Abstract
Background
The atherogenic index of plasma (AIP) has been shown to be positively correlated with cardiovascular events. However, it remains unclear whether hypertensive patients with long-term high AIP levels are at greater risk of developing heart failure (HF). Therefore, the aim of this study was to investigate the association between AIP trajectory and the incidence of HF in hypertensive patients.
Methods
This prospective study included 22,201 hypertensive patients from the Kailuan Study who underwent three waves of surveys between 2006 and 2010. Participants were free of HF or cancer before or during 2010. The AIP was calculated as the logarithmic conversion ratio of triglycerides to high-density lipoprotein cholesterol. Latent mixed modeling was employed to identify different trajectory patterns for AIP during the exposure period (2006–2010). Cox proportional hazard models were then used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for incident HF among different trajectory groups.
Results
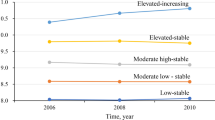
Four distinct trajectory patterns were identified through latent mixture modeling analysis: low-stable group (n = 3,373; range, -0.82 to -0.70), moderate-low stable group (n = 12,700; range, -0.12 to -0.09), moderate-high stable group (n = 5,313; range, 0.53 to 0.58), and elevated-increasing group (n = 815; range, 1.22 to 1.56). During a median follow-up period of 9.98 years, a total of 822 hypertensive participants experienced HF. After adjusting for potential confounding factors, compared with those in the low-stable group, the HR and corresponding CI for incident HF in the elevated-increasing group, moderate-high stable group, and moderate-low stable group were estimated to be 1.79 (1.21,2.66), 1.49 (1.17,1.91), and 1.27 (1.02,1.58), respectively. These findings remained consistent across subgroup analyses and sensitivity analyses.
Conclusion
Prolonged elevation of AIP in hypertensive patients is significantly associated with an increased risk of HF. This finding suggests that regular monitoring of AIP could aid in identifying individuals at a heightened risk of HF within the hypertensive population.
Similar content being viewed by others
Background
Heart failure (HF) poses a significant threat to global public health due to its substantial morbidity and mortality rates, diminished quality of life, and exorbitant medical expenses [1, 2]. It is estimated that HF affects more than over 64.3 million individuals worldwide, with an alarming one-year mortality rate ranging from 15 to 30% in the absence of appropriate treatment [3, 4]. Among the multitude of risk factors contributing to HF development, hypertension has emerged as one of the most influential modifiable factors [5]. The prevalence of hypertension in China has experienced a staggering surge over the past five decades, with approximately one out of every four Chinese adults currently afflicted by this condition [6]. Patients with hypertension face a two- to three-fold heightened risk for incident HF compared to those with normal blood pressure levels [7]. Moreover, some hospitalized HF patients have a history of hypertension upon screening, and these coexisting patients often exhibit an unfavorable prognosis and elevated mortality rates [8]. Consequently, it becomes imperative to implement viable strategies aimed at identifying high-risk populations susceptible to HF and effectively preventing its incidence.
In addition to the impact of blood pressure on vascular health, dyslipidemia commonly observed in hypertensive patients is also a pathogenic factor contributing to the development of HF [9]. Hyperlipidemia typically induces coronary atherosclerosis in individuals with hypertension, leading to the occurrence of coronary heart disease and subsequent HF episodes during advanced stages [10]. Recently, the atherogenic index of plasma (AIP), calculated as the logarithmic conversion ratio of triglyceride (TG) to high-density lipoprotein cholesterol (HDL-C), has emerged as a novel marker for assessing atherosclerosis and cardiovascular disease (CVD) [11, 12]. From the aforementioned research evidence, it could be inferred that AIP may serve as a potential risk factor for HF. However, there is a paucity of previous studies investigating the association between AIP and HF. Only one cross-sectional study has reported a plausible inverse relationship between AIP and HF in the general population [13], but the limitations of this study lie in its cross-sectional design and sole reliance on a single measurement of AIP. Furthermore, no long-term relationship has been observed between dynamic changes in AIP and HF, particularly among hypertensive individuals. To date, it remains uncertain whether individuals with persistently elevated long-term AIP levels among hypertensive patients face an increased risk of developing HF.
To address this knowledge gap, we used data from the Kailuan Study, a prospective population-based cohort study, to investigate the association between AIP trajectory patterns over 4 years and the risk of developing HF in hypertensive patients.
