Abstract
Background
Epicardial adipose tissue (EAT) accumulation is associated with multiple cardiometabolic risk factors and prognosis of heart failure with preserved ejection fraction (HFpEF). The correlation between EAT density and cardiometabolic risk and the effect of EAT density on clinical outcome in HFpEF remain unclear. We evaluated the relationship between EAT density and cardiometabolic risk factors, also the prognostic value of EAT density in patients with HFpEF.
Methods
We included 154 HFpEF patients who underwent noncontrast cardiac computed tomography (CT) and all patients received follow-up. EAT density and volume were quantified semi-automatically. The associations of EAT density and volume with cardiometabolic risk factors, metabolic syndrome and the prognostic impact of EAT density were analyzed.
Results
Lower EAT density was associated with adverse changes in cardiometabolic risk factors. Each 1 HU increase in fat density, BMI was 0.14 kg/m2 lower (95% CI 0.08–0.21), waist circumference was 0.34 cm lower (95% CI 0.12–0.55), non-HDL-cholesterol was 0.02 mmol/L lower (95% CI 0–0.04), triglyceride was 0.03 mmol/L lower (95% CI 0.01–0.04), fasting plasma glucose was 0.05 mmol/L lower (95% CI 0.02–0.08), TyG index was 0.03 lower (95% CI 0.02–0.04), Log2(TG/HDL-C) was 0.03 lower (95% CI 0.02–0.05), METS-IR was 0.36 lower (95% CI 0.23–0.49), MetS Z-score was 0.04 lower (95% CI 0.02–0.06), and Log2(CACS + 1) was 0.09 lower (95% CI 0.02–0.15). After adjusting for BMI and EAT volume, the associations of non-HDL-cholesterol, triglyceride, fasting plasma glucose, insulin resistance indexes, MetS Z-score, and CACS with fat density remained significant. The area under the curve (AUC) for the presence and severity of metabolic syndrome was greater in EAT density than volume (AUC: 0.731 vs 0.694, 0.735 vs 0.662, respectively). Over a median follow-up of 16 months, the cumulative incidence of heart failure readmission and composite endpoint increased with lower level of EAT density (both p < 0.05).
Conclusions
EAT density was an independent impact factor of cardiometabolic risk in HFpEF. EAT density might have better predictive value than EAT volume for metabolic syndrome and it might have prognostic value in patients with HFpEF.
Similar content being viewed by others
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a syndrome with substantial pathophysiological heterogeneity [1]. Patients with HFpEF have high incidence of comorbidities associated with metabolic syndrome components, including obesity, hypertension, dyslipidemia, and type 2 diabetes [2, 3]. Epicardial adipose tissue (EAT), located between the myocardium and the visceral pericardium, is considered to be a clinical biomarker of cardiometabolic diseases [4]. Accumulating data proposed that it has a significant impact on chronic inflammation, dyslipidaemia, insulin resistance, type 2 diabetes and atherosclerotic calcification probably through the mechanism of endocrine or paracrine [5,6,7]. But it remains unclear whether this is simply caused by obesity or the function and effects beyond epicardial fat tissue itself.
EAT density, which is measured by computed tomography (CT) tissue attenuation, has been recently used to represent the quality of epicardial fat tissue [8]. Adipose tissue density could be used to describe the lipid content and size of adipocyte, reflecting the inflammation and fibrosis of local tissue indirectly [9]. In the general population or in patients with high risk for cardiovascular diseases, lower EAT density was reported to be related with an adverse metabolic profile, independent of EAT volume [10, 11]. In addition, decreased EAT density was supposed to play a role for prognosis in asymptomatic individuals [12,13,14]. However, there is no study evaluating the relationship between EAT density with cardiometabolic risk and the prognosis value of EAT density in patients with HFpEF.
To explore whether EAT density and volume played a role in cardiometabolic risk and whether EAT density had a prognosis value in patients with HFpEF, we performed a prospective cohort study to explore the relationship between EAT density with cardiometabolic risk and clinical outcomes in HFpEF individuals.
