Abstract
Background
In the last years, the classical pattern of diabetic kidney disease (DKD) has been partially overcome, because of the uncovering of a new DKD phenotype with significant renal dysfunction without presence of albuminuria: the non-albuminuric DKD (NA-DKD). To date, the cardiovascular risk associated with this phenotype is still debated. We investigated the cardiovascular risk and renal injury profile of NA-DKD subjects in comparison with other DKD phenotypes.
Methods
Pulse wave velocity (PWV), intima-media thickness, presence of carotid atherosclerotic plaque, renal resistive index (RRI), and a panel of urinary biomarkers of kidney injury were evaluated in 160 subjects with type 2 diabetes, stratified according to estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR) into four groups: controls (UACR < 30 mg/g and eGFR ≥ 60 mL/min/1.73 m2), A-DKD (Albuminuric-DKD, UACR ≥ 30 mg/g and eGFR ≥ 60 mL/min/1.73 m2), NA-DKD (UACR < 30 mg/g and eGFR < 60 mL/min/1.73 m2), AL-DKD (Albuminuric and Low eGFR-DKD; UACR ≥ 30 mg/g and eGFR < 60 mL/min/1.73 m2).
Results
Subjects with NA-DKD showed a higher PWV (11.83 ± 3.74 m/s vs. 10.24 ± 2.67 m/s, P = 0.045), RRI (0.76 ± 0.11 vs. 0.71 ± 0.09, P = 0.04), and prevalence of carotid atherosclerotic plaque (59% vs. 31%, P = 0.009) compared with controls. These characteristics were similar to those of subjects with AL-DKD, whereas the profile of A-DKD subjects was closer to controls. After multiple regression analyses, we found that RRI, that is in turn influenced by eGFR (β = − 0.01, P = 0.01), was one of the major determinants of PWV (β = 9.4, P = 0.02). Urinary TreFoil Factor 3, a marker of tubular damage, was higher in NA-DKD subjects vs. controls (1533.14 ± 878.31 ng/mL vs. 1253.84 ± 682.17 ng/mL, P = 0.047). Furthermore, after multiple regression analyses, we found that urinary osteopontin was independently associated with PWV (β = 2.6, P = 0.049) and RRI (β = 0.09, P = 0.006).
Conclusions
Our data showed a worse cardiovascular and renal injury profile in NA-DKD subjects. This finding emphasizes the central role of eGFR in the definition of cardiovascular risk profile of diabetic subjects together with albuminuria.
Similar content being viewed by others
Background
Diabetic kidney disease (DKD) is a complication that occurs in about 40% of patients with type 2 diabetes and it is the leading cause of end-stage renal disease in developed countries [1].
In the last few years, the classical pattern of DKD with the progressive worsening of albuminuria and the subsequent decline of estimated glomerular filtration rate (eGFR) has been partially overcome because of the evidence of different patterns of DKD [2]. Traditionally, the diagnosis of DKD was based on the detection of albuminuria (Albuminuric DKD, A-DKD), which is an essential marker of renal damage in diabetes. However, recent epidemiological studies have uncovered a subgroup of patients with diabetes who exhibit significant renal dysfunction without elevated levels of urinary albumin excretion—Non-albuminuric DKD (NA-DKD). Indeed, several studies have shown a decreased prevalence of A-DKD and an increased prevalence of NA-DKD as the current trend in DKD epidemiology [3,4,5].
Several factors may have contributed to the rising prevalence of NA-DKD: a higher prevalence of hypertension and obesity, a reduction in smoking and the use of multifactorial interventions leading to improved glucose, blood pressure, and lipid management [6].
NA-DKD is a challenging clinical entity as it is rather poorly understood and often misdiagnosed because of its atypical presentation. To date, it is not clear whether there are differences in the cardiovascular risk and renal injury profile of patients affected by NA-DKD. Data in the literature have reported conflicting results: studies on type 1 and type 2 diabetes have shown that both albuminuria and low eGFR are independently associated with cardiovascular and renal events; however, they provide conflicting estimates of outcome rates associated with different phenotypes. In a post-hoc analysis of the Action in Diabetes and Vascular disease: preterAx and diamicroN modified release Controlled Evaluation (ADVANCE) study the risk for cardiovascular events was lower in patients with normoalbuminuria and stage 3 chronic kidney disease (CKD) than in those with albuminuria and stage 2 CKD [7]; in contrast, a post-hoc analysis of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study showed that the non-albuminuric phenotype was associated with a higher risk of cardiovascular death, compared to microalbuminuria with an eGFR > 60 mL/min/1.73 m2 and macroalbuminuria with eGFR > 90 mL/min/1.73 m2 [8], and accordingly, in the Renal Insufficiency and Cardiovascular Events (RIACE) study all-cause mortality risk was higher in the non-albuminuric group with eGFR < 45 mL/min/1.73 m2 than in the group with albuminuria alone [9].
