Abstract
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease (ESRD). Although both albuminuria and glomerular filtration rate (GFR) are well-established diagnostic/prognostic biomarkers of DKD, they have important limitations. There is, thus, increasing quest to find novel biomarkers to identify the disease in an early stage and to improve risk stratification. In this review, we will outline the major pitfalls of currently available markers, describe promising novel biomarkers, and discuss their potential clinical relevance. In particular, we will focus on the importance of recent advancements in multi-omic technologies in the discovery of new DKD biomarkers. In addition, we will provide an update on new emerging approaches to explore renal function and structure, using functional tests and imaging.


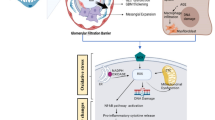
Adapted from Tonneijck et al. JASN 2017, 28:1023-1039
Similar content being viewed by others
Change history
29 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00592-021-01816-5
References
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12:2032–2045. https://doi.org/10.2215/CJN.11491116
Penno G, Solini A, Bonora E et al (2011) Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. https://doi.org/10.1097/HJH.0b013e3283495cd6
Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A (2013) Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabet 4:245–255. https://doi.org/10.4239/wjd.v4.i6.245
Caramori ML (2017) Should all patients with diabetes have a kidney biopsy? Nephrol Dial Transplant 32:3–5. https://doi.org/10.1093/ndt/gfw389
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. CJASN 12:2032–2045. https://doi.org/10.2215/CJN.11491116
Fioretto P, Mauer M (2007) Histopathology of diabetic nephropathy. Semin Nephrol 27:195–207. https://doi.org/10.1016/j.semnephrol.2007.01.012
MacIsaac RJ, Ekinci EI, Jerums G (2014) Progressive diabetic nephropathy. How useful is microalbuminuria?: contra. Kidney Int 86:50–57. https://doi.org/10.1038/ki.2014.98
Angiano Gómez L, Lei Y, Devarapu SK, Anders HJ (2018) The diabetes pandemic suggests unmet needs for ‘CKD with diabetes’ in addition to ‘diabetic nephropathy’—implications for pre-clinical research and drug testing. Nephrol Dial Transplan 33:1292–1304. https://doi.org/10.1093/ndt/gfx219
Anders HJ, Huber TB, Isermann B, Schiffer M (2018) CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 14:361–377. https://doi.org/10.1038/s41581-018-0001-y
Tonneijck L, Muskiet MHA, Smits MM et al (2017) Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28:1023–1039. https://doi.org/10.1681/ASN.2016060666
Niewczas MA, Gohda T, Skupien J et al. (2012) Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23:507–515. https://doi.org/10.1681/ASN.2011060627
Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA (2015) Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 87:812–819. https://doi.org/10.1038/ki.2014.330
Barr ELM, Barzi F, Hughes JT et al (2018) High baseline levels of tumor necrosis factor receptor 1 are associated with progression of kidney disease in indigenous Australians with diabetes: the eGFR follow-up study. Diabet Care 41:739–747. https://doi.org/10.2337/dc17-1919
Forsblom C, Moran J, Harjutsalo V et al (2014) Added value of soluble tumor necrosis factor-α receptor 1 as a biomarker of ESRD risk in patients with type 1 diabetes. Diabet Care 37(8): 2334 2342
Skupien J, Warram JH, Niewczas MA et al (2014) Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5–18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabet Care 37:2601–2608. https://doi.org/10.2337/dc13-1983
Krolewski AS, Niewczas MA, Skupien J et al (2014) Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabet Care 37:226–234. https://doi.org/10.2337/dc13-0985
Gohda T, Niewczas MA, Ficociello LH et al (2012) Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23:516–524. https://doi.org/10.1681/ASN.2011060628
Pavkov ME, Weil EJ, Fufaa GD et al (2016) Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int 89:226–234. https://doi.org/10.1038/ki.2015.278
Heerspink HJL, Perco P, Mulder S et al (2019) Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 62:1154–1166. https://doi.org/10.1007/s00125-019-4859-4
Yamanouchi M, Skupien J, Niewczas MA et al (2017) Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int 92:258–266. https://doi.org/10.1016/j.kint.2017.02.010
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009) Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668–1671. https://doi.org/10.2337/db09-0014
Jalal DI, Rivard CJ, Johnson RJ et al (2010) Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the coronary artery calcification in type 1 diabetes study. Nephrol Dial Transplant 25:1865–1969. https://doi.org/10.1093/ndt/gfp740
Ficociello LH, Rosolowsky ET, Niewczas MA et al (2010) High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabet Care 33:1337–1343. https://doi.org/10.2337/dc10-0227
Zoppini G, Targher G, Chonchol M et al (2012) Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabet Care 35:99–104. https://doi.org/10.2337/dc11-1346