Methods
Study design and participants
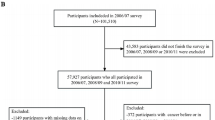
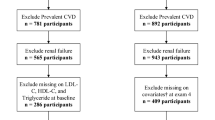
The Kailuan study is an ongoing prospective cohort study (trial registration number: ChiCTR-TNRC-11001489). In brief, all 155,418 active and retired employees aged 18 years or older of the Kailuan Group, Tangshan, China, were invited to participate in 2006. A total of 101,510 participants (81,110 males and 20,400 females, aged 18–98 years) agreed to participate and completed the first survey from June 2006 to October 2007. There were no significant demographic differences between those who agreed to participate in the study and those who declined, except for a change in gender distribution (79.9% of the participants were male, while 85.9% of the Kailuan Group employees were male). All participants underwent questionnaire assessment, physical examination, and laboratory tests at 11 hospitals responsible for Kailuan community. These participants were followed up every two years to update their status on the above parameters [14]. Details of the Kailuan Study cohort design, methods, and data collection have been published previously [15, 16]. In brief, a total of 101,510 community residents aged 18 years and older were recruited from 2006 to 2007 and received standardized questionnaires and clinical examinations at 11 community health service facilities. Of these, 23,060 were identified as having hypertension at baseline (2006–2007). 452 participants with a history of heart failure or cancer before 2006 and during 2006–2011 were excluded. In addition, 407 participants with TG or HDL-C deficiency before the third physical examination (2010–2011) were excluded. A total of 22,201 participants were finally included (Fig. 1). The AIP index trajectory was established for the exposure period (2006 to 2010) to predict the risk of HF for participants from 2010 to 2020. This study was approved by the Ethics Committee of Kailuan General Hospital according to the guidelines outlined in the Declaration of Helsinki. All participants agreed to participate in this study and provided written informed consent.
Data collection and definition
Face-to-face interviews were conducted with all participants, and structured questionnaires were used to collect information on demographic characteristics (e.g., age, sex), self-reported medical history (e.g., cardiovascular disease, hypertension, diabetes, ) medication history (e.g., antihypertensive, lipid-lowering, hypoglycemic drugs), and lifestyle (e.g., smoking status, drinking status, physical activity). Current smokers were defined as having smoked for more than 1 year, with an average of ≥ 1 cigarette per day, and were still smoking in the last year. Current drinkers were defined as having consumed alcohol for more than 1 year, with an average alcohol consumption ≥ 100 ml/ day, and were still drinking in the last year. Smoking and drinking status were graded as yes or no. Active physical exercise was defined as exercise ≥ 4 times a week, each time duration at least 20 minutes [17]. Body mass index (BMI) was calculated as weight (kg)/ height (m2). Education level was divided into junior high school or below and senior high school or above. Blood pressure (BP) was measured by a trained physician according to a standardized protocol, using a calibrated mercury sphygmomanometer to detect blood pressure (BP) in the left upper arm at least twice while the participant was seated. If the difference between the two measurements was ≥ 5 mmHg, BP was measured again and the average of the three BP measurements was recorded [18]. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, or/and diastolic blood pressure (DBP) ≥ 90 mmHg, or/and a history of clearly diagnosed hypertension or/and taking antihypertensive drugs [19]. Diabetes was defined as FPG ≥ 7.0 mmol/L, or/and a definite history of diabetes or/and use of hypoglycemic drugs [20].
Biochemical indicators and AIP index
A blood sample of 5 mL was taken from the anterior elbow vein at 7–9 a.m. on the day of the physical examination after the participants had fasted for at least 8 h, and the blood was injected into a vacuum tube containing EDTA. The supernatant was centrifuged at room temperature and measured within 4 h. Biochemical indices such as total cholesterol (TC), C-reactive protein (hs-CRP), FPG, TG were measured by reviewers using an automated analyzer (Hitachi 747, Tokyo, Japan) in the hospital central laboratory [21]. AIP index was calculated as the Log transformation of the ratio of triglyceride to high-density lipoprotein cholesterol (AIP = log (TG/HDL-C)) as previously described [11].