Methods
Study participants
The study recruited 382 patients who were diagnosed with HFpEF and admitted to the Cardiology Department of the First Affiliated Hospital of Chongqing Medical University from Oct. 2019 to Jun. 2022. HFpEF diagnostic criteria included typical signs and symptoms of heart failure, left ventricular ejection fraction (LVEF) ≥ 50%, HFA-PEFF score ≥ 5 [15]. We excluded 39 patients with the history of pericardial diseases, severe liver or renal insufficiency, carcinoma, autoimmune disease, hypercortisolism, or had undergone transthoracic surgery. Then, 189 patients were excluded because no cardiac CT scan was performed or CT slice thickness over 2.0 mm. According to the above inclusion and exclusion criteria, 154 patients were finally enrolled in the study (Fig. 1). The present study was approved by the Human Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and strictly adhered to the Declaration of Helsinki.
Clinical data and biochemical measurements
Information on demographic characteristics and comorbidities were obtained via a face-to-face validated questionnaire. Blood samples were collected in the morning from participants who had fasted for more than 8 h prior to the blood draw. Fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), serum urea, serum creatinine, N-terminal pro brain natriuretic peptide (NT-proBNP), and high-sensitivity C-reactive protein (hsCRP) were measured using automated enzymatic methods. All biomarker measurements were performed by investigators who were blinded to patients’ characteristics and outcomes.
Definition of terms
Body mass index (BMI) was computed as weight in kilograms divided by the square of height in meters. Waist circumference (WC) was measured midway between the 12th rib and the iliac crest. Traditional methods for detecting insulin resistance (IR) such as the homeostatic model assessment of IR and the quantitative insulin sensitivity check index require insulin measurements or invasive methods. Therefore, we selected the surrogates of IR, including TG/HDL-C, triglyceride and glucose (TyG) index, and metabolic score for IR (METS-IR) to evaluate IR levels as previously reported [16]. These indexes were calculated by the following formulas: TG/HDL-C = TG (mg/dL) ÷ HDL-C (mg/dL), TyG = Ln [TG (mg/dL) × FPG (mg/dL) ÷ 2], and METS-IR = Ln [(2 × FPG (mg/dL)) + TG (mg/dL)] × BMI (kg/m2) ÷ Ln [HDL-C(mg/dL)]. To classify metabolic syndrome, we used the recent definition proposed in a joint statement of the International Diabetes Federation (IDF) and American Heart Association (AHA)/National Heart, Lung and Blood Institute (NHLBI) [17]. The metabolic syndrome severity Z-score (MetS Z-score) was automatically calculated by the MetS Severity Calculator, which is an HTML and JavaScript implementation using established and well-researched equations (https://metscalc.org/).
Cardiac computed tomography
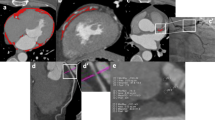
Electron beam CT scans were performed with GE (Healthcare, Milwaukee, USA) or Siemens (Healthineers, Erlangen, Germany) scanners without the use of contrast media. Each scan was analyzed using the calcium scoring software (IntelliSpace Portal, Philips Healthcare, Netherlands) to measure the total Agatston coronary artery calcification score (CACS), as described in detail previously [18]. EAT was defined as the fat tissue between the outer wall of the myocardium and the visceral layer of the pericardium [19]. We used the pulmonary artery bifurcation as the superior limit and the end of the left ventricular apex as the inferior limit of the heart. The pericardium was manually traced using a workstation with dedicated volumetric software (IntelliSpace Portal, Philips Healthcare, Netherlands). Then the software reconstructed EAT into a three-dimensional region and automatically measured EAT volume and average attenuation by including contiguous three-dimensional fat voxels ranged from − 190 to − 30 Hounsfield units (HU) as previously described [10] (Fig. 2).
Echocardiography
Cardiac structure and function parameters, including right atrium (RA) diameter, right ventricle (RV) diameter, tricuspid annular plane systolic excursion (TAPSE), systolic velocity of tricuspid annular tissue displacement (TAPSE-S), left atrium (LA) diameter, left atrial volume index (LAVI), left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic dimension (LVESD), left ventricular posterior wall end diastolic thickness (LVPW), left ventricular mass index (LVMI), early mitral inflow velocity (E-wave), late or atrial mitral inflow velocity (A-wave), peak early E-wave and late A-wave ratio (E/A ratio), septal mitral annular early diastolic peak velocities (Septal e′), lateral mitral annular early diastolic peak velocities (Lateral e′), average septal-lateral E/e′ ratio (Mean E/e′), fractional shortening (FS), and LVEF were measured using Vivid E95 ultra edition (GE Healthcare, Waukesha, WI, USA) by a professional ultrasound doctor.
