Abstract
Background
Women have a high risk of frailty independently of age and menopause state. Diabetes and hypertension increase the risk of frailty and cognitive impairment. Metformin has been employed in post-menopausal women and some reports have shown encouraging effects in terms of attenuated frailty. However, the impact on cognitive performance of a recently introduced extended-release formulation of metformin has never been explored.
Methods
We studied consecutive frail hypertensive and diabetic older women presenting at the ASL (local health authority of the Italian Ministry of Health) Avellino, Italy, from June 2021 to August 2022, who were treated or not with extended-release metformin. We included a control group of frail older males with diabetes and hypertension treated with extended-release metformin and a control group of frail older women with diabetes and hypertension treated with regular metformin.
Results
A total of 145 patients successfully completed the study. At the end of the 6-month follow-up, we observed a significantly different cognitive performance compared to baseline in the group of frail women treated with extended-release metformin (p: 0.007). Then, we compared the follow-up groups and we observed significant differences between frail women treated vs. untreated (p: 0.041), between treated frail women and treated frail men (p: 0.016), and between women treated with extended-release metformin vs. women treated with regular metformin (p: 0.048). We confirmed the crucial role of extended-release metformin applying a multivariable logistic analysis to adjust for potential confounders.
Conclusions
We evidenced, for the first time to the best of our knowledge, the favorable effects on cognitive impairment of extended-release metformin in frail women with diabetes and hypertension.
Similar content being viewed by others
Background
Frailty is a multidimensional condition typical of older adults driving worst outcomes including cognitive and functional decline, hospitalization, and death [1,2,3,4]. We and others have previously shown the association between frailty and hypertension [5,6,7,8,9,10,11]. Equally important, diabetes is a condition extremely common in frailty and represents a distinctive determinant of cognitive decline [12,13,14]. In a recent report, our group highlighted a strong correlation between physical and cognitive impairment in frail older adults [15].
Metformin is an oral antidiabetic drug that remains a cornerstone of the first-line therapeutic approach in both elder and younger populations [16,17,18,19]. Interestingly, recent studies have also suggested beneficial effects in post-menopausal women [20,21,22] and some reports have shown advantageous actions in terms of attenuated parameters of frailty [23,24,25].
The recently introduced extended-release formulation of metformin has shown less gastrointestinal effects compared to the regular (immediate-release) metformin and this aspect might be critical in elders, which are often overtreated [26,27,28,29,30,31,32]. Previous studies have reported differences in cognitive function when comparing extended-release to regular formulations [33,34,35,36]. However, to our knowledge the effects of extended-release metformin have never been assessed in frail older patients with diabetes and hypertension.
Women have a higher risk of frailty during lifespan [37] and previous investigations have evidenced that this aspect is independent of age and menopause state [38, 39]. Thus, also based on its increased tolerability and decreased side effects [40, 41] we hypothesized that extended-release metformin may be helpful in frail women with diabetes and hypertension. On these grounds, we investigated the effects of extended-release metformin on cognitive performance in this population.
Methods
We designed the LEOPARDESS (extended-reLease mEtformin and cOgnitive imPAirment in fRail olDer womEn with hypertenSion and diabeteS) study, enrolling consecutive frail hypertensive older women with diabetes presenting at the ASL (local health authority of the Italian Ministry of Health) Avellino, Italy, from June 2021 to August 2022. As a control population, we evaluated an age-matched group of frail hypertensive older males with diabetes and hypertension treated with extended-release metformin, a formulation of metformin hydrochloride suspended within a polymer matrix that dissolves over hours as the tablet passes through the gastrointestinal tract.
Inclusion criteria:
-
1.
Diagnosis of diabetes;
-
2.
Diagnosis of hypertension with no clinical or laboratory evidence of secondary causes;
-
3.
Age > 65 years;
-
4.
Montreal Cognitive Assessment (MoCA) Score < 26.
-
5.
Diagnosis of frailty.
Exclusion criteria:
-
1.
Age ≤ 65 years;
-
2.
Absence of frailty;
-
3.
MoCA score ≥ 26;
-
4.
Absence of diabetes and hypertension;
-
5.
Glomerular Filtration Rate (GFR) < 30.
Diabetes was defined according to the guidelines of the American Diabetes Association (ADA) [42]. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg on repeated measurements, or use of antihypertensive medications [43, 44]. All patients had blood samples drawn for measuring glycemia, HbA1c, and creatinine. The study was approved by the local Ethical Committee (Campania Nord). All subjects signed an informed consent.