HF and follow-up
The primary outcome was the first occurrence of HF events, with follow-up starting at the 2010 annual physical examination and continuing until the date of HF onset, death, or the end of follow-up (December 31, 2020), whichever came first. We used ICD-10 revision code I50.x to identify HF cases [22]. Discharge records from 11 local hospitals were collected and reviewed annually by specialized teams to identify patients with suspected HF. The definition of HF was revised according to the diagnostic criteria of the Chinese Guidelines for the Diagnosis and Treatment of Heart Failure [23]. A thorough review of the chart identified the following: (1) the presence of symptoms of HF, such as dyspnea, fatigue, and fluid retention. NYHA class II, III, IV or Killip class II, III, IV; (2) Left ventricular ejection fraction ≤ 50% measured by two-dimensional and Doppler echocardiography (modified Simpson method); (3) increased plasma level of natriuretic peptide (NT-proBNP). Diagnosis of HF requires at least condition (1) and at least one of conditions (2) and (3).
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). We used AIP trajectories from 2006 to 2010 as the primary exposure. AIP trajectories were determined by latent mixture modeling in the SAS Proc-Traj program [24,25,26]. The Bayesian Information Criterion (BIC) was used to assess model fit and the model with four patterns was determined to be the best fit. Continuous and normally distributed data were described by mean ± standard deviation (x̅±s). One-way ANOVA or Kruskal-Wallis test was used for comparison between groups. Continuous data with skewed distribution were described as medians and interquartile ranges (25% and 75%), and comparisons between groups were performed with the use of the Wilcoxon rank-sum test. Categorical variables were summarized as numbers and percentages (%), and chi-square tests were used for between-group comparisons. The cumulative incidence of HF for each trajectory group was calculated with the use of the Kaplan-Meier method and compared with the use of the log-rank test. In addition, Cox proportional hazard models were developed to analyze HR and 95%CI for HF in the different trajectory groups, and the proportional hazards assumption was met. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, BMI, hs-CRP, TC, eGFR, current smokers, current drinkers, physical activity, education level, hypertension, and diabetes. Further adjustments for the use of antihypertensive, hypoglycemic, and lipid-lowering drugs were made in model 3. Subgroup analyses were performed by sex (male vs. female), age (< 65 years vs. ≥65 years), BMI (< 28 kg/m2 vs. ≥28 kg/m2), BP (< 140/90 mmHg Vs. ≥140/90 mmHg) and diabetes (Yes vs. No). We performed several sensitivity analyses to test the robustness of our results. First, to minimize possible reverse causality, participants with an event of HF within 2 years were excluded. Second, we excluded participants who had a previous myocardial infarction, given the possible effect on heart failure after a myocardial infarction. Third, participants using antihypertensive, hypoglycemic, or lipid-lowering drugs were excluded, respectively, to account for the effect of different drugs on the results. Finally, to explore whether the association between AIP index trajectories and outcomes could be attributed to the effect of a single measurement of AIP during the period from 2006 to 2010, we further adjusted for AIP measurements in 2006 and 2010. In this study, two-sided p values < 0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the participants were shown in Table 1. A total of 22,201 participants were included in this study, with an average age of 57.54 ± 11.18 years, 18,258 men (82.2%) and 3,943 women (17.8%). In this study, we identified four different trajectory patterns through latent mixture modeling (Fig. 2): Low-stable group (participants who maintained low AIP levels, n = 3,373; mean AIP range, -0.82 to -0.70), moderate-low stable group (participants who maintained moderate-low AIP levels, n = 12,700; mean AIP range, -0.12 to -0.09), moderate-high stable group (participants who maintained moderate-high AIP levels, n = 5,313; mean AIP range, 0.53 to 0.58) and elevated-increasing group (AIP levels increased initially and then increased slightly, n = 815; mean AIP range, 1.22 to 1.56). Compared with those in the low-stable group, participants in the elevated-increasing group were more likely to be young, male, physically inactive, current smokers, current drinkers, have diabetes, and were more likely to take antihypertensive, hypoglycemic, and lipid-lowering drugs. They also had higher levels of BMI, SBP, DBP, LDL-C, TG, TC, Hs-CRP and lower level of HDL-C.
Relationship between HF risk and AIP index trajectory
During a median follow-up of 9.98 (9.49, 10.28) years, 822 hypertensive participants experienced an event of HF. Kaplan-meier curves showed that participants in the elevated-increasing group had a higher risk of HF than those in the other trajectory groups (log-rank test, p < 0.01, Fig. 3). After adjusting for potential confounding factors, compared with the low-stable group, the HR and 95%CI of the elevated-increasing group, moderate-high stable group, and moderate-low stable group were 1.79 (1.21,2.66), 1.49 (1.17,1.91), and 1.27 (1.02,1.58), respectively (Table 2).