This finding raises serious concerns about the pathophysiology and mechanisms underlying the elevated cardiovascular risk found in NA-DKD patients. In the last few years, interest in identifying biomarkers that may improve diagnosis and risk stratification has increased. These biomarkers should capture inflammation, fibrosis, glomerular and tubular involvement, and mitochondrial impairment [10,11,12]. Additionally, biomarkers could be coupled with kidney structure evaluation obtained by abdominal ultrasound, which is a non-invasive procedure that can provide information about renal morphology and vascularity.
The aim of this study was to investigate the cardiovascular risk profile of subjects with NA-DKD compared with other DKD phenotypes; our fist aim was to investigate early markers of vascular damage such as arterial stiffness and carotid atherosclerosis. In addition, we characterized the renal profile of these patients with ultrasound measurements of intrarenal resistive index (RRI) and renal volume (RV); finally, we investigated the association between a panel of urinary markers strictly linked to renal injury.
Methods
Study subjects
One hundred sixty subjects with type 2 patients who attended our university hospital for diabetes and cardiovascular risk evaluation were recruited in this study. The inclusion criterion was an age range 45–75 years. The exclusion criteria were malignancies, rheumatological diseases, drug abuse, and corticosteroids therapy. All patients underwent a physical examination and review of clinical history, smoking status, and medications.
Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Blood pressure (BP) was measured with a calibrated sphygmomanometer after 10 min resting. Venous blood samples were drawn from the antecubital vein on the morning after an overnight fast. Baseline venous blood samples were obtained for the measurement of clinical biochemistry parameters. LDL cholesterol concentrations were estimated using the Friedewald formula. A sample of spot urine was collected.
Biochemical analyses
Plasma glucose, serum total cholesterol, triglycerides, and HDL cholesterol were measured using available enzymatic methods [13].
HbA1c was measured via high performance liquid chromatography using a National Glycohemoglobin Standardization Program and was standardized to the Diabetes Control and Complications Trial (DCCT) [14] assay reference. Chromatography was performed using a certified automated analyzer (HLC-723G7 hemoglobin HPLC analyzer; Tosoh Corp.) (normal range 4.25%–5.9%).
Albuminuria determination was performed as albumin-to-creatinine ratio (UACR) in a spot urine sample and the mean of two different values obtained in a period of 3–6 months was considered [15].
Estimated glomerular filtration rate (eGFR) was assessed with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [16].
Study groups
Subjects were assigned to four different groups according to UACR and eGFR as follows: controls (UACR < 30 mg/g and eGFR ≥ 60 mL/min/1.73 m2), A-DKD (UACR ≥ 30 mg/g and eGFR ≥ 60 mL/min/1.73 m2), NA-DKD (UACR < 30 mg/g and eGFR < 60 mL/min/1.73 m2), AL-DKD (Albuminuric and Low eGFR DKD; UACR ≥ 30 mg/g and eGFR < 60 mL/min/1.73 m2).
Carotid ultrasound examination
Ultrasound scans were performed using a high-resolution B-mode ultrasound system. All ultrasound examinations were performed by a single physician who was blinded to the clinical and laboratory characteristics of the patients. Longitudinal B-mode (60 Hz, 128 radiofrequency lines) images of the right common carotid artery 2 cm below the carotid bulb were obtained using a high-precision echo tracking device (MyLab Alpha, Esaote, Maastricht, NL) paired with a high-resolution linear array transducer (13 MHz) to acquire quality intima-media thickness (qIMT) using the built-in echo tracking software.
Pulse wave velocity
The SphygmoCor CvMS (AtCor Medical, Sydney, Australia) system was used for the determination of the pulse wave velocity (PWV) [17]. This system uses a tonometer and two different pressure waves obtained at the common carotid artery (proximal recording site) and at the femoral artery (distal recording site). An electrocardiogram was used to determine the start of the pulse wave. The PWV was determined as the difference in travel time of the pulse wave between the two different recording sites and the heart, divided by the travel distance of the pulse waveform. The PWV was calculated on the mean basis of 10 consecutive pressure waveforms to cover a complete respiratory cycle.