Frailty assessment
As previously described [12, 45], we diagnosed frailty when at least three of the following five criteria were present:
-
Weight loss (unintentional loss ≥ 4.5 kg in the past year).
-
Weakness (handgrip strength in the lowest 20% quintile at baseline, adjusted for sex and body mass index, BMI).
-
Exhaustion (poor endurance and energy, self-reported).
-
Slowness (walking speed under the lowest quintile adjusted for sex and height).
-
Low physical activity level (lowest quintile of kilocalories of physical activity during the past week).
Global cognitive evaluation
We screened global cognitive function using the MoCA Test, a cognitive test largely considered the best option to diagnose mild cognitive impairment [46]. We defined cognitive impairment by cut-off values < 26, as specified in the inclusion criteria [47, 48].
Extended-release metformin treatment
Our population was divided in three different groups: patients receiving 500 mg extended-release metformin, patients receiving 500 mg regular metformin, and patients not receiving metformin; we also examined a control group of age-matched frail older males with diabetes and hypertension, receiving 500 mg extended-release metformin. All patients were followed-up for six months.
Statistical analysis
Data are presented as means ± SD. Based on our preliminary findings, we had calculated the number of patients required for the study to have 95% power with a two-tailed type I error at the 0.05 level of significance; the minimum sample size was predicted using G_POWER software, yielding an estimated sample of 90 patients. Multivariable regression models fitted to assess the association between cognitive impairment and covariates. All calculations were performed using SPSS 26 (IBM, Armonk, NY) and GraphPad Prism 9.0 (San Diego, CA).
Results
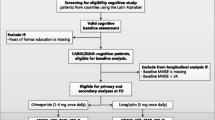
We screened 153 consecutive cognitively impaired frail older women with diabetes and hypertension. We excluded 29 women because they did not meet the afore-mentioned inclusion criteria. Hence, we enrolled 124 women. Of these, 40 were assigned to be treated with extended-release metformin, 36 received regular metformin, and 38 did not receive metformin (Fig. 1). At the end of the 6-month follow-up, data from a total of 10 women were not available (4 from the untreated group, and 3 each from the groups receiving regular or extended-release metformin); so, 114 women successfully completed the study. We also examined a control group of age-matched 31 frail older males with diabetes and hypertension who received extended-release metformin. The baseline characteristics of these patients are reported in Table 1.
At the end of the 6-month follow-up, we observed a significant (p: 0.007) difference compared to baseline in the frail women group treated with extended-release metformin (Fig. 2). Remarkably, we found no significant difference between baseline and follow-up in the other groups.
Then, we compared the follow-up groups to verify the presence of differences among groups. We found a significant difference at follow-up between frail women treated with extended-release metformin vs. non-treated (p: 0.041), between treated frail women vs. treated frail men (p: 0.016), and between women treated with extended-release metformin vs. women treated with regular metformin (p: 0.048).
We confirmed the critical role of extended-release metformin by a multivariable logistic analysis after adjusting for potential confounders (Table 2), including age, BMI, systolic and diastolic blood pressure, heart rate, blood glucose, and serum creatinine; these covariates were selected because they have been previously associated with cognitive impairment [7, 49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
Discussion
In the present study we have demonstrated, for the first time to the best of our knowledge, the favorable effects of extended-release metformin on cognitive impairment in frail women with diabetes and hypertension.
Sex differences are pivotal in the management of frailty and cognitive impairment. In this context, extended-release metformin may play a decisive role in reducing cognitive impairment in frail women with hypertension and diabetes. Many drugs have been investigated in post-menopausal women [65,66,67] and metformin has been proposed as an anti-aging drug [68, 69]. Intriguingly, preclinical assays in rodents had evidenced differences in the effects of metformin on lifespan [70, 71]. We conjectured that the extended-release formulation could be decisive in reducing the adverse effects of metformin in frail women. Indeed, gastroenteric effects of normal metformin are often key determinants in discontinuing therapy. Additionally, metformin has been shown to have pleiotropic effects in women with polycystic ovary syndrome [72] and in preventing breast cancer [73, 74]. On these grounds, we believe that extended-release metformin exerted beneficial effects in our population because of its safe profile and multifactorial functions. We can only speculate on the potential molecular mechanisms underlying the observed phenotype, especially considering that although metformin and other biguanides are known to work by inhibiting hepatic gluconeogenesis, its precise mechanisms of action remain widely debated. Nir Barzilai and collaborators have shown that 6 weeks of metformin can improve age-associated metabolic derangements in glucose intolerant older adults: the treatment had tissue‐specific effects on gene expression and influenced not only metabolic genes and pathways, but also collagen and mitochondrial genes in adipose tissue, and DNA repair genes in skeletal muscle, emphasizing its targeting of multiple hallmarks of aging [75]. Most recently, researchers from Stanford University [76] have examined the genome-wide DNA methylation profile of metformin users, concluding that epigenetic modifications may underlie the previously reported anti-aging role of this drug. Metformin has been shown to affect the receptors for adiponectin, insulin, and several cytokines, and, all pathways that when inhibited are associated with longevity; at the intracellular level, biguanids inhibit inflammatory pathways and activate AMPK, thereby inhibiting mTOR, a major target in aging-related processes [77, 78]. Metformin also regulates protein synthesis, stress defense, mitochondrial function, oxidative stress, and autophagy, phenomena that are known to be associated with aging/longevity [79, 80]. Henceforth, a sustained action of metformin achieved via the extended-release formulation could be affecting any of the above-mentioned mechanisms.