Subgroup and sensitivity analysis
The results of the subgroup analyses are shown in Table 3, we found significant interactions between AIP index trajectories and age, but not with sex, BMI, BP or diabetes in hypertensive participants. In sensitivity analyses that excluded outcome events that occurred during the first year of follow-up (n = 158) or participants with a history of myocardial infarction (n = 71), the results remained consistent with the main results (Additional file: Tables S1-S2). Results were similar to the primary analyses after exclusion of participants treated with antihypertensive drugs (n = 5,550), hypoglycemic drugs (n = 1,335), or lipid-lowering drugs (n = 373) (Additional file: Tables S3). In addition, the relationship between the AIP Index trajectory and HF risk did not change substantially after additional adjustments to the AIP Index in 2006 or 2010. (Additional file: Tables S4-S5).
Discussion
In this prospective study of 22,201 participants with hypertension, we identified four distinct trajectories of AIP associated with incident HF. Hypertensive patients with a long-term high AIP level had a significantly higher risk of HF compared to those in the low-stable group, independent of baseline AIP. These findings were consistent across subgroup and sensitivity analyses.
To our knowledge, previous studies have primarily focused on the association between AIP and CVD in the general population, but limited research has explored the relationship between AIP and incident HF, specifically among individuals with hypertension. In previous studies, Zhang et al. [27] found that high baseline AIP and long-term AIP were associated with an increased risk of myocardial infarction after analyzing a general population that included 98,861 people from northern China. However, in contrast to the present study, the population included by Zhang et al. had a lower baseline AIP value than the hypertensive population in the present study, and their risk of outcome was relatively low. Moreover, a study involving 181,133 US adults showed a J-shaped relationship between baseline AIP levels and all-cause mortality in the US general population, with a significant positive association between AIP and all-cause mortality risk when baseline AIP ≥ 0.0905 [28]. Similarly, lower AIP values and lower risk for outcomes were observed in the general US population compared with the hypertensive population in our study. The above results suggest that people with hypertension may have higher AIP values and a higher risk of adverse events compared to the general population. In addition, another large-scale nationwide cohort study demonstrated that high AIP level was associated with an increased risk of major adverse cardiovascular events (including myocardial infarction, stroke, and CVD mortality) in the general population beyond traditional risk factors [29]. However, they only consider a single measurement of AIP. Given that HF often represents an inevitable manifestation of CVD progression such as myocardial infarction and coronary heart disease at later stages, previous evidence suggests that AIP may serve as a potential risk factor for HF development [30,31,32]. However, Xue et al. conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey, which paradoxically showed an inverse correlation between AIP and HF in the general population [33], yet this analysis may be limited by its nature and lack of long-term follow-up on changes in AIP levels. In contrast to prior research limitations, we employed a trajectory model to illustrate the patterns of AIP changes over a 4-year period and examined its long-term impact on the risk of HF among hypertensive patients. After adjusting for potential confounders, our study expands the existing database by demonstrating that hypertensive patients with persistently elevated AIP levels over time face an increased risk of HF compared to those with consistently low AIP levels. Notably, this association remains robust even after additional adjustment for baseline AIP level. Therefore, regular monitoring of the AIP index may serve as a valuable tool in identifying individuals at higher risk of HF within the hypertensive population, thereby providing novel evidence for HF prevention in clinical practice.
Notably, we observed a significant interaction between the trajectory of AIP and age. We found that young hypertensive patients with elevated AIP levels face a higher risk of HF compared to older hypertensive patients, indicating that the association is more pronounced among younger individuals with hypertension. In recent years, there has been an evident trend towards hypertension occurring at younger ages, with numerous studies demonstrated the heightened CVD risk faced by young individuals with hypertension, high TG or metabolic abnormalities [18, 34, 35]. AIP is calculated as the logarithmic conversion ratio of TG to HDL-C, and young hypertensive patients commonly exhibit dyslipidemia characterized by high TG levels and low HDL-C levels, leading to increased AIP values and more severe arteriosclerosis. Previous studies had demonstrated that AIP plays a crucial role in explaining metabolic disorders and CVD risk in younger populations [36]. A cross-sectional study involving 52,380 community residents revealed that AIP level had a stronger impact on carotid atherosclerosis prevalence in participants under 60 years old than those aged 60 or above in the general population [37], supporting our findings indirectly.