Pulse wave analysis
All measurements were made from the right radial artery by applanation tonometry using a Millar tonometer (SPC-301; Millar Instruments, Houston, TX, USA). The measurements were performed by a single investigator with the subject in the supine position. The data were collected directly with a desktop computer and processed with SphygmoCorCvMS (AtCor Medical, Sydney, Australia). The aortic waveform has two systolic pressure peaks, the second is caused by wave reflection from the periphery. With arterial stiffening, both the PWV and the amplitude of the reflected wave are increased such that the reflected wave arrives earlier and adds to (or augments) the central systolic pressure. The aortic waveform in the pulse wave analysis was subjected to further analysis for the calculation of the aortic Aug and AugI (calculated by dividing augmentation by pulse pressure). Pulse pressure is the difference between the systolic and diastolic BPs [18].
Renal Resistive Index (RRI)
RRI was derived from the Doppler spectrum of intrarenal arteries as the difference between maximum (peak systolic) and minimum (end-diastolic) flow velocity to maximum flow velocity, using a high-precision echo tracking device (MyLab Alpha, Esaote, Maastricht, NL) paired with a convex transducer [19]. The RRI was calculated as the mean of 3 different measurements.
Renal volume (RV)
RV of the right kidney was calculated with the ellipsoid formula derived from the cranio-caudal, mediolateral, and anteroposterior diameters, using a high-precision echo tracking device (MyLab Alpha, Esaote, Maastricht, NL) paired with a convex transducer. Values were adjusted for body surface area (BSA) [20].
Quantification urinary renal function biomarkers
The BioPlex® (Bio-Rad, Hercules, California, USA) platform was used for the determination of urinary biomarkers. The commercial package Bio-Plex Pro™ RBM Human Kidney Toxicity Assay Panel 2 (albumin, β2 microglobulin [u-B2M], cystatin C [u-Cys], Neutrophil Gelatinase-Associated Lipocalin [u-NGAL], osteopontin [u-OPN], and TreFoil Factor 3 [u-TFF3]) was used to evaluate the protein content in the urinary samples, according to the manufacturer’s protocol. Briefly, urine samples were thawed on ice and, after centrifugation at 500×g for 5 min, were diluted 1:50. After blockade of nonspecific binding sites, 30 µL of standards, controls or diluted samples were added to 96-well plates and incubated with 10 µL of fluorescently dyed magnetic microspheres covalently coupled to specific antibodies for the desired biomarkers. The plates were incubated for 1 h at RT and washed three times with wash buffer. Afterward, 40 µL of biotinylated detection antibody was added to the wells and the plates were incubated for an additional hour at RT. The final detection complex was completed with the addition of conjugated streptavidin–phycoerythrin. The median relative fluorescence units from the antibody reactions were acquired using a BioPlex 200 analyzer (Bio-Rad) and BioPlex Manager Software™ version 6.2 Build 175 (Bio-Rad).
Statistical analyses
The sample size was calculated based on PWV using a level of significance (α) set to 5% and a power (1 − β) set to 80%. The estimated sample size was 38 patients per group.
Statistical comparisons of clinical and biomedical parameters were performed using Stat View 6.0 for Windows. The data are presented as mean ± SD or median (interquartile range). Each variable’s distributional characteristics, including normality, were assessed using the Kolmogorov–Smirnov test. One-way ANOVA for clinical and biological data was performed to test the differences among groups, and the Bonferroni post hoc test for multiple comparisons was also performed. The χ2 test was used for categorical variables. A P value < 0.05 was considered significant. When necessary, numerical variables were logarithmically transformed to reduce skewedness.
To identify variables independently associated with variations of PWV and RRI, we performed three multivariate regression models: the first model included cardiovascular risk factors (age, sex, BMI, smoking status, systolic BP [SBP], history of cardiovascular Disease [CVD]); variables reaching significance in the first model were included in a second model including biochemical variables (LDL and HDL cholesterol, HbA1c, eGFR, UACR > 30 mg/g); finally, variables reaching significance in the second model were included in a third model with renal variables (RRI—not in the analysis for RRI -, u-B2M, u-Cys, u-NGAL, u-OPN, and u-TFF3). The same models were used to perform a multiple logistic regression analysis to identify variables independently associate with the presence of atherosclerotic plaques.
Furthermore, we performed a multivariate logistic regression model in order to identify variables related to alterations in PWV and RRI. We used a cut-off of 10 m/s for PWV and 0.70 for RRI [21, 22]. The considered independent variables were age, sex, smoking status, BMI, being in secondary prevention, PAS, use of sodium-glucose cotransporter-2 inhibitors (SGLT-2i), use of Angiotensin Converting enzyme inhibitors (ACEi) or Angiotensin Receptor Blockers, HbA1c, eGFR, UACR > 30 mg/g.
The variance inflation factor (VIF) was used to check for the problem of multicollinearity among the predictor variables in multiple regression analysis. Any variable with a VIF that exceeded 4 was excluded from the model. No variable was detected with a VIF greater than 4.