Our results are noteworthy but not exempts of limitations, including the relatively small sample size and the brief follow-up. However, our population was a real-world homogeneous cluster and we had performed an a priori power analysis; additionally, observing effects already at 6-months could be seen as a plus. Nevertheless, further studies in large populations and with a longer follow-up are warranted to confirm our results. Moreover, since our study was performed in medical centers and health units that mostly see White patients, our findings may not be applicable to subjects with a different ethnical background.
Conclusions
We evidenced the favorable effects on cognitive impairment of extended-release metformin in frail women with diabetes and hypertension.
Data availability
All data and materials are available from the first author upon reasonable request.
References
Galluzzo L, Noale M, Maggi S, Feraldi A, Baldereschi M, Di Carlo A, Onder G, Group IW. Frailty Prevalence, Incidence, and Association with Incident Disability in the italian longitudinal study on aging. Gerontology. 2023;69(3):249–60.
Chao CT, Wang J, Chien KL. Group COoGNiNs: both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):130.
Mangin D, Lawson J, Risdon C, Siu HY, Packer T, Wong ST, Howard M. Association between frailty, chronic conditions and socioeconomic status in community-dwelling older adults attending primary care: a cross-sectional study using practice-based research network data. BMJ Open. 2023;13(2):e066269.
Stolz E, Mayerl H, Freidl W. Fluctuations in frailty among older adults. Age Ageing. 2019;48(4):547–52.
Benetos A, Petrovic M, Strandberg T. Hypertension management in older and Frail older patients. Circ Res. 2019;124(7):1045–60.
Mone P, Pansini A, Frullone S, de Donato A, Buonincontri V, De Blasiis P, Marro A, Morgante M, De Luca A, Santulli G. Physical decline and cognitive impairment in frail hypertensive elders during COVID-19. Eur J Intern Med. 2022;99:89–92.
Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–44.
Mone P, Pansini A, Calabro F, De Gennaro S, Esposito M, Rinaldi P, Colin A, Minicucci F, Coppola A, Frullone S, et al. Global cognitive function correlates with P-wave dispersion in frail hypertensive older adults. J Clin Hypertens (Greenwich). 2022;24(5):638–43.
Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, Poon C, Schaeffer S, Segarra SG, Korbelin J, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76(3):795–807.
Aprahamian I, Sassaki E, Dos Santos MF, Izbicki R, Pulgrossi RC, Biella MM, Borges ACN, Sassaki MM, Torres LM, Fernandez IS, et al. Hypertension and frailty in older adults. J Clin Hypertens (Greenwich). 2018;20(1):186–92.
Bromfield SG, Ngameni CA, Colantonio LD, Bowling CB, Shimbo D, Reynolds K, Safford MM, Banach M, Toth PP, Muntner P. Blood pressure, antihypertensive polypharmacy, Frailty, and risk for serious fall injuries among older treated adults with hypertension. Hypertension. 2017;70(2):259–66.
Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, Frullone S, Santulli G. Empagliflozin improves cognitive impairment in Frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022;45(5):1247–51.
Li G, Prior JC, Leslie WD, Thabane L, Papaioannou A, Josse RG, Kaiser SM, Kovacs CS, Anastassiades T, Towheed T, et al. Frailty and Risk of Fractures in patients with type 2 diabetes. Diabetes Care. 2019;42(4):507–13.
Ida S, Kaneko R, Imataka K, Murata K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18(1):81.
Mone P, Gambardella J, Lombardi A, Pansini A, De Gennaro S, Leo AL, Famiglietti M, Marro A, Morgante M, Frullone S, et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc Diabetol. 2022;21(1):10.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. Older adults: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):216–S229.
