserved EF (HFpEF) [15,16,17]. Nevertheless, the impact of EAT on LV reverse remodeling and myocardial recovery remains unknown. In the present study, we sought to investigate the association between EAT assessed by cardiac CT and the incidence of HFimpEF.
Methods
Study population
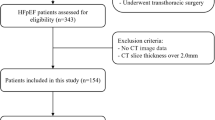
The study consecutively enrolled 402 patients who were diagnosed with HFrEF (LVEF ≤ 40%) and underwent coronary CT angiography (CCTA) during index hospitalization between November 2011 and December 2022 in Shanghai Ruijin Hospital. A total of 98 patients comorbid with pericardial diseases (n = 24), malignant tumor (n = 19), heart transplantation (n = 1), prior cardiac surgery (n = 51), and in-hospital death (n = 3) were excluded. The enrolled patients were routinely followed up and underwent repeat echocardiograms. During follow-up, there were 15 deaths and 86 patients lost to echocardiogram follow-up, who were also excluded (Fig. 1).
The primary outcome was the development of HFimpEF, which was diagnosed based on repeat echocardiograms ≥ 3 months apart according to the working definition of HFimpEF: (1) ≥ 10% absolute LVEF improvement; and (2) a second LVEF > 40%.
This study complies with the Declaration of Helsinki. The study protocol was approved by Shanghai Ruijin Hospital ethics committee, and written informed consent was obtained from all participants.
Clinical and biochemical assessments
Detailed information of medical history and lifestyles including smoking habits was obtained using a standard questionnaire by trained physicians on admission. Body mass index (BMI) was calculated as weight/height2 (kilograms per square meter). Body surface area (BSA) was calculated by Stevenson’s formula: 0.0061 × height + 0.0128 × weight − 0.1529. Hypertension was diagnosed according to the seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (BP; JNC 7). The diagnosis of diabetes was made according to the criteria of American Diabetes Association.
All the blood samples were drawn after overnight fasting. Plasma glucose, liver and renal function, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were assessed (HITACHI 912 Analyzer, Roche Diagnostics, Germany). The estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration equation. Blood glycated hemoglobin A1c (HbA1c) was measured using ion-exchange high performance liquid chromatography with Bio-rad Variant Hemoglobin Testing System (Bio-Rad Laboratories, USA).
Echocardiographic examination
Comprehensive transthoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI). The sonographers were blinded to this study. Two-dimensional echocardiography and Doppler flow imaging were recorded from standard parasternal and apical transducer positions.
LVEF was calculated using the modified Simpson’s biplane technique. The LV length was measured in an apical 4-chamber view. To facilitate application of clinical normality cut points, LV end-diastolic volume (EDV) and end-systolic volume (ESV) were indexed by BSA calculated at the study time point. LV mass was estimated from M-mode measurements by the formula: LV mass = \(\:0.8\times\:1.04\times\:\left[{\left(EDD+IVST+PWT\right)}^{3}-{EDD}^{3}\right]+0.6\), and was indexed by BSA, where EDD is LV end-diastolic diameter, IVST is interventricular septal thickness, PWT is LV posterior wall thickness.
CT scans
CCTA scans were performed on dual-source CT systems (SOMATOM® Definition Flash or SOMATOM® Force, Siemens Healthineers) with the use of iodine contrast agent. Non-contrast images were routinely acquired by a prospectively electrocardiogram-triggered high-pitch spiral acquisition mode followed by contrast CT scans. Adhering to the common practice for EAT assessment in previous studies, this study utilized non-contrast cardiac CT data with the following parameters. SOMATOM® Definition Flash (256-slice CT): axial scan; X-ray exposure times, 148 ms; tube voltage, 120 kV; tube currents, 429 mA; center of imaging window, 70% of R-R interval. SOMATOM® Force (384-slice CT): axial scan; X-ray exposure times, 285 ms; tube voltage, 120 kV; tube currents, 858 mA; center of imaging window, 70% of R-R interval. For image reconstruction, a B35f (SOMATOM® Definition Flash) or Qr36f (SOMATOM® Force) convolution kernel was used for non-contrast scans with a slice thickness of 3.0 mm and an increment of 1.5 mm. All the CT images were transferred to a dedicated workstation (Xcelera, Philips) for image post-processing.
