Abstract
Background
A high triglyceride-glucose index (TyG) is associated with a higher risk of incident heart failure. However, the effects of longitudinal patterns of TyG index on the risk of heart failure remain to be characterized. Therefore, in the present study, we aimed to characterize the relationship between the trajectory of TyG index and the risk of heart failure.
Methods
We performed a prospective study of 56,149 participants in the Kailuan study who attended three consecutive surveys in 2006–2007, 2008–2009, and 2010–2011 and had no history of heart failure or cancer before the third wave survey (2010–2011). The TyG index was calculated as ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2], and we used latent mixture modeling to characterize the trajectory of the TyG index over the period 2006–2010. Additionally, Cox proportional risk models were used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for incident heart failure for the various TyG index trajectory groups.
Results
From 2006 to 2010, four different TyG trajectories were identified: low-stable (n = 13,554; range, 7.98–8.07), moderate low-stable (n = 29,435; range, 8.60–8.65), moderate high-stable (n = 11,262; range, 9.31–9.30), and elevated-stable (n = 1,898; range, 10.04–10.25). A total of 1,312 new heart failure events occurred during a median follow-up period of 10.04 years. After adjustment for potential confounders, the hazard ratios (HRs) and 95% confidence intervals (CIs) for incident heart failure for the elevated-stable, moderate high-stable, and moderate low-stable groups were 1.55 (1.15, 2.08), 1.32 (1.08, 1.60), and 1.17 (0.99, 1.37), respectively, compared to the low-stable group.
Conclusions
Higher TyG index trajectories were associated with a higher risk of heart failure. This suggests that monitoring TyG index trajectory may help identify individuals at high risk for heart failure and highlights the importance of early control of blood glucose and lipids for the prevention of heart failure.
Similar content being viewed by others
Background
Heart failure (HF) is a growing epidemic causing significant morbidity and mortality globally, with an estimated prevalence of more than 64.3 million worldwide [1, 2]. Epidemiologic studies have shown that approximately 1-2% of adults suffer from HF in developed countries, and this prevalence rises to more than 10% in people aged 70 years or older [3, 4]. In China, the prevalence of HF in adults increased from 0.9% in 2000 to 1.3% in 2015, representing an increase of more than 9 million cases [5, 6]. Given the enormous socioeconomic and public health burdens associated with HF, it is critical to identify risk factors and high-risk populations for HF and implement effective preventive measures as early as possible.
Insulin resistance (IR) is important in the pathogenesis of type 2 diabetes and the metabolic syndrome and is associated with adverse ventricular remodeling and dysfunction [7]. Previous molecular studies have provided ample evidence for the etiologic role of IR in the development of HF [8, 9]. The hyperinsulinemic-euglycemic clamp is currently considered to be the gold-standard method for the evaluation of IR, but it is time-consuming, labor-intensive, and invasive, and therefore is difficult to implement in large-scale clinical practice [10]. However, the triglyceride-glucose index (TyG), which can be calculated using the fasting plasma glucose (FPG) and fasting triglyceride (TG) concentrations, has been shown to be a simple and reliable surrogate marker of IR [11, 12]. Previous studies have shown that a high TyG index is associated with higher risks of various cardiovascular diseases, including HF, stroke, and myocardial infarction [13,14,15]. However, most previous studies of the relationship between TyG index and HF only used TyG index calculated on a single measurement and did not explore the longitudinal relationship between changes in TyG index over time and HF events in the general population. Therefore, in the present study, we aimed to characterize the relationship between the longitudinal pattern of TyG index and the risk of HF in the general population by using the TyG index trajectory of the Kailuan large community cohort study.