Endpoints and follow-up
Primary outcome was defined as readmission for heart failure. The secondary outcome was composite endpoint of all-cause death or heart failure readmission. The participants were followed up by telephone or visiting our out-patient clinic every 3 months. All patients were followed up until death, or the end of follow-up, which was December 30, 2022. For each patient, the time to death or heart failure readmission was calculated from the initial date of follow-up to the date that the primary or secondary outcome occurred.
Statistical analysis
Continuous variables were tested for normal distribution using the Shapiro–Wilk test and were expressed as mean ± SD for normally distributed data or median and quartiles (Q1–Q3) for non-normally distributed data. Categorical variables were described as cases (n) and percentages (%). Spearman correlation analyses were used to test the cross-sectional relationship between EAT density and volume with cardiometabolic risk markers. For TG/HDL-C and CACS, data were log-transformed to improve the skewed distribution. We constructed three linear regression models to estimate how cardiometabolic risk factors were associated with EAT density and whether their relation was independent of BMI and EAT volume. The first model adjusted for age and gender. The second model included the same covariates from model 1 as well as BMI. In model 3, we adjusted for the covariates in model 1 as well as EAT volume. Receiver Operating Characteristics (ROC) curves were constructed to evaluate the predictive value of EAT density and volume for the presence and severity of metabolic syndrome. The area under the ROC curve (AUC) was used to quantify response prediction and the optimal cut-off point was determined by maximizing the Youden-index. Cumulative survival estimates were calculated using the Kaplan–Meier method and compared using the log-rank test. Statistical analyses were performed using SPSS version 26.0 (IBM SPSS Statistics, Armonk, New York).
Results
Patient characteristics
Patient characteristics are displayed in Table 1. Of the 154 included HFpEF individuals, 61.7% were women and the median age was 74 years (range: 66 to 81 years) at the baseline. Comorbidities including hypertension and coronary artery disease were present in nearly half patients (50.0% and 51.9%, respectively), type 2 diabetes and atrial fibrillation were present in nearly one-third patients (31.8% and 31.2%, respectively), chronic obstructive pulmonary disease was present in 15.6% patients. The median HFA-PEFF score was 6 (range: 5 to 6). The median EAT volume was 145.7 cm3 (range: 105.9 to 185.7 cm3) and the median fat density was − 76.2HU (range: − 81.4 to − 70.4 HU).
Correlations of EAT density and volume with cardiometabolic risk markers
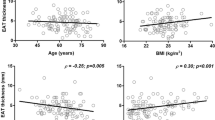
EAT density was inversely correlated with EAT volume and all cardiometabolic risk factors, including age, BMI, WC, FPG, HbA1c, TC, non-HDL-C, TG, CACS, and insulin resistance indexes (all p < 0.05, Table 2, Fig. 3), the absolute values of Spearman correlation coefficients ranged from 0.161 to 0.473, indicating weak to moderate associations between EAT density and cardiometabolic risk factors. On the contrary, EAT volume was positively correlated with those risk factors (all p < 0.05, Table 2, Fig. 3) except HbA1c, TC, non-HDL-C, and CACS, which showed no correlation with EAT volume.
Correlations between EAT characters with insulin resistance indexes and MetS Z-score. Correlation coefficient (r) and p value were acquired by Spearman rank correlation test. EAT epicardial adipose tissue, METS‐IR metabolic score for insulin resistance, MetS Z-score metabolic syndrome severity Z score, TyG triglyceride and glucose index
Correlations of EAT density and volume with echocardiography parameters
Table 3 presents the associations of EAT density and volume with echocardiography parameters. Increases in EAT density was correlated with higher levels of RA diameter, RV diameter, FS, and LVEF, but with lower levels of LVEDD, LVESD, LVPW, and LVMI (all p < 0.05). Moreover, increases in EAT volume was correlated with lower levels of RA diameter, RV diameter, FS, and LVEF, but with higher levels of LA diameter, LVEDD, LVESD, LVPW, and LVMI (all p < 0.05).