The study was approved by the local ethics committee (Comitato Etico Catania 2, protocol n. 270/C.E. 26 April 2022). Informed consent was obtained from each participant.
Results
Study population characteristics
According to the eligibility criteria, 160 patients were included in this study. The study population was divided into the following four groups (based on UACR and eGFR levels): 40 subjects without DKD (Controls), 41 subjects with albuminuria (A-DKD), 41 subjects with eGFR reduction without albuminuria (NA-DKD), and 38 subjects with both albuminuria and eGFR reduction (AL-DKD). As shown in Table 1, the four groups were homogeneous for blood pressure, fasting glycemia and LDL cholesterol. Age was significantly higher in NA-DKD and AL-DKD patients. The groups were homogeneous also for the number of patients with history of CVD (myocardial infarction, stroke and peripheral artery disease), with the exception of the AL-DKD group, which showed a significantly higher percentage of subjects with a history of CVD in comparison with controls and the NA-DKD group. In the study population there was a major prevalence of males, especially in A-DKD patients. Regarding medications, we found statistically significant differences among the groups, with a greater use of metformin in controls and the A-DKD group and a higher use of SGLT-2i in AL-DKD group, as expected from clinical indications.
Cardiovascular profile of the study population according to UACR and eGFR
As shown in Table 2, patients with NA-DKD showed higher PWV compared with controls (11.83 ± 3.74 m/s vs. 10.24 ± 2.67 m/s, P = 0.045); no differences were observed between patients with NA-DKD and AL-DKD (11.83 ± 3.74 m/s vs. 11.99 ± 3.98 m/s, P = 0.86). In the simple regression analysis PWV was associated with age (r = 0.28; P = 0.0012), eGFR (r = − 0.22; P = 0.01) and RRI (r = 0.36; P < 0.0001).
Furthermore, we found that in NA-DKD patients there was a significantly higher prevalence of carotid atherosclerotic plaque in comparison with controls (59% vs. 31%, P = 0.009). No differences were observed between patients with NA-DKD and AL-DKD (59% vs. 74%, P = 0.16). In the simple logistic regression analysis, the presence of atherosclerotic plaque was associated with age (OR 1.08, 95% CI 1.01–1.16), eGFR (OR 0.98, 95% CI 0.96–0.99), history of CVD (OR 3.46, 95% CI 1.28–9.35).
Finally, we found increased qIMT in the NA-DKD group compared with controls, without statistical significance (0.83 ± 0.12 mm vs. 0.81 ± 0.14 mm, P = 0.60) (Table 2). No differences were observed between patients with A-DKD and controls (0.81 ± 0.14 mm vs. 0.81 ± 0.15 mm, P = 0.91).
Renal profile of the study population according to UACR and eGFR
The RRI was significantly increased in patients with NA-DKD compared with controls (0.76 ± 0.11 vs. 0.71 ± 0.09, P = 0.04) and A-DKD patients (0.76 ± 0.11 vs. 0.71 ± 0.08, P = 0.02). Moreover, we found no difference between NA-DKD and AL-DKD patients (0.76 ± 0.11 vs. 0.77 ± 0.08, P = 0.51) (Table 2). In the simple regression analysis RRI was associated with age (r = 0.41, P < 0.0001), history of CVD (r = 0.18, P = 0.04), eGFR (r = − 0.37; P < 0.0001), PWV (r = 0.36; P < 0.0001).
RV/BSA was significantly lower in NA-DKD vs. A-DKD patients (78.57 ± 17.04 mL/m2 vs. 91.45 ± 29.94 mL/m2, P = 0.04). In addition, RV/BSA was higher in A-DKD patients than in controls, without statistical significance (Table 2).
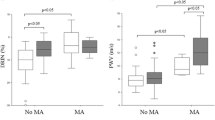
As concerns urinary biomarkers of kidney damage (Fig. 1), u-TFF3 was higher in NA-DKD patients versus controls (1533.14 ± 878.31 ng/mL vs. 1253.84 ± 682.17 ng/mL, P = 0.047). In addition, u-Cys was lower in NA-DKD versus A-DKD patients (12.06 [8.09–19.51] ng/mL vs. 22.11 [11.13–27.93] ng/mL, P = 0.01) and AL-DKD patients (12.06 [8.09–19.51] ng/mL vs. 18.20 [11.85–42.93], P = 0.01) and u-B2M was higher in AL-DKD patients vs. controls (52.90 [9.06–369.19] ng/mL vs. 13.83 [4.18–33.41] ng/mL, P = 0.001) and A-DKD patients versus controls (33.53 [7.71–66.33] ng/mL vs. 13.83 [4.18–33.41] ng/mL, P = 0.048). Finally, no statistically significant differences were observed for u-NGAL and u-OPN.