Methods
Study design and participants
The Kailuan study is a community-based prospective cohort study being conducted in Tangshan, China, that aims to identify risk factors for chronic non-communicable diseases such as cardiovascular and cerebrovascular diseases. The study design and methods have been described in detail elsewhere [16, 17]. In 2006 and 2007, 101,510 employees and retirees of the Kailuan Group were recruited to participate in the baseline survey, then a comprehensive examination was performed every 2 years, which included the completion of questionnaires (concerning the demographic characteristics and lifestyle of the participants), a physical examination (including the measurement of body mass, height, and blood pressure), and laboratory testing (including the measurement of FPG and lipid concentrations). In the present study, we designated 2006–2010 as the “trajectory identification period” and 2010–2020 as the “survival analysis period,” with the aim of determining the effect of TyG trajectory on the risk of HF in the general population by following participants who underwent examinations in 2006, 2008, and 2010 (Fig. 1A).
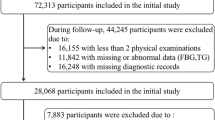
A total of 57,927 participants participated in the first three surveys (2006–2007, 2008–2009, and 2010–2011), 257 participants who had HF and 372 participants who had cancer before 2006 or between 2006 and 2010 were excluded. In addition, 1,149 participants with missing TG or FPG data before the third wave of the survey (2010–2011) were also excluded. Finally, a total of 56,149 participants were included in this study (Fig. 1B). In addition, the trajectories of the TyG index in each participant over the exposure period (2006–2010) were characterized, to assess their utility for the prediction of the risk of HF in the general population during 2010–2020. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kailuan General Hospital. All participants agreed to participate in research and to provide a written informed consent.
Data collection and definitions
All the participants underwent baseline and follow-up surveys at one of 11 hospitals in the Kailuan community. A structured questionnaire was used to collect information regarding patient demographics, including their age, sex, educational level and coal mine occupational history; their medical history, for example of hypertension, diabetes mellitus, and arrhythmia; their history of medication, including anti-hypertensive, lipid-lowering, and hypoglycemic drugs; and their lifestyle, including their physical activity, smoking status, and drinking status.
Smoking and drinking status were categorized into two levels: current and never or previous. Physically active individuals were defined as those who exercised ≥ 4 times per week for at least 20 min on each occasion [16]. Body mass index (BMI) was calculated as body mass (kg)/height squared (m2). The blood pressure of each participant was measured at least twice using a calibrated mercury sphygmomanometer while they were seated, and if the difference between the two measurements was ≥ 5 mmHg, the blood pressure was measured again, and the mean of the three values was recorded. Hypertension was defined using a systolic blood pressure (SBP)/diastolic blood pressure (DBP) ≥ 140/90 mmHg, the use of antihypertensive medication, or a self-reported history of hypertension [18]. Diabetes mellitus was defined using an FPG ≥ 7.0 mmol/L, the use of hypoglycemic medication, or a self-reported history of diabetes [19]. Biochemical indices, such as TG, FPG, total cholesterol (TC), high-sensitivity C-reactive protein (hs-CRP), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C), were measured by professional technicians using an automated analyzer (Hitachi 747, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) of each participant was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI 2009) equation [20]. The TyG index was calculated as ln [TG (mg/dL) × FPG (mg/dL)/2] [21].
Assessment of heart failure
The primary outcome of the study was the first occurrence of HF, which was assessed annually during follow-up from the baseline examination until the date of HF onset, death, or the end of follow-up (December 31, 2020), whichever came first. Potential HF events were also identified using information obtained from four additional sources: (1) the municipal social security agency, (2) the hospital discharge registry, (3) death certificates, and (4) a questionnaire that was completed every 2 years from 2006. The diagnostic criteria used for HF were those in the Guidelines for the Diagnosis of Chronic Heart Failure of the European Society of Cardiology [22]; and the following were obtained through a review of the medical records: (1) clinical symptoms of HF, including dyspnea, fatigue, and fluid retention, NYHA heart function classes II, III, and IV, and Killip heart function classes II, III, and IV; (2) high plasma natriuretic peptide (NT-proBNP) concentrations; and (3) left ventricular ejection fraction ≤ 50%, measured using two-dimensional and the modified Simpson method of Doppler echocardiography. For a diagnosis of HF to be made, (1) and at least one of (2) and (3) were required.