Multivariable-adjusted regressions of EAT density and volume with cardiometabolic risk factors
EAT density was inversely associated with all risk factors after adjusting for age and gender (all p < 0.05, Table 4). For 1HU increment in EAT density value, we observed a 0.14 kg/m2 decrease in BMI (95% CI 0.08–0.21), a 0.34 cm decrease in WC (95% CI 0.12–0.55), a 0.02 mmol/L decrease in non-HDL-C (95% CI 0–0.04), a 0.03 mmol/L decrease in TG (95% CI 0.01–0.04), a 0.05 mmol/L decrease in FPG (95% CI 0.02–0.08), a 0.03 decrease in TyG (95% CI 0.02–0.04), a 0.03 decrease in Log2(TG/HDL-C) (95% CI 0.02–0.05), a 0.36 decrease in METS-IR (95% CI 0.23–0.49), a 0.04 decrease in MetS Z-score (95% CI 0.02–0.06), and a 0.09 decrease in Log2(CACS + 1) (95% CI 0.02–0.15). After adjusting for BMI and EAT volume, the direction and significance of the associations between non-HDL-C, TG, FPG, insulin resistance indexes, MetS Z-score, and CACS with EAT density still remained. However, EAT volume was related only to BMI, WC, and METS-IR in the last model (all p < 0.05, Table 4).
ROC curves for metabolic syndrome prediction
In ROC curve analyses (Fig. 4A), the AUC for the presence of metabolic syndrome was greater in EAT density (AUC: 0.731) than EAT volume (AUC: 0.694). EAT density ≤ − 76.0HU and volume ≥ 143.5cm3 were the best cut-off values to identify the presence of metabolic syndrome. Moreover, the AUC for the more severe metabolic syndrome was greater in EAT density (AUC: 0.735) than EAT volume (AUC: 0.662). EAT density ≤ − 72.1HU and volume ≥ 134.3cm3 were the best cut-off values to identify the more severe metabolic syndrome in HFpEF patients (Fig. 4B).
Receiver operating characteristic (ROC) curve comparison. A ROC curves of EAT density and volume in predicting the presence of metabolic syndrome. The area under the curve (AUC) in EAT density was 0.731 (95% CI 0.639–0.823), the best cutoff point was − 76.0HU with the sensitivity of 66.2% and the specificity of 74.5%, the predictive positive value (PPV) was 71.6%. The AUC in EAT volume was 0.694 (95% CI 0.597–0.791), the best cutoff point was 143.5cm3 with the sensitivity of 66.2% and the specificity of 72.5%, the PPV was 64.7%. B ROC curves of EAT density and volume in predicting the more severe metabolic syndrome. The AUC in EAT density was 0.735 (95% CI 0.649–0.822), the best cutoff point was − 72.1HU with the sensitivity of 87.2% and the specificity of 54.5%, the PPV was 73.7%. The AUC in EAT volume was 0.662 (95% CI 0.569–0.755), the best cutoff point was 134.3cm3 with the sensitivity of 69.2% and the specificity of 61.8%, the PPV was 62.4%. All p < 0.01. CI = confidence interval, EAT epicardial adipose tissue
EAT density and heart failure readmission/composite endpoint
Over a median follow-up of 16 months, 39 (25.3%) heart failure readmission and 6 (3.9%) all-cause death were recorded. Kaplan–Meier survival analysis showed that the cumulative incidence of heart failure readmission and composite endpoints increased with lower level of EAT density (both p < 0.05) (Fig. 5).
Kaplan–Meier survival curves of freedom from heart failure readmission (A) and composite endpoints. (B) after a median follow-up of 16-month in total HFpEF patients. Median value of EAT density was used to divide the cohort into two groups: EAT ≤ − 76.2HU and EAT > − 76.2HU. P value was calculated by log-rank test. EAT epicardial adipose tissue
Discussion
The present study assessed the associations of EAT density and volume with cardiometabolic risk and the prognostic value of EAT density in patients with HFpEF. We reported three main findings from this prospective cohort study. First, EAT density was significantly associated with multiple cardiometabolic risk factors, independent of BMI and EAT volume. Second, EAT density had a better predictive value than EAT volume in the development and severity of metabolic syndrome. Finally, EAT density was associated with the risk of heart failure readmission and composite endpoints in patients with HFpEF. The findings suggested that epicardial fat density might be more closely associated with cardiometabolic risk than EAT volume and had a prognostic value of clinical outcomes in patients with HFpEF.