EAT measurement
One observer with extensive experience in cardiac CT analysis who was blinded to the patients’ information did the analysis. EAT volume was quantified on non-contrast-enhanced images using an open-source medical imaging processing software 3D Slicer (Boston, USA, 5.2.2 version). EAT was defined as the fat tissue between the outer wall of the myocardium and the visceral layer of the pericardium, which was quantified using a region of interest (ROI) manually defined by tracing the pericardium from the pulmonary artery trunk to the LV apex. Then the software reconstructed EAT into a three-dimensional region and automatically measured EAT volume and average attenuation by including contiguous three-dimensional fat voxels ranged from − 190 to − 30 Hounsfield units (HU) in the ROI as previously described [18] (Fig. 2).
EAT on non-enhanced series of CCTA in HFrEF and HFimpEF patients. Shown are representative axial, sagittal and coronal images of EAT in patients with persistent HFrEF and HFimpEF. EAT density was converted into pseudo-color according to CT attenuation ranging from − 190 to − 30 HU. 3D visualization of EAT was performed by reconstruction of EAT volumes. A color scale bar is shown aside. A EAT in HFrEF; B EAT in HFimpEF. CCTA coronary CT angiography, EAT epicardial adipose tissue, HFimpEF heart failure with improved ejection fraction, HFrEF heart failure with reduced ejection fraction, HU Hounsfield units
Statistical analyses
Continuous variables were presented as median (interquartile range [IQR]) or mean ± standard deviation (SD), and categorical data were summarized as frequencies (percentages). Normal distribution of continuous variables was evaluated by Shapiro-Wilk test. For non-normally distributed continuous variables, differences were analyzed by Mann-Whitney U test or Kruskal-Wallis test. Differences among groups were analyzed by Student’s t-test or one-way analysis of variance (ANOVA). Correlation between EAT properties and changes in LVEF was determined by Spearman’s correlation test. Univariate logistic regression analysis was performed to identify predictors for HFimpEF. Afterwards, multivariate regression models were constructed to interrogate the association between EAT properties and HFimpEF. Traditional risk factors, adiposity-associated index (BMI) and predictors with P < 0.10 in the univariate analysis were selected as confounding factors in the multivariate analysis. In Model 1, age and sex were adjusted. In Model 2, additional adjustment was performed for smoking habits, BP, BMI, HF etiology, as well as history of myocardial infarction, hypertension and diabetes. In Model 3, we further adjusted for hemoglobin, HbA1c, triglyceride, blood urea nitrogen and eGFR. In Model 4, guideline-directed therapies including beta-blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor-neprilysin inhibitors (ARNI), spironolactones as well as sodium-glucose cotransporter 2 (SGLT2) inhibitors were additionally adjusted. In Model 5, baseline EDV index and ESV index were further adjusted. In these models, the association between HFimpEF and EAT volume (models with a suffix ‘a’) or density (models with a suffix ‘b’) were analyzed respectively. In Model 6, both EAT volume and density were enrolled based on Model 5.
All statistical analyses were performed using the R statistical package v.4.0.3 (R Project for Statistical Computing, Vienna, Austria). A 2-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
A total of 203 HFrEF patients were finally enrolled in the study. The mean age was 58.4 ± 13.2 years and 82.3% were male patients. During a median follow-up of 8.6 (4.9 ~ 13.3) months, 104 (51.2%) patients developed HFimpEF, whereas 99 (48.8%) remained persistent HFrEF. The baseline characteristics of the study population are shown in Table 1. Compared with persistent HFrEF, HFimpEF patients tended to be female, younger, non-current smokers, with higher diastolic BP and better renal function. HFimpEF subjects were less frequently with an ischemic etiology, history of revascularization and anti-platelet therapies. Both groups received optimal medical HF therapies unless intolerance or contra-indications. There was no significant difference in BMI, systolic BP, history of type 2 diabetes, hypertension, prior myocardial infarction, atrial fibrillation, HbA1c, lipid profiles, NT-proBNP and other medical therapies between the two groups. Echocardiography analysis showed that baseline LV chamber tended to be smaller in HFimpEF compared to persistent HFrEF patients. Significant LV reverse remodeling accompanied by LVEF improvement was observed in HFimpEF patients (Supplementary Table I).
EAT volume and density
In the overall population, the median EAT volume was 111.51 (IQR 101.33 ~ 184.36) mL and mean EAT density was − 76.79 ± 6.83 HU. EAT density was inversely correlated with EAT volume (Spearman’s r= -0.33, P < 0.001). Compared with HFrEF patients, HFimpEF patients had lower EAT volume (115.36 [IQR 87.08 ~ 154.78] mL vs. 169.67 [IQR 137.22 ~ 218.89] mL, P < 0.001) and higher EAT density (-74.92 ± 6.84 HU vs. -78.76 ± 6.28 HU, P < 0.001) (Fig. 3and Table 2). BMI-indexed EAT volume was also significantly lower in HFimpEF than HFrEF patients (P < 0.001; Table 2 and Supplementary Figure I).