Statistical analysis
Statistical analyses were performed using SAS v.9.4 (SAS Institute, Inc, Cary, NC, USA) software. The trajectories in TyG index between 2006 and 2010 were characterized by latent mixture modeling using the SAS Proc-Traj program [23, 24]. The Bayesian Information Criterion (BIC) was used to assess model fit, and a model with four patterns was determined to show the best fit. In the baseline description, normally distributed continuous variables were described as mean ± standard deviation (SD), skewed continuous variables were described as median within the interquartile range (25-75%), and frequencies and percentages (%) were used to describe categorical variables. Continuous variables were compared according to distribution using ANOVA or Kruskal-Wallis tests, and categorical variables were compared using chi-square tests. Incidence rate was measured as the number of events divided by the total number of person-years of follow-up and is expressed as the number of events per 1,000 person-years.
The Kaplan-Meier method was used to calculate the cumulative incidence of HF for each trajectory group and these were compared using the log-rank test. In addition, in order to evaluate the relationship between the TyG index and the risk of HF for these groups, multivariate Cox proportional hazards regression analysis was performed, and the proportional hazards assumption was met. Model 1 was adjusted for sex and age; Model 2 was further adjusted for BMI, hs-CRP, eGFR, HDL-C, LDL-C, coal miner, smoking status (current smoker or not), drinking status (current drinker or not), physical activity status (active or not), educational level (junior high school or below, or senior high school or above), hypertension (yes or no), and diabetes mellitus (yes or no); and Model 3 was further adjusted for the use of antihypertensive drugs (yes or no), lipid-lowering drugs (yes or no), and hypoglycemic drugs (yes or no). Subgroup analyses were performed following stratification according to sex (male vs. female), age (< 60 years vs. ≥ 60 years), BMI (< 28 kg/m2vs. ≥ 28 kg/m2), and hypertension (yes vs. no).
Finally, several sensitivity analyses were performed to test the robustness of the results. First, to minimize the possibility of reverse causality, participants who experienced an HF event within the first year were excluded. Second, given the possible effect of myocardial infarction or the presence of NAFLD on HF, participants with a history of myocardial infarction or NAFLD were excluded. Third, to account for the effect of different medication, four sensitivity analyses were performed, by excluding participants who were taking hypoglycemic drugs, antihypertensive, lipid-lowering drugs, or beta-blockers. Additionally, the incident HF analysis was repeated using the Cause-Specific model, using death owing to non-HF causes as a competing risk. To determine whether this association could be explained by the TyG index during the follow-up period, additional adjustments were made according to the values of TyG in 2006 and 2010. A two-sided P < 0.05 was considered to represent statistical significance.
Results
Baseline characteristics
The baseline characteristics of the participants are shown in Table 1. A total of 56,149 eligible participants were included in the present study. Their mean age was 53.47 ± 12.05 years, and 13,141 were women (23.4%), and 43,008 were men (76.6%). Four distinct trajectory patterns were identified by latent mixture modeling, based on the pattern of change in TyG index between 2006 and 2010 (Fig. 2): low-stable (mean TyG ranged from 7.98 in 2006 to 8.07 in 2010); moderate low-stable (mean TyG ranged from 8.60 in 2006 to 8.65 in 2010); moderate high-stable (mean TyG ranged from 9.31 in 2006 to 9.30 in 2010); and elevated-stable (mean TyG ranged from 10.04 in 2006 to 10.25 in 2010). Compared with the low-stable group, participants in the moderate low-stable, moderate high-stable and elevated-stable group were male; had higher BMI, SBP, DBP, TG, LDL-C, FPG and hs-CRP; were more likely to be coal miners, current smokers and drinkers; had a higher prevalence of diabetes mellitus; and were more likely to be taking medications.
Relationship between the risk of heart failure and the trajectory of TyG index
During a median follow-up of 10.04 years, 1,312 HF events occurred. Kaplan-Meier analysis showed that participants in the elevated-stable TyG group were at a higher risk of developing HF than the other trajectory groups, and the differences in cumulative incidence of HF among the groups were statistically significant (log-rank test, P < 0.01) (Fig. 3). After adjusting for potential confounding factors, and compared with the low-stable group, the HRs and 95% CIs of the elevated-stable group, moderate high-stable group, and moderate low-stable group were 1.55 (1.15, 2.08), 1.32 (1.08, 1.60), and 1.17 (0.99, 1.37), respectively (Table 2).