Box plots of urinary biomarkers of kidney injury according to UACR and eGFR. The x-axis shows the study groups, while the y-axis shows the log-transformed concentrations of urinary biomarkers. UACR: urinary albumin to creatine ratio; eGFR: estimated glomerular filtration rate; A-DKD: albuminuric diabetic kidney disease (UACR ≥ 30 mg/g and eGFR ≥ 60 mL/min/1.63 m2); NA-DKD: non-albuminuric diabetic kidney disease (UACR < 30 mg/g and eGFR < 60 mL/min/1.63 m2); AL-DKD: albuminuric and low estimated glomerular filtration rate diabetic kidney disease (UACR ≥ 30 mg/g and eGFR < 60 mL/min/1.63 m2); u-B2M: urinary β2 microglobulin; u-Cys: urinary Cystatin C; u-NGAL: urinary Neutrophil Gelatinase-Associated Lipocalin; u-OPN: urinary osteopontin; u-TFF3: urinary TreFoil Factor 3. *P < 0.05; **P < 0.001
Multiple regression analysis to identify variables independently associated with PWV, AugI, and RRI variations
We performed a multiple regression analysis (Table 3) in order to identify variables independently associated with PWV and RRI variations.
In the first model PWV showed an association with age (β = 0.20, P = 0.0003), while in the second model, the variables that remained significantly related with PWV were age (β = 0.14, P = 0.02) and HbA1c (β = 0.25, P = 0.008). Finally, in the third model variables that showed a significant association with PWV were RRI (β = 9.4, P = 0.02), and u-OPN (β = 2.6, P = 0.049).
RRI was associated with age (β = 0.006, P < 0.0001) in the first model. Then, in the second model variables that remained significantly related to RRI were age (β = 0.29, P = 0.004), HbA1c (β = 0.23, P = 0.007), and eGFR (β = − 0.24, P = 0.01). Finally, in the third model variables related to RRI were age (β = 0.002, P = 0.047), HbA1c (β = 0.014, P = 0.008), eGFR (β = − 0.01, P = 0.01), and u-OPN (β = 0.09, P = 0.006).
Logistic regression analysis to identify variables independently associated with the presence of carotid atherosclerotic plaque
In the first model, the presence of carotid atherosclerotic plaque was associate with age (OR 1.10, 95% CI 1.01–1.20, P = 0.02); while in the second model it was related to history of CVD (OR 4.11, 95% CI 1.08–15.62, P = 0.04). Finally, in the third model age remained significantly associated with the presence of carotid atherosclerotic plaque (OR 1.09, 95% CI 1.03–1.18, P = 0.04) and the history of CVD (OR 1.93, 95% CI 1.02–6.04, P = 0.048).
Logistic regression analysis to identify variables independently associated with PWV > 10 m/s and RRI > 0.70
In the logistic regression model values of PWV > 10 m/s were associated with age (OR 1.12, 95% CI 1.02–1.23, P = 0.01), SBP (OR 1.04 95% CI 1.01–1.07, P = 0.02), and HbA1c (OR 2.02, 95% CI 1.31–3.12, P = 0.001).
As concerns values of RRI > 0.70, they were significantly associated with SBP (OR 1.04, 95% CI 1.01–1.07, P = 0.01), HbA1c (OR 1.61, 95% CI 1.08–2.40, P = 0.02), and eGFR (OR 0.97, 95% CI 0.94–0.99, P = 0.03].
Discussion
The increasing clinical relevance of NA-DKD as a distinct phenotype of DKD with its own set of challenges has recently been a topic of growing interest among researchers and clinicians.
In this study, we investigated the cardiovascular risk profile of patients with NA-DKD, using arterial stiffness and qIMT, which are well known early markers of cardiovascular disease and predictive of cardiovascular events. In addition, we investigated the renal injury profile by measuring RRI and dosing urinary biomarkers strictly linked to renal injury.
We found that patients with NA-DKD exhibited higher PWV compared with controls; PWV was similar in NA-DKD patients in comparison with AL-DKD patients. Furthermore, we found a higher prevalence of carotid atherosclerotic plaques in patients with NA-DKD and AL-DKD compared with A-DKD patients and controls.
Previous studies focused on the cardiovascular risk profile of patients with DKD; Kourtidou et al. have recently reported that patients with DKD appear to have higher arterial stiffness and carotid atherosclerosis than patients with type 2 diabetes and preserved kidney function [23]; other authors have reported a relationship between IMT, vascular function, eGFR and stages of diabetic nephropathy [24, 25]. However, none of these studies evaluated markers of cardiovascular disease in different phenotypes of DKD, to identify in which of them the cardiovascular risk is greater. Our results suggest that patients with NA-DKD exhibited a higher risk of developing cardiovascular disease compared with diabetic patients without kidney disease and similar to those with AL-DKD.