HFimpEF patients had lower EAT volume but higher EAT density compared with persistent HFrEF patients. Shown are EAT volume (A) and EAT density (B) in patients with persistent HFrEF and HFimpEF. Horizontal lines in the box: upper, 75% percentile; middle, median; lower, 25% percentile. Upper whisker, 95% percentile; lower whisker, 5% percentile. EAT epicardial adipose tissue, HFimpEF heart failure with improved ejection fraction, HFrEF heart failure with reduced ejection fraction, HU Hounsfield units
Furthermore, correlation analyses (Fig. 4) showed that changes in LVEF were inversely correlated to EAT volume (Spearman’s r = -0.35, P < 0.001) while positively correlated to EAT density (Spearman’s r = 0.18, P = 0.009). Of note, EAT volume exhibited a stronger correlation with LV reverse remodeling than EAT density (Supplementary Table II).
The association between EAT volume or density and HFimpEF
Univariate regression analysis (Supplementary Table III) revealed that predictors for HFimpEF were younger age, female sex, non-current smokers, higher diastolic BP, non-ischemic etiology, better renal function, smaller LV chamber size, as well as lower EAT volume and higher EAT density.
Multivariate regression analysis (Table 3) demonstrated that EAT volume and density were both significantly associated with the development of HFimpEF after adjustment for conventional risk factors, pharmacological therapies and baseline anthropometric parameters including BMI. In the full adjustment model (Model 6) that enrolled both EAT properties, EAT volume and density were both independently associated with HFimpEF after multivariate adjustment. Every 10 mL increase in EAT volume corresponded to a 11.5% decreased likelihood of HFimpEF (OR: 0.885 [95%CI 0.822 ~ 0.947]), whereas each 10 HU increase in EAT mean density was associated with an 84.5% greater incidence of HFimpEF (OR: 1.845 [95%CI 1.023 ~ 3.437]).
Furthermore, subgroup analysis (Table 4) exhibited that the associations of HFimpEF with EAT volume and density were independent of age, BMI, and HF etiology.
Discussion
The major findings of the present study are that HF patients with lower EAT volume and higher EAT density are more likely to develop HFimpEF. EAT volume and density are independent predictors for HFimpEF, irrespective of HF etiology and BMI.
HFimpEF has been increasingly emphasized for the lower cardiovascular mortality, less re-hospitalization rate, and improved health-related life quality compared to HFrEF or HFpEF [19,20,21]. Existing evidence reveals that myocardial recovery from failing heart is a complex process driven by coordinated gene reprogramming, switch of cellular phenotypes and metabolic pathways [4,5,6]. A number of cohort studies showed that this recovery process is more readily to occur in certain clinical conditions after guideline-directed medical therapies [1, 22, 23]. Previously, we also showed that cardiometabolic factors such as insulin resistance, glycemic levels and variability, are implicated in the development of HFimpEF [24,25,26]. In this study, 51.2% of the hospitalized HF patients developed HFimpEF by optimal medical therapy during a median of 8.6-month follow-up. In line with previous reports, we showed by univariate analysis that predisposing factors for HFimpEF were younger age, female sex, non-current smokers, higher diastolic BP, non-ischemic etiology, better renal function and smaller LV chamber size.
Myocardial function and remodeling are affected not only by systemic factors but also by local environment. EAT has been recognized as a metabolic sensor and transducer, secreting adipokines, cytokines, or pro/anti-inflammatory mediators to exert exocrine and paracrine effects on the myocardium [7, 13]. The prevailing evidence supports a role of EAT as an independent risk factor for the incidence of HF. The risk of HF increased by 34%~43% per SD increase in EAT volume/thickness over the general population [27,28,29]. Additionally, HF patients with elevated EAT volume suffered a 2-fold higher all-cause mortality (HR: 2.06 [95% CI, 1.26–3.37], P = 0.004) and a 54% greater risk of HF hospitalization (HR: 1.54 [95% CI, 1.04–2.30], P = 0.03) [16]. However, the so-far understanding of the relationship between EAT and HF is mainly derived from HFpEF. Previous studies revealed that EAT volume differs between HFpEF with HFrEF patients. Compared to healthy controls, total EAT volume or thickness is higher in HFpEF but lower in HFrEF irrespective of aetiology [15, 30,31,32,33]. Pugliese et al. revealed that EAT has different impacts on HFrEF and HFpEF in terms of cardiovascular hemodynamics, metabolic profile and prognosis [31]. Reduced EAT thickness in HFrEF, in contrast to HFpEF, has been shown to be associated with elevated inflammatory response and myocardial damage characterized by higher hs-CRP, troponins as well as NT-proBNP levels [31]. However, data on EAT parameters in HFimpEF patients are still limited.