EAT density is associated with adverse cardiometabolic risk, independent of general obesity and EAT volume. In the present study, we found EAT volume was positively associated with age, BMI, WC, FPG, non-HDL-C, TG, and insulin resistance indexes. It is in good agreement with the results of recently published PROMIS-HFpEF study, which described that HFpEF patients with more epicardial fat were more likely to have higher levels of age, BMI, WC, TG, and insulin resistance [7]. We also found lower EAT attenuation was associated with higher levels of BMI, WC, FPG, non-HDL-C, TG, insulin resistance indexes, and CACS. After adjusting for BMI and EAT volume, the associations of FPG, non-HDL-C, TG, insulin resistance indexes, and CACS with EAT density remained significant. It indicated that lower EAT density might represent higher levels of cardiometabolic risk factors. Similar results were observed in a cross-sectional analysis from the offspring and third generation cohort of Framingham Heart Study [11], which showed lower attenuation of visceral adipose tissue was associated with impaired fasting glucose, metabolic syndrome and insulin resistance independent of adipose tissue volume. Lower EAT attenuation was also reported to be correlated with fasting glucose and metabolic syndrome in patients at high risk of cardiovascular disease independent of EAT volume [10]. Together with our results, these findings support the potential role for EAT density as a valid marker in relation to cardiometabolic risk and it cannot be fully explained by EAT volume in patients with HFpEF.
The pathogenic effect of epicardial fat does not only correspond to its adipocyte size or the number of adipocytes. Our results and previous works have shown that patients with HFpEF display an increase in epicardial fat thickness and accompany changed global EAT density [20].
From CT imaging, it is uncertain whether the decreased attenuation and expansion of epicardial fat in patients with HFpEF stem from adipocyte hypertrophy or proliferation, increased interstitial fibrosis or reduced capillary density. It is presumed that reduced epicardial fat attenuation represented more lipid dense fat tissue, larger adipocyte size, and poorer vascularity [11]. However, it has been controversial whether the expansion of epicardial adipose depots is driven by the increase in adipocyte size (hypertrophy) or by the formation of new adipocytes (hyperplasia). Both the female visceral adipose tissue area and attenuation derived from CT analysis were reported correlated with visceral adipocyte hypertrophy [21], but the correlation between EAT thickness and adipocyte size has not been detected in another study [22]. Although lipogenic capacity of EAT is still contentious, a growing number of studies prefer adipocyte proliferation as the main cause of EAT expansion. Our study presented that lower EAT density was characterized by increased EAT volume as well as cardiometabolic risk profile. Epicardial fat tissue is a potential source of inflammatory mediators, including interleukin (IL)-1β, IL-6, and tumour necrosis factor (TNF)-α. It changes its biological property and takes on many of the characteristics of white adipose tissue in chronic inflammatory disorders [23]. CT derived fat attenuation was reported to be related to local and systemic inflammatory markers [24, 25]. Therefore, we speculate that the decrease of epicardial fat density and the increase of volume might both resulted from the expansion of white adipose tissue, which is denser, larger and secretes more inflammatory factors compared with brown adipose tissue [10, 26]. However, the mechanisms underlying the association between epicardial fat attenuation and cardiometabolic risk still need more in-depth studies.
Both EAT density and volume can be used to predict the presence and the severity of metabolic syndrome in patients with HFpEF. Notably, the density of epicardial fat exhibited a better predictive value than EAT volume. MetS Z-score is the first metabolic syndrome scoring system among adults, which represents the severity of metabolic syndrome and have proven to be related to long-term risk for type 2 diabetes and cardiovascular diseases [27]. Several studies have used the MetS Z-score to evaluate the severity of metabolic syndrome among Chinese population [28,29,30]. In our research, lower EAT density and higher EAT volume were associated with higher levels of MetS Z-score. The EAT density presented a better predictive value than EAT volume for both the occurrence and severity of metabolic syndrome in ROC analyses, which indicated that the density of EAT might be a more sensitive marker in predicting metabolic syndrome.