Results of the subgroup and sensitivity analysis
The results of the subgroup analyses are shown in Table 3. We found a significant interaction of TyG trajectory with age, but no significant interactions with sex, BMI, or hypertension. With respect to the sensitivity analyses, after the exclusion of participants who experienced an HF event within the first year, the results were consistent with those of the primary analysis (Table S1). Moreover, the results remained stable after the exclusion of participants with a history of myocardial infarction or NAFLD (Table S2 and S3). In addition, the results were consistent after the exclusion of participants who were taking hypoglycemic drugs, anti-hypertensive drugs, lipid-lowering drugs, or beta-blockers (Table S4 and S5). Considering that non-HF-related death is a competing event, we assessed the relationship between the TyG index trajectory and new-onset HF using cause-specific model, and the result was consistent with the primary results (Table S6). Finally, the relationship between TyG index trajectory and heart failure risk did not change substantially after additional adjustments for TyG index in 2006 or 2010 (Table S7 and S8).
Discussion
In this prospective cohort study of 56,149 participants, we identified four different patterns of TyG trajectory. Compared with the low-stable group, participants with a long-term high TyG were at a higher risk of HF after 10 years of follow-up, independent of the baseline TyG index. Similar findings were observed in both subgroup and sensitivity analyses.
The relationship between TyG index and the risk of developing several conditions (including HF, CVD, CAD, etc.) in the general population has been widely studied in the past. The results of our study are generally consistent with those of previous studies. Amirmohammad Khalaji et al. [25] conducted a systematic review and meta-analysis including 772,809 individuals, showing that an increase of one unit in the TyG index raised the risk of HF development in normal populations. Liu and Liang et al. separately reported that a higher TyG index was associated with an increased risk of incident CVD or CAD compared with a lower TyG index [26, 27]. A study including 18 studies and 592,635 patients showed that an elevated TyG index is significantly associated with increased risk of ischemic stroke, stroke recurrence, and death in the general population [28]. Other studies have also shown that elevated TyG index levels are risk factors for atrial fibrillation and obstructive sleep apnea [29, 30]. However, these results are based on studies investigating TyG index at a single time point, which may not reflect long-term exposure. In the present study, we used trajectories to present the pattern of TyG changes over 4 years to observe its long-term effect on the risk of HF in the general population. We found that after adjustment for potential confounders, participants with a long-term elevated TyG level were at a higher risk of HF than those with a low-stable TyG level. Moreover, this association was not affected by additional adjustment for the baseline TyG level. Thus, regular monitoring and maintenance of an appropriate TyG may help identify and prevent HF in the general population.
Another important finding of the present study was that age is an important moderator of the relationship between TyG trajectory and the risk of HF. Specifically, the association between the trajectory in TyG index and HF risk was stronger in participants aged ≥ 60 years than in those aged < 60 years. Previous studies have shown that HF due to elevated TyG index is more common in older age groups [14, 31]. In the present study, the risk of HF in the elevated-stable group of TyG index was still higher for those participants aged ≥ 60 years than for those aged < 60 years, which is consistent with the previous findings. This may suggest that we should pay more attention to the longitudinal effects of TyG in the elderly population while focusing on the general population.
The mechanisms underlying the relationship between the long-term change in TyG index and the risk of HF are unclear, but they may be related to IR. First, IR is associated with high concentrations of free fatty acids and triglycerides in the circulation, which have lipotoxic effects in the heart via the production of toxic lipid intermediates, resulting in a reduction in metabolic efficiency [32, 33]. This metabolic dysfunction increases the susceptibility of patients to pressure overload and ischemia, thereby causing the development or progression of HF [34, 35]. In addition, IR is associated with sympathetic nervous system activation, hyperinsulinemia, and an upregulation of angiotensin II responses [36], which may promote cardiac remodeling through oxidative stress, inflammation, and cardiac fibrosis [37, 38]. Finally, IR increases the risk of many chronic metabolic diseases, including diabetes, hypertension, and hyperlipidemia [39, 40], which are important risk factors for HF [41], and a combination of these may further increase the risk of HF. Nonetheless, to date, the mechanisms linking insulin resistance and HF are not fully understood and this remains an important area for further investigation.