In this study, we clearly demonstrated an inverse association between EAT volume and HFimpEF after adjustment for traditional risk factors. Every 10 mL increase in EAT volume corresponded to a 11.5% decreased likelihood of HFimpEF. Moreover, subgroup analysis revealed the association between EAT volume and HFimpEF persisted significant irrespective of age, general adiposity reflected by BMI, as well as the presence of ischemic etiology. These data jointly support the notion that higher EAT volume is associated with compromised development of HFimpEF. Some plausible mechanisms may provide explanation. In the setting of HF, EAT can be transformed into a hypertrophic and inflamed state, leading to sustained inflammation, fibrosis, cardiomyocyte disarray and apoptosis in the myocardium via secreting pro-inflammatory and profibrotic cytokines or mediators [7, 13]. In addition, accumulating EAT may infiltrate the contiguous myocardium, resulting in direct mechanical compression and lipotoxic effects, thereby worsening both LV diastolic and systolic function [17, 34, 35]. Indeed, an animal study performed in obese rats subjected to acute myocardial infarction showed that surgical resection of cardiac fat was associated with better LVEF compared with those without fat resection [36]. Moreover, SGLT2 inhibitors have been shown to not only reduce the quantity of EAT, but also ameliorate adipose inflammation [7, 11], which might partly account for its salutary role in HF across the whole spectrum of EF.
On the other hand, we further revealed that higher EAT density is associated with myocardial recovery independent of EAT volume and HF etiology. Every 10 HU increase in EAT mean density was associated with an 84.5% greater incidence of HFimpEF. EAT density, measured by CT tissue attenuation, to some extent reflects the composition of EAT by adipocytes, stromal cells and infiltrated inflammatory cells [37]. Adult EAT is mainly composed of white adipose tissue (WAT) and small portion of brown adipose tissue (BAT), the latter is of smaller size, higher density, and cardioprotective effects. The proportion of BAT was shown to decrease with age or under various pathological conditions [18, 38, 39]. In the setting of HF, EAT is prone to be ‘whitening’ (more WAT and less BAT), expanded and inflamed, thus presenting with higher volume and lower CT attenuation [14, 17, 37]. Based on prior basic studies, CT is able to distinguish between BAT and WAT based on CT attenuation values and it is rational to hypothesize that EAT with higher density represents higher proportion of BAT, which confers beneficial paracrine and cardiometabolic effects, and facilitates myocardium structural and functional restoration [14, 38]. Actually, current evidence suggests ‘re-browning’ of EAT may improve the hypoxic and inflammatory microenvironment, favoring a better outcome [17]. From another perspective, EAT density is reflective of both local and systemic inflammatory status [14, 40, 41]. In inflamed adipose tissue, local adipogenesis is inhibited, thereby leading to a shift towards higher tissue density from the lipid phase to the aqueous phase [41]. Thus, higher EAT density is indicative of less adipocyte hypertrophy and hyperplasia, and more content of water, fibrosis and inflammatory cells [41]. Previous studies displayed conflicting results on the association between EAT density and different types of cardiovascular diseases [37, 40, 41]. According to the findings in our study, EAT density was significantly higher in HFimpEF than HFrEF patients. Hence, it is reasonable to propose that moderate inflammation in EAT might provide salutary effects for myocardial reparation and LV reverse remodeling. However, these hypotheses await to be validated and in-depth research.
Limitations
Our findings should be interpreted in the context of the following limitations. First, this study is a retrospective analysis from a single center with relatively small size. Second, anthropometric parameters such as waist circumstance and waist-to-hip ratio were not recorded though the association between EAT and HF has been previously shown to be independent of global adiposity [27]. Third, the proportion of active BAT can only be accurately measured by positron emission tomography (PET) acquisition rather than CT scans. Therefore, the extent to which BAT exerts beneficial effect cannot be evaluated from existing data and is our important future goal. Fourth, hard endpoints were not recorded due to the study design. Prospective studies are warranted to analyze the causal link between EAT and the development of HFimpEF.