De Cosmo S, Viazzi F, Pacilli A et al (2015) Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol 10(11):1929. https://doi.org/10.2215/CJN.03140315
Ahola AJ, Sandholm N, Forsblom C, Harjutsalo V, Dahlström E, Groop PH (2017) The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int 91(1178):1185. https://doi.org/10.1016/j.kint.2016.11.025
Pilemann-Lyberg S, Hansen TW, Tofte N et al (2019) Uric acid is an independent risk factor for decline in kidney function, cardiovascular events, and mortality in patients with type 1 diabetes. Diabet Care 42:1088–1094. https://doi.org/10.2337/dc18-2173
Doria A, Galecki AT, Spino C et al (2020) Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. https://doi.org/10.1056/NEJMoa1916624
Velho G, Bouby N, Hadjadj S et al (2013) Plasma copeptin and renal outcomes in patients with type 2 diabetes and albuminuria. Diabet Care 36:3639–3645. https://doi.org/10.2337/dc13-0683
Velho G, Boustany RE, Lefèvre G et al (2016) Plasma copeptin, kidney outcomes, ischemic heart disease, and all-cause mortality in people with long-standing type 1 diabetes. Diabet Care 39:2288–2295. https://doi.org/10.2337/dc16-1003
Boertien WE, Riphagen IJ, Drion I et al (2013) Copeptin, a surrogate marker for arginine vasopressin, is associated with declining glomerular filtration in patients with diabetes mellitus (ZODIAC-33). Diabetologia 56:1680–1688. https://doi.org/10.1007/s00125-013-2922-0
Pikkemaat M, Melander O, Bengtsson Boströmd J (2015) Association between copeptin and declining glomerular filtration rate in people with newly diagnosed diabetes. The skaraborg diabetes register. J Diabet Complicat 29:1062–1065. https://doi.org/10.1016/j.jdiacomp.2015.07.006
Wannamethee SG, Welsh P, Lennon L, Papacosta O, Whincup PH, Sattar N (2016) Copeptin and the risk of incident stroke, CHD and cardiovascular mortality in older men with and without diabetes: The british regional Heart study. Diabetologia 59:1904–1912. https://doi.org/10.1007/s00125-016-4011-7
Velho G, Ragot S, Boustany RE et al (2018) Plasma copeptin, kidney disease, and risk for cardiovascular morbidity and mortality in two cohorts of type 2 diabetes. Cardiovasc Diabetol 17:110. https://doi.org/10.1186/s12933-018-0753-5
Sabbisetti VS, Waikar SS, Antoine DJ et al (2014) Blood kidney injury molecule-1 Is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25:2177–2186. https://doi.org/10.1681/ASN.2013070758
Nowak N, Skupien J, Niewczas MA et al (2016) Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89:459–467. https://doi.org/10.1038/ki.2015.314
Wu L, Li XQ, Chang DY et al (2020) Associations of urinary epidermal growth factor and monocyte chemotactic protein-1 with kidney involvement in patients with diabetic kidney disease. Nephrol Dial Transplant 35:291–297. https://doi.org/10.1093/ndt/gfy314
Satirapoj B, Dispan R, Radinahamed P, Kitiyakara C (2018) Urinary epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrol. https://doi.org/10.1186/s12882-018-1043-x
Nowak N, Skupien J, Smiles AM et al (2018) Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int 93:1198–1206. https://doi.org/10.1016/j.kint.2017.11.024
Mayer G, Heerspink HJL, Aschauer C et al (2017) Systems biology-derived biomarkers to predict progression of renal function decline in type 2 diabetes. Diabet Care. https://doi.org/10.2337/dc16-2202
Bjornstad P, Pyle L, Cherney DZI et al (2018) Plasma biomarkers improve prediction of diabetic kidney disease in adults with type 1 diabetes over a 12-year follow-up: CACTI study. Nephrol Dial Transplant 101(333):340. https://doi.org/10.1093/ndt/gfx255
Pezzolesi MG, Satake E, McDonnell KP, Major M, Smiles AM, Krolewski AS (2015) Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 64:3285–3293. https://doi.org/10.2337/db15-0116
Argyropoulos C, Wang K, Bernardo J et al (2015) Urinary microRNA profiling predicts the development of microalbuminuria in patients with type 1 diabetes. J Clin Med 4:1498–1517. https://doi.org/10.3390/jcm4071498
Regmi A, Liu G, Zhong X et al (2019) Evaluation of Serum microRNAs in patients with diabetic kidney disease a nested case-controlled study and bioinformatics analysis. Med Sci Monit https://doi.org/10.12659/MSM.913265
Assmann TS, Recamonde-Mendoza M, Costa AR et al (2019) Circulating miRNAs in diabetic kidney disease: case-control study and in silico analyses. Acta Diabetol 56:55–65. https://doi.org/10.1007/s00592-018-1216-x
Barutta F, Tricarico M, Corbelli A et al (2013) Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One 8:e73798. https://doi.org/10.1371/journal.pone.0073798
Uil M, Hau CM, Ahdi M et al (2019) Cellular origin and microRNA profiles of circulating extracellular vesicles in different stages of diabetic nephropathy. Clini Kidney J. https://doi.org/10.1093/ckj/sfz145
Prabu P, Rome S, Sathishkumar C et al (2019) MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the Asian Indian phenotype. Diabet Metabolism. https://doi.org/10.1016/j.diabet.2018.08.004