These results appear to be in contrast with a recent observational cohort study: Yokohama et al. reported that the risks of death and cardiovascular disease in Japanese patients with type 2 diabetes were not higher in NA-DKD patients than those without renal impairment [26]. Conversely, in line with our data, Penno et al. reported NA-DKD as a strong predictor of mortality [9]. One possible explanation for the inconsistency of these results may be the different rate of macrovascular complications; in fact, the incidence of cardiovascular disease in Japan is significantly lower than in the Caucasian population.
As concerns renal injury profile, we found that patients with NA-DKD exhibited significantly higher RRI compared with controls and A-DKD patients, and similar to those of AL-DKD patients. This is not the first study reporting an increase of RRI in NA-DKD patients: Garofolo et al. found increased RRI in patients with type 2 diabetes and different DKD phenotypes [27]. Accordingly, Afsar et al. have shown higher RRI in patients with both albuminuria and reduced eGFR and a lower RRI in those patients with normoalbuminuria and preserved eGFR [28]. These results are also consistent with previous studies showing a higher RRI in patients with lower eGFR in comparison to those with preserved eGFR [29, 30]. These data demonstrate that increased RRI is present in DKD regardless of albuminuric status; accordingly, it appears to be a clinical feature of NA-DKD. Moreover, we found that RRI ≥ 0.7 inversely correlates with eGFR and directly with SBP and HbA1c in multiple logistic analyses, and we found a relationship between PWV and RRI in multiple regression analyses. These data are consistent with other studies that demonstrated a link between RRI alteration, arterial stiffness and atherosclerosis, with a predictive role for cardiovascular events in diabetic subjects [31, 32].
The increased RRI in NA-DKD is not surprising, considering that RRI may represent a marker of tubulointerstitial injury [33]. In fact, a possible explanation of increased RRI in NA-DKD patients could be the presence of a greater tubulointerstitial rather than glomerular involvement, which is typical of this phenotype [34].
As concerns RV, our results are consistent with another recent study by Garofolo et al. [27], where total renal volume was lower in NA-DKD than in A-DKD patients. Furthermore, the authors reported that the use of 3D US showed no differences than the widely used 2D US in the assessment of RV.
In our study u-TFF3 was higher in NA-DKD patients compared with controls. Interestingly, TFF3 is highly expressed in renal tubules, and it might play a role in epithelial regeneration [35]. Thus, considering the pivotal role of tubular damage in NA-DKD pathogenesis, the increase of this peptide in patients with this specific phenotype of DKD is not surprising. Other studies investigated the role of u-TFF3 in patients with CKD with and without diabetes. Astor et al. showed that u-TFF3 levels were higher in diabetic patients; furthermore, increased u-TFF3 levels were linked to a higher risk of developing DKD [36]. Similarly, Yamanari et al. showed a predictive role of this peptide in CKD progression [37].
No statistically significant differences were observed for u-OPN between different DKD phenotypes. However, in the multiple regression analyses, we found that u-OPN was independently associated with PWV and RRI. OPN is known to be involved in atherosclerosis [38]. In addition, previous studies highlighted a relationship between plasma levels of OPN and arterial stiffness in various clinical scenarios, including patients with coronary artery disease [39] and geriatric subjects [40]. Unfortunately, no studies are available concerning the relationship between u-OPN and arterial stiffness or RRI. Considering that animal studies identified a higher expression of OPN on tubular epithelium and in renal interstitium with a possible role in DKD progression [41], the association of urinary levels of this molecule and arterial stiffness and RRI in this field is justified.
This study presents some strengths and limitations. As concern strengths, we tried to obtain a complete cardiovascular and renal profile of different DKD phenotypes, which is still an underexplored field. In addition, we used well validated measurements, such as PWV, qIMT, the presence of atherosclerotic plaques, and RRI.
On the other hand, this was a cross-sectional study, thus a longitudinal causal relationship cannot be established. In addition, study groups presented different sex distributions.
Conclusions
In conclusion, our study analyzed the differences existing in various DKD phenotypes, highlighting the possible central role of eGFR in the definition of cardiovascular risk profile of diabetic patients over the more studied albuminuria. The NA-DKD phenotype presents a clinical challenge in early diagnosis and management for its implications not only for kidney-specific therapy, but also for cardiovascular risk. Thus, a timely diagnosis and a multidisciplinary management should be pursued to optimize global patient outcomes.