Conclusions
In conclusion, our findings suggest that lower EAT volume and higher EAT density are associated with the development of HFimpEF independent of BMI and HF etiology. Therapies targeted at reducing EAT quantity and improving its quality might provide favorable effects on myocardial recovery in HF patients.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACEI:
-
Angiotensin converting enzyme inhibitors
- ANOVA:
-
Analysis of variance
- ARB:
-
Angiotensin receptor blockers
- ARNI:
-
Angiotensin receptor-neprilysin inhibitors
- BAT:
-
Brown adipose tissue
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BSA:
-
Body surface area
- CCTA:
-
Coronary CT angiography
- EAT:
-
Epicardial adipose tissue
- EF:
-
Ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- EDD:
-
End-diastolic diameter
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- HbA1c:
-
Glycated hemoglobin A1c
- HDL:
-
High-density lipoprotein
- HF:
-
Heart failure
- HFimpEF:
-
Heart failure with improved ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- HU:
-
Hounsfield units
- IQR:
-
Interquartile range
- IVST:
-
Interventricular septal thickness
- LDL:
-
Low-density lipoprotein
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- PET:
-
Positron emission tomography
- PWT:
-
Posterior wall thickness
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SGLT2:
-
Sodium-glucose cotransporter 2
- WAT:
-
White adipose tissue
References
Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1(5):510–8.
Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129(23):2380–7.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the management of Heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2022;145(18):e895–1032.
Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15(2):83–96.
Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection Fraction: JACC Scientific Expert Panel. J Am Coll Cardiol. 2020;76(6):719–34.
Adamo L, Yu J, Rocha-Resende C, Javaheri A, Head RD, Mann DL. Proteomic Signatures of Heart Failure in relation to left ventricular ejection Fraction. J Am Coll Cardiol. 2020;76(17):1982–94.
Rossi VA, Gruebler M, Monzo L, Galluzzo A, Beltrami M. The Different Pathways of Epicardial Adipose Tissue across the Heart Failure Phenotypes: From Pathophysiology to Therapeutic Target. Int J Mol Sci. 2023;24(7):6838.
Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153(6):907–17.
Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94(9):3611–5.
Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4(6):416–29.
Packer M. Epicardial Adipose tissue May Mediate Deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71(20):2360–72.
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6.
Antonopoulos AS, Papastamos C, Cokkinos DV, Tsioufis K, Tousoulis D. Epicardial adipose tissue in myocardial disease: from physiology to heart failure phenotypes. Curr Probl Cardiol. 2023;48(10):101841.
Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606.
van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20(11):1559–66.
van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, Rienstra M, Westenbrink BD, Gorter TM. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ Heart Fail. 2022;15(3):e009238.
van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail. 2022;24(12):2238–50.
Franssens BT, Nathoe HM, Leiner T, van der Graaf Y, Visseren FL. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur J Prev Cardiol. 2017;24(6):660–70.
Su K, Li M, Wang L, Tian S, Su J, Gu J, Chen S. Clinical characteristics, predictors, and outcomes of heart failure with improved ejection fraction. Int J Cardiol. 2022;357:72–80.
Wohlfahrt P, Nativi-Nicolau J, Zhang M, Selzman CH, Greene T, Conte J, Biber JE, Hess R, Mondesir FL, Wever-Pinzon O, et al. Quality of life in patients with heart failure with recovered ejection fraction. JAMA Cardiol. 2021;6(8):957–62.
Zhang X, Sun Y, Zhang Y, Chen F, Dai M, Si J, Yang J, Li X, Li J, Xia Y, et al. Characteristics and outcomes of heart failure with recovered left ventricular ejection fraction. ESC Heart Fail. 2021;8(6):5383–91.
Agra Bermejo R, Gonzalez Babarro E, López Canoa JN, Varela Román A, Gómez Otero I, Oro Ayude M, Parada Vazquez P. Gómez Rodríguez I, Díaz Castro O, González Juanatey JR: Heart failure with recovered ejection fraction: clinical characteristics, determinants and prognosis. CARDIOCHUS-CHOP registry. Cardiol J. 2018;25(3):353–62.
Lupón J, Díez-López C, de Antonio M, Domingo M, Zamora E, Moliner P, González B, Santesmases J, Troya MI, Bayés-Genís A. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail. 2017;19(12):1615–23.
Yang CD, Aihemaiti M, Quan JW, Chen JW, Shu XY, Ding FH, Shen WF, Lu L, Zhang RY, Pan WQ, et al. HbA1c level is associated with the development of heart failure with recovered ejection fraction in hospitalized heart failure patients with type 2 diabetes. Int J Cardiol. 2023;371:259–65.