The mechanism underlying the relationship between long-term changes in AIP index and risk of HF is unclear, but the following may be involved. First, AIP can be used as a marker for the presence of low-density LDL (sd-LDL) [31]. The sd-LDL particles are more likely to penetrate the arterial wall and deposit in the vascular endothelium than larger, loose-grained LDL particles [32]. In addition, the sd-LDL is more sensitive to oxidative stress, which further aggravates atherosclerosis and leads to the occurrence of HF [33]. Second, AIP was also significantly associated with FERHDL [31], with the partial esterification rate of HDL cholesterol (FERHDL) defined as the percentage of HDL free cholesterol (HDLFC) after Apo b depletion during HDL development [38]. A clinical trial confirmed the association between FERHDL and cardiovascular disease risk factors [39]. Another study showed that FERHDL and LDL particle size are predictors of insulin resistance [40, 41]. Insulin resistance and its related hyperinsulinemia, hyperglycemia and adipocytokines can also cause vascular endothelial dysfunction, vascular inflammation, hypertension and dyslipidemia, which together promote the occurrence and development of HF. Finally, participants with long-term high AIP levels had higher SBP, DBP, BMI, TC, and TG, and were current smokers or drinkers, which are important risk factors for HF and may significantly increase the risk of HF.
The significance of our study lies in the finding that long-term elevated AIP levels are positively correlated with the risk of HF in hypertensive patients, thereby expanding the potential application of AIP in the population with hypertension. Beyond conventional risk factors, AIP is considered an effective, cost-efficient, rapid, specific, and non-invasive mass screening method. It has been endorsed by the National Cholesterol Education Program as a marker for plasma atherosclerosis and a reliable predictor for CVD [42]. Although hospitalizations for HF have shown a slight decline since the 1990s, these trends exhibit variations across different age groups [43]. Generally speaking, HF is a prevalent latent disease associated with substantial morbidity and mortality worldwide, particularly among elderly individuals. However, recent research indicates an alarming increase in hospitalizations for HF among younger patients [44], emphasizing the importance of assessing HF risk in this population group. Our subgroup analysis on age revealed that young hypertensive patients with elevated AIP levels face an increased risk of developing HF. This further underscores the necessity to monitor AIP levels in younger hypertensive patients. In addition, previous studies have also shown that increased AIP index is associated with many CVD risks in the general population [12, 45]. AIP can be used as a potential early warning indicator to predict the risk of developing various CVD. Previous studies have shown an independent inverse association between moderate to vigorous physical activity and AIP [46]. It has also been shown that w86 flaxseed significantly reduces AIP and can be part of a heart-healthy and anti-AS diet in humans [47]. Compared with diet and exercise, the role of drugs is also very important. The combination therapy with ciprofibrate and statins and niacin can significantly reduce AIP [48]. Through medication, exercise, diet can reduce AIP value to a certain extent. Therefore, early monitoring of AIP value change, maintaining appropriate TG and HDL-C levels within the scope of the ideal, is beneficial to early discover and prevent the happening of cardiovascular events.
Our study possesses several notable strengths, including that the trajectories representing changes in AIP levels over time in hypertensive patients was established and this is the first large-scale prospective investigation with a 10-year follow-up duration to explore the association between longitudinal AIP patterns and incident HF in hypertensive patients. Furthermore, the Kailuan study cohort underwent rigorous biennial physical examinations to ensure strict follow-up, and the study population exhibited relative stability, enabling continuous monitoring of major outcomes throughout the entire process, thereby enhancing the reliability of the results to a certain extent. However, it is important to acknowledge certain limitations within our study. Firstly, as is true of any observational study, residual confounding may exist despite adjustments for potential covariates, and we could not determine a causal relationship between AIP trajectories and risk of HF. Secondly, the specific subtypes of HF were not extensively documented or identified in detail, which could potentially impact the clinical applicability of our findings. Lastly, generalizability may be limited as our participant pool consisted solely of northern Chinese adults residing in the Kailuan community, and fewer of them are women. Further investigations involving diverse ethnic populations are warranted to validate these results.