Availability of data and materials
The dataset used and/or analysed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- ACEi:
-
Angiotensin coverting enzyme inhibitors
- A-DKD:
-
Albuminuric diabetic kidney disease
- AL-DKD:
-
Albuminuric and low estimate glomerular filtration rate diabetic kidney disease
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BSA:
-
Body surface area
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- CVD:
-
Cardiovascular disease
- DKD:
-
Diabetic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- NA-DKD:
-
Non-albuminuric diabetic kidney disease
- PWV:
-
Pulse wave velocity
- qIMT:
-
Quality intima-media thickness
- RRI:
-
Renal resistive index
- RV:
-
Renal volume
- SBP:
-
Systolic blood pressure
- SGLT-2i:
-
Sodium-glucose cotransporert-2 inhibitors
- UACR:
-
Urinary albumin to creatinine ratio
- u-B2M:
-
Urinary β2-microglobulin
- u-Cys:
-
Urinary cystatin C
- u-NGAL:
-
Urinary neutrophil gelatinase-associated lipocalin
- u-OPN:
-
Urinary osteopontin
- u-TFF3:
-
Urinary trefoil factor 3
- VIF:
-
Variance inflation factor
References
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–45.
Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17:740–50.
Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant. 2009;24:1212–9.
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–10.
Doshi SM, Friedman AN. Diagnosis and management of type 2 diabetic kidney disease. CJASN. 2017;12:1366–73.
Scilletta S, Di Marco M, Miano N, Filippello A, Di Mauro S, Scamporrino A, et al. Update on diabetic kidney disease (DKD): focus on non-albuminuric DKD and cardiovascular risk. Biomolecules. 2023;13:752.
Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21.
Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54:32–43.
for the Renal Insufficiency And Cardiovascular Events (RIACE) Study Group, Penno G, Solini A, Orsi E, Bonora E, Fondelli C, et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia. 2018;61:2277–89.
Barutta F, Bellini S, Canepa S, Durazzo M, Gruden G. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol. 2021;58:819–30.
Di Mauro S, Scamporrino A, Filippello A, Di Marco M, Di Martino MT, Scionti F, et al. Mitochondrial RNAs as potential biomarkers of functional impairment in diabetic kidney disease. IJMS. 2022;23:8198.
Scamporrino A, Di Mauro S, Filippello A, Di Marco G, Di Pino A, Scicali R, et al. Identification of a new RNA and protein integrated biomarker panel associated with kidney function impairment in DKD: translational implications. Int J Mol Sci. 2023;24:9412.
Calanna S, Scicali R, Di Pino A, Knop FK, Piro S, Rabuazzo AM, et al. Alpha- and beta-cell abnormalities in haemoglobin A1c-defined prediabetes and type 2 diabetes. Acta Diabetol. 2014;51:567–75.
International Federation of Clinical Chemistry and Laboratory Medicine, IFCC Scientific Division, Mosca A, Goodall I, Hoshino T, Jeppsson JO, John WG, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med. 2007;45:1077–80.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 11. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46:S191–202.
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–49.
Di Pino A, Urbano F, Scicali R, Di Mauro S, Filippello A, Scamporrino A, et al. 1 h postload glycemia is associated with low endogenous secretory receptor for advanced glycation end product levels and early markers of cardiovascular disease. Cells. 2019;8:910.
Zanoli L, Lentini P, Briet M, Castellino P, House AA, London GM, et al. Arterial stiffness in the heart disease of CKD. JASN. 2019;30:918–28.
Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32:149–53.
Fiorini F, Barozzi L. The role of ultrasonography in the study of medical nephropathy. J Ultrasound. 2007;10:161–7.
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8.
Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885–92.
Kourtidou C, Rafailidis V, Varouktsi G, Kanakis E, Liakopoulos V, Vyzantiadis T-A, et al. Evaluation of subclinical vascular disease in diabetic kidney disease: a tool for personalization of management of a high-risk population. J Pers Med. 2022;12:1139.
Chan WB, Chan NN, Lai CWK, So WY, Lo MKW, Lee KF, et al. Vascular defect beyond the endothelium in type II diabetic patients with overt nephropathy and moderate renal insufficiency. Kidney Int. 2006;70:711–6.
Ito H, Komatsu Y, Mifune M, Antoku S, Ishida H, Takeuchi Y, et al. The estimated GFR, but not the stage of diabetic nephropathy graded by the urinary albumin excretion, is associated with the carotid intima-media thickness in patients with type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2010;9:18.
Yokoyama H, Araki S-I, Kawai K, Yamazaki K, Shirabe S-I, Sugimoto H, et al. The prognosis of patients with type 2 diabetes and nonalbuminuric diabetic kidney disease is not always poor: implication of the effects of coexisting macrovascular complications (JDDM 54). Diabetes Care. 2020;43:1102–10.