Kim H, Bae YU, Jeon JS et al (2019) The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J Transl Med 17:236. https://doi.org/10.1186/s12967-019-1983-3
Ghai V, Wu X, Bheda-Malge A et al (2018) Genome-wide profiling of urinary extracellular vesicle microRNAs associated with diabetic nephropathy in type 1 diabetes. Kidney Int Rep 3:555–572. https://doi.org/10.1016/j.ekir.2017.11.019
Good DM, Zürbig P, Argiles A et al (2010) Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteom 9(11):2424–2437
Roscioni SS, de Zeeuw D, Hellemons ME et al (2013) A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia. 56:259–267. https://doi.org/10.1007/s00125-012-2755-2
Zürbig P, Jerums G, Hovind P et al (2012) Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes 61:3304–3313. https://doi.org/10.2337/db12-0348
Lindhardt M, Persson F, Zürbig P et al (2017) Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients, a sub-study of the DIRECT-Protect 2 study. Nephrol Dial Transplant 32:1866–1873. https://doi.org/10.1093/ndt/gfw292
Schanstra JP, Zurbig P, Alkhalaf A et al (2015) Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol 26:1999–2010. https://doi.org/10.1681/ASN.2014050423
Pontillo C, Jacobs L, Staessen JA et al (2017) A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol Dial Transpl 32:1510–1516. https://doi.org/10.1093/ndt/gfw239
Zurbig P, Mischak H, Menne J, Haller H (2019) CKD273 enables efficient prediction of diabetic nephropathy in nonalbuminuric patients. DIABET Care 42:e4–e5. https://doi.org/10.2337/dc18-1322
Tofte N, Lindhardt M, Adamova K et al (2020) Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabet Endocrinol 8:301–312. https://doi.org/10.1016/S2213-8587(20)30026-7
Siwy J, Klein T, Rosler M, von Eynatten M (2019) Urinary proteomics as a tool to identify kidney responders to dipeptidyl peptidase-4 Inhibition a hypothesis-generating analysis from the MARLINA-T2D Trial. PROTEOMICS-Clin Appl. https://doi.org/10.1002/prca.201800144
Niewczas MA, Pavkov ME, Skupien J et al (2019) A signature of circulating inflammatory proteins and development of end stage renal disease in diabetes. Nat Med. https://doi.org/10.1038/s41591-019-0415-5
Hocher B, Adamski J (2017) Metabolomics for clinical use and research in chronic kidney disease. Nat Rev Nephrol 13:269–284. https://doi.org/10.1038/nrneph.2017.30
Niewczas MA, Mathew AV, Croall S et al (2017) Circulating modified metabolites and a risk of ESRD in patients with type 1 diabetes and chronic kidney disease. Diabet Care 40:383–390. https://doi.org/10.2337/dc16-0173
Tofte N, Suvitaival T, Trost K et al (2019) Metabolomic assessment reveals alteration in polyols and branched chain amino acids associated with present and future renal impairment in a discovery cohort of 637 persons with type 1 diabetes. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2019.00818
Tofte N, Suvitaival T, Ahonen L et al (2019) Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci Rep 9:16398. https://doi.org/10.1038/s41598-019-52916-w
Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E (2016) Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab 101(2):696–704
Afshinnia F, Nair V, Lin J et al (2019) Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI insight 4:e130317. https://doi.org/10.1172/jci.insight.130317
Krolewski AS (2015) Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabet Care 38:954–962. https://doi.org/10.2337/dc15-0184
Porrini E, Ruggenenti P, Luis-Lima S et al (2019) Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 15:177–190. https://doi.org/10.1038/s41581-018-0080-9
Inker LA, Mondal H, Greene T et al (2016) Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am J Kidney Dis 68:392–401. https://doi.org/10.1053/j.ajkd.2016.02.042
Holtkamp FA, de Zeeuw D, Thomas MC et al (2011) An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80:282–287. https://doi.org/10.1038/ki.2011.79
Mora-Gutiérrez MJ, Garcia-Fernandez N, Roblero MFS et al (2017) Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25717
Wang YC, Feng Y, Lu CQ, Ju S (2018) Renal fat fraction and diffusion tensor imaging in patients with early-stage diabetic nephropathy. Eur Radiol 28:3326–3334. https://doi.org/10.1007/s00330-017-5298-6
Inoue T, Kozawa E, Okada H et al (2011) Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22:1429–1434. https://doi.org/10.1681/ASN.2010111143
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81:684–689. https://doi.org/10.1038/ki.2011.455
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors
Informed consent
For this type of study, formal consent is not required.
Additional information
This article belongs to the topical collection Diabetic Nephropathy, managed by Giuseppe Pugliese.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Error in table 1 reference corrected.
Rights and permissions
About this article
Cite this article
Barutta, F., Bellini, S., Canepa, S. et al. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol 58, 819–830 (2021). https://doi.org/10.1007/s00592-020-01656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01656-9