Conclusions
In conclusion, hypertensive patients with consistently elevated levels of AIP were at greater risk of HF, independent of baseline AIP and possibly age-dependent, with the association being more pronounced in younger age groups. Therefore, our findings highlight the importance of continuous monitoring of changes in AIP levels among patients with hypertension in clinical practice for the preventing of HF occurrence.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AIP:
-
Atherogenic index of plasma
- HF:
-
Heart failure
- CVD:
-
Cardiovascular disease
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglyceride
- BMI:
-
Body mass index
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein-cholesterol
- LDL-C:
-
Low-density lipoprotein-cholesterol
- hs-CRP:
-
High-sensitivity C-reactive protein
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Adamo M, Gardner RS, McDonagh TA, Metra M. The ‘Ten commandments’ of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2022;43(6):440–1.
Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25.
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–87.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Di Palo KE, Barone NJ. Hypertension and heart failure: prevention, targets, and treatment. Heart Fail Clin. 2020;16(1):99–106.
Wang JG, Zhang W, Li Y, Liu L. Hypertension in China: epidemiology and treatment initiatives. Nat Rev Cardiol. 2023;20(8):531–45.
Pfeffer MA. Heart failure and hypertension: importance of prevention. Med Clin North Am. 2017;101(1):19–28.
Georgiopoulou VV, Kalogeropoulos AP, Butler J. Heart failure in hypertension: prevention and treatment. Drugs. 2012;72(10):1373–98.
Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes Mellitus, Hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e535–78.
Yao YS, Li TD, Zeng ZH. Mechanisms underlying direct actions of hyperlipidemia on myocardium: an updated review. Lipids Health Dis. 2020;19(1):23.
Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–94.
Min Q, Wu Z, Yao J, Wang S, Duan L, Liu S, Zhang M, Luo Y, Ye D, Huang Y, et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc Diabetol. 2024;23(1):54.
Xue J, He L, Xie H, Xie X, Wang H. An inverse correlation between the atherogenic index of plasma and heart failure: an analysis of the National Health and Nutrition Examination Survey 2017-March 2020 pre-pandemic data. J Cardiovasc Dev Dis. 2022;9(12):42.
Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5(4):487–93.
Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris-Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;2(5):e194758.
Zhou H, Ding X, Lan Y, Chen S, Wu S, Wu D. Multi-trajectories of triglyceride-glucose index and lifestyle with cardiovascular disease: a cohort study. Cardiovasc Diabetol. 2023;22(1):341.
Xia X, Chen S, Tian X, Xu Q, Zhang Y, Zhang X, Li J, Wu S, Wang A. Association of triglyceride-glucose index and its related parameters with atherosclerotic cardiovascular disease: evidence from a 15-year follow-up of Kailuan cohort. Cardiovasc Diabetol. 2024;23(1):208.
Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75(23):2921–30.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7.
Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, Wu S. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. 2020;127(12):1491–8.
Huo Z, Huang Z, Feng J, Li J, Chen S, Wang G, Peng Y, Huang L, Wu S, Gao X, et al. Life’s Essential 8 and heart failure among patients with chronic kidney disease: the Kailuan Cohort Study. Eur J Prev Cardiol. 2024;31(7):824–31.
Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association; Chinese Heart Failure Association of Chinese Medical Doctor Association; Editorial Board of Chinese Journal of Cardiology. [Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46(10):760–89.
Jones BLND, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–93.
Jones BLND. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–71.
Huang Z, Ding X, Yue Q, Wang X, Chen Z, Cai Z, Li W, Cai Z, Chen G, Lan Y, et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):141.
Zhang Y, Wu S, Tian X, Xu Q, Xia X, Zhang X, Li J, Chen S, Liu F, Wang A. Elevated atherogenic index of plasma increased the risk of myocardial infarction in a general population. Ann Epidemiol. 2024;90:1–8.
Qin M, Chen B. Association of atherogenic index of plasma with cardiovascular disease mortality and all-cause mortality in the general US adult population: results from NHANES 2005–2018. Cardiovasc Diabetol. 2024;23(1):255.
Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, Huh JH, Kang JG, Lee SJ, Ihm SH. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. 2022;21(1):81.
Nam JS, Kim MK, Nam JY, Park K, Kang S, Ahn CW, Park JS. Association between atherogenic index of plasma and coronary artery calcification progression in Korean adults. Lipids Health Dis. 2020;19(1):157.
Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apob-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–8.
Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis. 1996;124(2):261–71.
Frohlich J, Dobiásová M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49(11):1873–80.
Huang Z, Wang X, Ding X, Cai Z, Li W, Chen Z, Fang W, Cai Z, Lan Y, Chen G, et al. Association of age of metabolic syndrome onset with cardiovascular diseases: the Kailuan study. Front Endocrinol (Lausanne). 2022;13:857985.