The homeostatic model assessment of insulin resistance (HOMA-IR) and the insulin detection methods require laboratory data on insulin levels, and their implementation is expensive, laborious, and cannot be performed in daily practice, making them difficult to perform in resource-limited countries. Another simpler method that does not require the use of insulin assays is the TyG index, which is closely related to the HOMA-IR and insulin clamp test, and is an easy-to-measure, reliable surrogate marker for the diagnosis of IR that is superior to the HOMA-IR for the assessment of IR [42]. This method requires only simple laboratory parameters such as triglycerides and glucose, and has the advantages of convenient measurement, low cost, and high sensitivity, which makes it more suitable for large-scale application in the clinic [43, 44].
Study strengths and limitations
The present study had several strengths. It was a prospective cohort study performed in a large population with a long follow-up period, so the relationship between different TyG index trajectories and the risk of HF can be studied by monitoring the long-term changes in TyG index. Additionally, the results of multiple subgroup analyses and sensitivity analyses were consistent with those of the principal analysis, which further strengthens the present findings. However, the study also had certain limitations. First, we did not calculate HOMA-IR or perform a hyperinsulinemic-euglycemic clamp to evaluate IR, so we could not compare the effects of HOMA and TyG index on the risk of HF. Second, our study evaluated the relationship between peripheral insulin resistance (IR) and HF, but not myocardial insulin resistance, which may have influenced our results. Whether IR (either peripheral or myocardial) is merely a marker of disease severity or whether it actually contributes to the progression of HF remains to be determined. Moreover, some hypoglycemic drugs (such as GLP1RAs and DPP4i) can increase the risk of HF, but due to our lack of specific type of hypoglycemic drugs, this could affect our results. Third, we did not assess the onset and progression of T2DM by appropriate tests such as HbA1c, repeated glycemic control, and adherence to antidiabetic therapy, but instead used a single FPG measure, which may have led to an over- or underestimation of the actual prevalence of T2DM. In addition, a previous study has shown that T2DM can lead to the occurrence of heart failure, and patients with HF are susceptible to IR, which increases the risk of T2DM. However, since this study is an observational study, we may not be able to infer the causal relationship between T2DM and HF, and more studies are needed to verify their relationship in the future. Last but not least, approximately one-third of the participants in our study were coal miners, whose lifestyles and work environments are different from those of other participants, and the results may not be generalizable to other populations. However, the population we studied was very homogeneous, which to some extent makes our findings more robust.
Conclusions
In conclusion, we have shown that higher TyG index trajectories were associated with a higher risk of HF. These results suggest that monitoring TyG index trajectory may help identify individuals at high risk for HF and emphasize the importance of early glycemic and lipid control to prevent the onset of HF.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- TyG index:
-
Triglyceride-glucose index
- IR:
-
Insulin resistance
- HF:
-
Heart failure
- CVD:
-
Cardiovascular disease
- CAD:
-
Coronary artery disease
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglyceride
- FPG:
-
Fasting plasma glucose
- BMI:
-
Body mass index
- TC:
-
Total cholesterol
- eGFR:
-
estimated glomerular filtration rate
- HDL-C:
-
High-density lipoprotein-cholesterol
- LDL-C:
-
Low-density lipoprotein-cholesterol
- hs-CRP:
-
High-sensitivity C-reactive protein
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–78.
Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–90.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of Acute and Chronic Heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847.
Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–59.
Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, Zheng C, Kang Y, Jiang L, Zhu Z, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail. 2019;21(11):1329–37.
Gu DHG, He J. Investigation of prevalence and distributing feature of chronic heart failure in Chinese adult population. In: Zhonghua Xin Xue Guan BingZa Zhi 2003;31:3–6.
Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR Jr., et al. Association of Insulin Resistance and glycemic metabolic abnormalities with LV structure and function in Middle Age: the CARDIA Study. JACC Cardiovasc Imaging. 2017;10(2):105–14.
Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–69.
Velez M, Kohli S, Sabbah HN. Animal models of insulin resistance and heart failure. Heart Fail Rev. 2014;19(1):1–13.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60(12):1673–6.
Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, Tian X, Wang P, Meng X, Wu S, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46.
Xu L, Wu M, Chen S, Yang Y, Wang Y, Wu S, Tian Y. Triglyceride-glucose index associates with incident heart failure: a cohort study. Diabetes Metab. 2022;48(6):101365.
Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, Wang A. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19.
Huang Z, Ding X, Yue Q, Wang X, Chen Z, Cai Z, Li W, Cai Z, Chen G, Lan Y, et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):141.
Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris-Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of Trajectory of Cardiovascular Health Score and Incident Cardiovascular Disease. JAMA Netw Open. 2019;2(5):e194758.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The Seventh Report of the Joint National Committee on Prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7.
Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transpl. 2017;32(3):497–507.
Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72(5):986–8.
Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the diagnosis and treatment of Chronic Heart failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115–40.
Sharashova E, Wilsgaard T, Ball J, Morseth B, Gerdts E, Hopstock LA, Mathiesen EB, Schirmer H, Løchen ML. Long-term blood pressure trajectories and incident atrial fibrillation in women and men: the Tromsø Study. Eur Heart J. 2020;41(16):1554–62.
Jones BL, Nagin DS. Advances in Group-based trajectory modeling and an SAS Procedure for estimating them. Sociol Methods Res. 2007;35(4):542–71.
Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, Vinciguerra C, Cannavo A. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):244.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, Huang H, He Y. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170.
Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, Jiang S, Wang J, Yang Y, Pan G, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):2.
Azarboo A, Behnoush AH, Vaziri Z, Daneshvar MS, Taghvaei A, Jalali A, Cannavo A, Khalaji A. Assessing the association between triglyceride-glucose index and atrial fibrillation: a systematic review and meta-analysis. Eur J Med Res. 2024;29(1):118.
Behnoush AH, Khalaji A, Ghondaghsaz E, Masrour M, Shokri Varniab Z, Khalaji S, Cannavo A. Triglyceride-glucose index and obstructive sleep apnea: a systematic review and meta-analysis. Lipids Health Dis. 2024;23(1):4.
Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, Zhou J, Hui JMH, Lee YHA, Satti DI, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229.
Kolwicz SC Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113(5):603–16.
Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:277–304.
Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364(9447):1786–8.
Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, et al. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123(18):1953–62.
Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: Molecular mechanisms. Heart Fail Clin. 2012;8(4):609–17.
Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26(8):2433–41.
Fiordaliso F, Cuccovillo I, Bianchi R, Bai A, Doni M, Salio M, De Angelis N, Ghezzi P, Latini R, Masson S. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79(2):121–9.
Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a Meta-analysis. Front Cardiovasc Med. 2021;8:644035.
Reaven GM. The insulin resistance syndrome. Curr Atheroscler Rep. 2003;5(5):364–71.
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46.
Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Acknowledgements
We sincerely thank all the research participants and members of the Kailuan Research Group for their contributions.
Funding
This work has been supported by Science and Technology Innovation Strategy Special Project of Guangdong (No. 221117237489175) and Shantou Medical Health Science and Technology Plan (NO. 210624106490813).
Author information
Authors and Affiliations
Contributions
The study idea was designed by ZH, WS and CY; ZH, WK, WW, HZ and WX analyzed and interpreted the data; ZH, FP, WY, CZ, CZ, LY and CZ were responsible for drafting the manuscript. The manuscript was reviewed by WS and CY. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05). All participants agreed to participate in the study and provided informed written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, H., Wu, K., Wu, W. et al. Relationship of the trajectory of the triglyceride-glucose index with heart failure: the Kailuan study. Lipids Health Dis 23, 257 (2024). https://doi.org/10.1186/s12944-024-02254-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02254-2