From our results, lower EAT density was associated with increased risk of heart failure readmission and composite endpointsin patients with HFpEF. This finding was in good agreement with a large population-based prospective study, which showed that EAT density was significantly associated with major adverse cardiovascular events (MACE) risk in asymptomatic individuals [14]. Likewise, a community-based cohort study [12], drawn from EISNER trial, suggested a role of decreased EAT attenuation at baseline for myocardial infarction and cardiac death after adjusting for obesity measures in asymptomatic subjects. Moreover, another research [13] on asymptomatic subjects reported that EAT density was more significantly associated with myocardial infarction and cardiac death than EAT volume. These findings indicated that EAT density might add valuable information in the assessment of patient prognosis and it might not entirely attributable to the volume of adipose tissue.
Our study presented some limitations. Firstly, this is a small cohort of highly selected patients with HFpEF, and therefore our results may only apply to similar populations. Secondly, owing to the observational nature of the study, we could not establish a causal association between the measures of EAT and the clinical outcomes. Finally, all CT scans included in this study were non-electrocardiographically gated, so that the movement of the heart may lead to motion artefacts and inaccurate voxel density values for EAT.
In conclusion, EAT density measured by tissue attenuation on CT imaging might play a more important role in cardiometabolic risk than EAT volume in HFpEF patients. EAT density might have prognostic value for clinical outcomes in patients with HFpEF. HFpEF patients with decreasing EAT attenuation might receive more attention to prevent adverse clinical outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI/ARB:
-
Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker
- A-wave:
-
Peak late diastolic transmitral flow velocity
- BMI:
-
Body mass index
- CACS:
-
Coronary artery calcium score
- CCB:
-
Calcium channel blocker
- CI:
-
Confidence interval
- DBP:
-
Diastolic blood pressure
- E/A ratio:
-
E-Peak to A-Peak ratio
- EAT:
-
Epicardial adipose tissue
- E-wave:
-
The peak velocity of the filling peak in the early diastolic period
- FPG:
-
Fasting plasma glucose
- FS:
-
Fractional shortening
- HbA1c:
-
Glycosylated hemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- hsCRP:
-
High-sensitivity C-reactive protein
- LA:
-
Left atrium
- Lateral e′:
-
Lateral mitral annular early diastolic peak velocities
- LAVI:
-
Left atrial volume index
- LDL-C:
-
Low-density lipoprotein cholesterol
- LV:
-
Left ventricular
- LVEDD:
-
Left ventricular end-diastolic dimension
- LVEF:
-
Left ventricular ejection fraction
- LVESD:
-
Left ventricular end-systolic dimension
- LVMI:
-
Left ventricular mass index
- LVPW:
-
Left ventricular posterior wall end diastolic thickness
- Mean E/e′:
-
Average septal-lateral E/e′ ratio
- MetS Z-Score:
-
Metabolic syndrome severity Z score
- METS‐IR:
-
Metabolic score for insulin resistance
- NT-proBNP:
-
N-terminal pro brain natriuretic peptide
- PASP:
-
Pulmonary artery systolic pressure
- RA:
-
Right atrium
- RV:
-
Right ventricle
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- Septal e′:
-
Septal mitral annular early diastolic peak velocities
- Serum Cr:
-
Serum creatinine
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TAPSE-S:
-
Systolic velocity of tricuspid annular tissue displacement
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TG/HDL‐C:
-
The ratio of triglycerides divided by high‐density lipoprotein cholesterol
- TyG:
-
Triglyceride and glucose index
- WC:
-
Waist circumference
References
Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–79.
Abudureyimu M, Luo X, Wang X, Sowers JR, Wang W, Ge J, et al. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022;14(5):mjac028.
Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005.
Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11(6):363–71.
van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20(11):1559–66.
Bos D, Shahzad R, van Walsum T, van Vliet LJ, Franco OH, Hofman A, et al. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur Heart J Cardiovasc Imaging. 2015;16(11):1264–9.