Zhou H, Ding X, Yang Q, Chen S, Li Y, Zhou X, Wu S. Associations of hypertriglyceridemia onset age with cardiovascular disease and all-cause mortality in adults: a cohort study. J Am Heart Assoc. 2022;11(20):e026632.
Płaczkowska S, Sołkiewicz K, Bednarz-Misa I, Kratz EM. Atherogenic plasma index or non-high-density lipoproteins as markers best reflecting age-related high concentrations of small dense low-density lipoproteins. Int J Mol Sci. 2022;23(9):5089.
Huang Q, Liu Z, Wei M, Huang Q, Feng J, Liu Z, Xia J. The atherogenic index of plasma and carotid atherosclerosis in a community population: a population-based cohort study in China. Cardiovasc Diabetol. 2023;22(1):125.
Liu J, Yang R, Zhou M, Mao W, Li H, Zhao H, Wang S, Chen W, Dong J, He Q. Fractional esterification rate of cholesterol in high-density lipoprotein associates with risk of coronary heart disease. Lipids Health Dis. 2017;16(1):162.
Dong J, Yu S, Yang R, Li H, Guo H, Zhao H, Wang S, Chen W. A simple and precise method for direct measurement of fractional esterification rate of high density lipoprotein cholesterol by high performance liquid chromatography. Clin Chem Lab Med. 2014;52(4):557–64.
von Schenck H, Wallentin L, Lennmarken C, Larsson J. Lipoprotein metabolism following gastroplasty in obese women. Scand J Clin Lab Invest. 1992;52(4):269–74.
Gray RS, Robbins DC, Wang W, Yeh JL, Fabsitz RR, Cowan LD, Welty TK, Lee ET, Krauss RM, Howard BV. Relation of LDL size to the insulin resistance syndrome and coronary heart disease in American indians. The strong heart study. Arterioscler Thromb Vasc Biol. 1997;17(11):2713–20.
Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Chan DZL, Kerr A, Grey C, Selak V, Lee MAW, Lund M, Poppe K, Doughty RN. Contrasting trends in heart failure incidence in younger and older New Zealanders, 2006–2018. Heart. 2022;108(4):300–6.
Jain V, Minhas AMK, Khan SU, Greene SJ, Pandey A, Van Spall HGC, Fonarow GC, Mentz RJ, Butler J, Khan MS. Trends in HF Hospitalizations among young adults in the United States from 2004 to 2018. JACC Heart Fail. 2022;10(5):350–62.
Zhang Y, Chen S, Tian X, Wang P, Xu Q, Xia X, Zhang X, Li J, Liu F, Wu S, et al. Association between cumulative atherogenic index of plasma exposure and risk of myocardial infarction in the general population. Cardiovasc Diabetol. 2023;22(1):210.
Edwards MK, Loprinzi PD. Physical activity and diet on atherogenic index of plasma among adults in the United States: mediation considerations by central adiposity. Eur J Clin Nutr. 2018;72(6):826–31.
Króliczewska B, Miśta D, Ziarnik A, Żuk M, Szopa J, Pecka-Kiełb E, Zawadzki W, Króliczewski J. The effects of seed from Linum usitatissimum cultivar with increased phenylpropanoid compounds and hydrolysable tannin in a high cholesterol-fed rabbit. Lipids Health Dis. 2018;17(1):76.
Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7(2):203–19.
Acknowledgements
We sincerely thank all the research participants and members of the Kailuan Research Group for their contributions.
Funding
This work has been supported by Science and Technology Innovation Strategy Special Project of Guangdong (No. 221117237489175) and Shantou Medical Health Science and Technology Plan (NO. 210624106490813).
Author information
Authors and Affiliations
Contributions
The study idea was designed by HCZ, SLW and YRC; HCZ, ZGH, KYW and WQW analyzed and interpreted the data; HCZ, ZGH, XXW, PF, YXW, ZFC, ZKC, YLL and ZWC were responsible for drafting the manuscript. The manuscript was reviewed by SLW and YRC. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05). All participants agreed to participate in the study and provided informed written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, H., Huang, Z., Wu, K. et al. Association between the atherogenic index of plasma trajectory and risk of heart failure among hypertensive patients: a prospective cohort study. Cardiovasc Diabetol 23, 301 (2024). https://doi.org/10.1186/s12933-024-02375-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02375-z