Garofolo M, Russo E, Miccoli R, Lucchesi D, Giusti L, Sancho-Bornez V, et al. Albuminuric and non-albuminuric chronic kidney disease in type 1 diabetes: Association with major vascular outcomes risk and all-cause mortality. J Diabetes Complications. 2018;32:550–7.
Afsar B, Elsurer R. Comparison of renal resistive index among patients with Type 2 diabetes with different levels of creatinine clearance and urinary albumin excretion. Diabet Med. 2012;29:1043–6.
Boeri D, Derchi LE, Martinoli C, Simoni G, Sampietro L, Storace D, et al. Intrarenal arteriosclerosis and impairment of kidney function in NIDDM subjects. Diabetologia. 1998;41:121–4.
MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, et al. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29:1560–6.
Calabia J, Torguet P, Garcia I, Martin N, Mate G, Marin A, et al. The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: the link between macrocirculation and microcirculation. J Clin Hypertens (Greenwich). 2014;16:186–91.
Delsart P, Vambergue A, Ninni S, Machuron F, Lelievre B, Ledieu G, et al. Prognostic significance of the renal resistive index in the primary prevention of type II diabetes. J Clin Hypertens (Greenwich). 2020;22:223–30.
Prabahar MR, Udayakumar R, Rose J, Fernando EM, Venkatraman R, Balaraman V, et al. Prediction of tubulo-interstitial injury by Doppler ultrasound in glomerular diseases: value of resistive and atrophic indices. J Assoc Phys India. 2008;56:21–6.
Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013;36:3620–6.
Rinnert M, Hinz M, Buhtz P, Reiher F, Lessel W, Hoffmann W. Synthesis and localization of trefoil factor family (TFF) peptides in the human urinary tract and TFF2 excretion into the urine. Cell Tissue Res. 2010;339:639–47.
Astor BC, Köttgen A, Hwang S-J, Bhavsar N, Fox CS, Coresh J. Trefoil factor 3 predicts incident chronic kidney disease: a case-control study nested within the Atherosclerosis Risk in Communities (ARIC) study. Am J Nephrol. 2011;34:291–7.
Yamanari T, Sugiyama H, Tanaka K, Morinaga H, Kitagawa M, Onishi A, et al. Urine trefoil factors as prognostic biomarkers in chronic kidney disease. Biomed Res Int. 2018;2018:3024698.
Wolak T. Osteopontin—a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis. 2014;236:327–37.
Maniatis K, Siasos G, Oikonomou E, Vavuranakis M, Zaromytidou M, Mourouzis K, et al. Osteoprotegerin and osteopontin serum levels are associated with vascular function and inflammation in coronary artery disease patients. Curr Vasc Pharmacol. 2020;18:523–30.
Lee C-J, Wang J-H, Chen Y-C, Chen M-L, Yang C-F, Hsu B-G. Serum osteopontin level correlates with carotid-femoral pulse wave velocity in geriatric persons. Biomed Res Int. 2014;2014: 570698.
Kelly DJ, Wilkinson-Berka JL, Ricardo SD, Cox AJ, Gilbert RE. Progression of tubulointerstitial injury by osteopontin-induced macrophage recruitment in advanced diabetic nephropathy of transgenic (mRen-2)27 rats. Nephrol Dial Transplant. 2002;17:985–91.
Acknowledgements
We wish to thank the Scientific Bureau of University of Catania for language assistance.
Funding
We received no external funding for this work. This study was in keeping with the objectives of the project “DEGENER-action”, Department of Clinical and Experimental Medicine, University of Catania.
Author information
Authors and Affiliations
Contributions
M.D.M and A.D.P designed the study. M.D.M., S.S., N.Mi., P.T., G.B., F.D.G.B, A.M, and T.B. researched data and contributed to the discussion. S.D.M., A.F., A.S. stored and processed urine and blood samples and contributed to the discussion. N.Ma., A.N., and F.G. dosed the panel of urinary biomarkers. M.D.M and S.S. wrote the first draft of the manuscript. A.D.P., R.S. and S.P. contributed to the discussion, reviewed and edited the manuscript. L.F., P.C., and F.P. supervised the study and reviewed the manuscript. All authors approved the final version of the manuscript. A.D.P is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee (Comitato Etico Catania 2, protocol n. 270/C.E. 26 April 2022). Informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Di Marco, M., Scilletta, S., Miano, N. et al. Cardiovascular risk and renal injury profile in subjects with type 2 diabetes and non-albuminuric diabetic kidney disease. Cardiovasc Diabetol 22, 344 (2023). https://doi.org/10.1186/s12933-023-02065-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02065-2