Venkateshvaran A, Faxen UL, Hage C, Michaelsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: insights from the PROMIS-HFpEF study. Eur J Heart Fail. 2022;24(12):2251–60.
Pandey NN, Sharma S, Jagia P, Kumar S. Epicardial fat attenuation, not volume, predicts obstructive coronary artery disease and high risk plaque features in patients with atypical chest pain. Br J Radiol. 2020;93(1114):20200540.
Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):eaal2658.
Franssens BT, Nathoe HM, Leiner T, van der Graaf Y, Visseren FL, Group SS. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur J Prev Cardiol. 2017;24(6):660–70.
Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. J Am Coll Cardiol Img. 2013;6(7):762–71.
Eisenberg E, McElhinney PA, Commandeur F, Chen X, Cadet S, Goeller M, et al. Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging. 2020;13(2):e009829.
Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12(1):67–73.
Lin A, Wong ND, Razipour A, McElhinney PA, Commandeur F, Cadet SJ, et al. Metabolic syndrome, fatty liver, and artificial intelligence-based epicardial adipose tissue measures predict long-term risk of cardiac events: a prospective study. Cardiovasc Diabetol. 2021;20(1):27.
Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–317.
Wu Z, Cui H, Li W, Zhang Y, Liu L, Liu Z, et al. Comparison of three non-insulin-based insulin resistance indexes in predicting the presence and severity of coronary artery disease. Front Cardiovasc Med. 2022;9:918359.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
Mihl C, Loeffen D, Versteylen MO, Takx RA, Nelemans PJ, Nijssen EC, et al. Automated quantification of epicardial adipose tissue (EAT) in coronary CT angiography; comparison with manual assessment and correlation with coronary artery disease. J Cardiovasc Comput Tomogr. 2014;8(3):215–21.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19.
Cote JA, Nazare JA, Nadeau M, Leboeuf M, Blackburn L, Despres JP, et al. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte. 2016;5(1):35–42.
Aitken-Buck HM, Moharram M, Babakr AA, Reijers R, Van Hout I, Fomison-Nurse IC, et al. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte. 2019;8(1):412–20.
Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71(20):2360–72.
Torriani M, Oliveira AL, Azevedo DC, Bredella MA, Yu EW. Effects of Roux-en-Y gastric bypass surgery on visceral and subcutaneous fat density by computed tomography. Obes Surg. 2015;25(2):381–5.
Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213(2):649–55.
Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15(9):507–24.
DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: an analysis of the diabetes prevention program. Diabetes Care. 2018;41(11):2421–30.
Jiang B, Gu T, Zhou K, Zheng Y, Guo Y, Lu Y. Fatty liver as a potential surrogate for waist circumference in the diagnosis of metabolic syndrome: a population-based study among Chinese adults. Int J Endocrinol. 2018;2018:7903982.
Shu MJ, Zhai FF, Zhang DD, Han F, Zhou L, Ni J, et al. Metabolic syndrome, intracranial arterial stenosis and cerebral small vessel disease in community-dwelling populations. Stroke Vasc Neurol. 2021;6(4):589–94.
Chen SP, Li CR, Chang HC, Li YL, Pai HC. Relationship between metabolic syndrome severity and kidney function as related to gender: a population-based longitudinal study. Clin Nurs Res. 2020;29(6):355–62.
Acknowledgements
We thank all the investigators and subjects who participated in this project.
Funding
This work was supported by the National Natural Science Foundation of China (number 82270406, 81970203); Chongqing Health Commission (number 2022MSXM028);China Cardiovascular Health Alliance-Access Research Found (number 2021-CCA-ACCESS-130).
Author information
Authors and Affiliations
Contributions
LG, DZ, and JL contributed to the study design, ZL, LY, YZ, ZL contributed to the data acquisition, JL and QY contributed to the data analysis, LT, QD, ZZ provided technical support, JL drafted the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Human Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No.2021-473) and informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Yu, Q., Li, Z. et al. Epicardial adipose tissue density is a better predictor of cardiometabolic risk in HFpEF patients: a prospective cohort study. Cardiovasc Diabetol 22, 45 (2023). https://doi.org/10.1186/s12933-023-01778-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01778-8