Yang CD, Pan WQ, Feng S, Quan JW, Chen JW, Shu XY, Aihemaiti M, Ding FH, Shen WF, Lu L, et al. Insulin resistance is Associated With Heart failure with recovered ejection fraction in patients without diabetes. J Am Heart Assoc. 2022;11(19):e026184.
Yang CD, Chen JW, Quan JW, Shu XY, Feng S, Aihemaiti M, Ding FH, Shen WF, Lu L, Zhang RY, et al. Long-term glycemic variability predicts compromised development of heart failure with improved ejection fraction: a cohort study. Front Endocrinol (Lausanne). 2023;14:1211954.
Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, Burke GL, McClelland RL, Arai AE, Bluemke DA. Pericardial Fat and the risk of heart failure. J Am Coll Cardiol. 2021;77(21):2638–52.
Arshi B, Aliahmad HA, Ikram MA, Bos D, Kavousi M. Epicardial Fat volume, cardiac function, and Incident Heart failure: the Rotterdam Study. J Am Heart Assoc. 2023;12(1):e026197.
Choy M, Huang Y, Peng Y, Liang W, He X, Chen C, Li J, Zhu W, Wei FF, Dong Y, et al. Association between epicardial adipose tissue and incident heart failure mediating by alteration of natriuretic peptide and myocardial strain. BMC Med. 2023;21(1):117.
Ying W, Sharma K, Yanek LR, Vaidya D, Schär M, Markl M, Subramanya V, Soleimani S, Ouyang P, Michos ED, et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8(4):2535–45.
Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021;23(11):1858–71.
Doesch C, Haghi D, Flüchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12(1):40.
Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, Loh SY, Lee S, Jaufeerally F, Ling LH, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail. 2022;24(8):1346–56.
Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, Montgomery MK, Binny S, Watts T, Joshi SB, et al. Epicardial Adipose tissue Accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76(10):1197–211.
Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, Hoendermis ES, van Veldhuisen DJ. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8(8):667–76.
Kang KW, Ko JY, Lee H, Shin SY, Lee WS, Hong J, Kim SW, Lee SK, Oak MH. Surgically metabolic resection of Pericardial Fat to ameliorate myocardial mitochondrial dysfunction in Acute myocardial infarction obese rats. J Korean Med Sci. 2022;37(9):e55.
Liu J, Yu Q, Li Z, Zhou Y, Liu Z, You L, Tao L, Dong Q, Zuo Z, Gao L, et al. Epicardial adipose tissue density is a better predictor of cardiometabolic risk in HFpEF patients: a prospective cohort study. Cardiovasc Diabetol. 2023;22(1):45.
Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017;22(6):889–902.
Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15(9):507–24.
Monti CB, Capra D, Zanardo M, Guarnieri G, Schiaffino S, Secchi F, Sardanelli F. CT-derived epicardial adipose tissue density: systematic review and meta-analysis. Eur J Radiol. 2021;143:109902.
Salam B, Al-Kassou B, Weinhold L, Sprinkart AM, Nowak S, Theis M, Schmid M, Al Zaidi M, Weber M, Pieper CC, et al. CT-derived Epicardial adipose tissue inflammation predicts outcome in patients undergoing transcatheter aortic valve replacement. J Thorac Imaging. 2024;39(4):224–31.
Acknowledgements
None.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 82170423, 82000369, 82200512), the Opening Project Program of the National Research Center for Translational Medicine at Shanghai (Grant No. NRCTM(SH)-2023-13), National Key Research and Development Program of China (Grant No. 2022YFC2503502), Shanghai Jiao-Tong University School of Medicine– Gaofeng Clinical Medicine Grant Support (Grant No. 20240801), Natural Science Foundation of Shanghai (Grant No. 22YF1432400).
Author information
Authors and Affiliations
Contributions
CY and XW performed study design, data analysis and data interpretation. CY, JQ and XW performed manuscript writing. CY, JQ, GT, SF, HY, AA, ST, XW, RY and XW performed data collection. CY, RZ, LL and XW performed manuscript revision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Shanghai Ruijin Hospital ethics committee, and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, C.D., Quan, J.W., Tay, G.P. et al. Epicardial adipose tissue volume and density are associated with heart failure with improved ejection fraction. Cardiovasc Diabetol 23, 283 (2024). https://doi.org/10.1186/s12933-024-02376-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02376-y