Bromage DI, Yellon DM. The pleiotropic effects of metformin: time for prospective studies. Cardiovasc Diabetol. 2015;14:109.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. Diabetes Care in the hospital: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):267–S278.
Luo F, Das A, Chen J, Wu P, Li X, Fang Z. Metformin in patients with and without diabetes: a paradigm shift in cardiovascular disease management. Cardiovasc Diabetol. 2019;18(1):54.
Krysiak R, Kowalcze K, Okopien B. The impact of metformin on prolactin levels in postmenopausal women. J Clin Pharm Ther. 2021;46(5):1433–40.
Krysiak R, Szkrobka W, Okopien B. The Effect of Metformin on serum gonadotropin levels in Postmenopausal Women with Diabetes and Prediabetes: a pilot study. Exp Clin Endocrinol Diabetes. 2018;126(10):645–50.
Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9(2):419–46.
Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM, Lushchak O. Metformin as a geroprotector: experimental and clinical evidence. Biogerontology. 2019;20(1):33–48.
Espinoza SE, Musi N, Wang CP, Michalek J, Orsak B, Romo T, Powers B, Conde A, Moris M, Bair-Kelps D, et al. Rationale and Study Design of a Randomized Clinical Trial of Metformin to prevent Frailty in older adults with Prediabetes. J Gerontol A Biol Sci Med Sci. 2020;75(1):102–9.
Hazuda HP, Pan Q, Florez H, Luchsinger JA, Crandall JP, Venditti EM, Golden SH, Kriska AM, Bray GA. Association of Intensive Lifestyle and Metformin Interventions with Frailty in the diabetes Prevention Program Outcomes Study. J Gerontol A Biol Sci Med Sci. 2021;76(5):929–36.
Nabrdalik K, Skonieczna-Zydecka K, Irlik K, Hendel M, Kwiendacz H, Loniewski I, Januszkiewicz K, Gumprecht J, Lip GYH. Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: a systematic review, meta-analysis and meta-regression of randomized controlled trials. Front Endocrinol (Lausanne). 2022;13:975912.
Sun ML, Liu C, Bai HH, Wei YL, Zhang W, Liu HJ, Li YJ, Liu L, Wang Y, Tong YX, et al. Bioequivalence and safety evaluation of two preparations of metformin hydrochloride sustained-release tablets (Boke((R)) and glucophage((R))-XR) in healthy chinese volunteers: a randomized phase I clinical trial. Ann Med. 2022;54(1):2617–26.
Tan J, Wang Y, Liu S, Shi Q, Zhou X, Zhou Y, Yang X, Chen P, Li S. Long-acting Metformin Vs. Metformin Immediate Release in patients with type 2 diabetes: a systematic review. Front Pharmacol. 2021;12:669814.
Ji L, Liu J, Yang J, Li Y, Liang L, Zhu D, Li Q, Ma T, Xu H, Yang Y, et al. Comparative effectiveness of metformin monotherapy in extended release and immediate release formulations for the treatment of type 2 diabetes in treatment-naive chinese patients: analysis of results from the CONSENT trial. Diabetes Obes Metab. 2018;20(4):1006–13.
Guo LX, Liu GE, Chen L, Wang HF, Guo J, Zheng XL, Duan BH, Wang DZ, Zhu W, Wang K, et al. Comparison of clinical efficacy and safety of Metformin sustained-release tablet (II) (Dulening) and Metformin Tablet (glucophage) in treatment of type 2 diabetes Mellitus. Front Endocrinol (Lausanne). 2021;12:712200.
Longo M, Bellastella G, Maiorino MI, Meier JJ, Esposito K, Giugliano D. Diabetes and aging: from treatment goals to pharmacologic therapy. Front Endocrinol (Lausanne). 2019;10:45.
Abrilla AA, Pajes A, Jimeno CA. Metformin extended-release versus metformin immediate-release for adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;178:108824.
Riedel M, Schmitz M, Ostergaard PK, Ferrannini L, Franco MA, Alfano V, Vansvik ED. Comparison of the effects of quetiapine extended-release and quetiapine immediate-release on cognitive performance, sedation and patient satisfaction in patients with schizophrenia: a randomised, double-blind, crossover study (eXtRa). Schizophr Res. 2015;162(1–3):162–8.
Pearson DA, Santos CW, Aman MG, Arnold LE, Lane DM, Loveland KA, Mansour R, Ward AR, Casat CD, Jerger S, et al. Effects of extended-release Methylphenidate Treatment on Cognitive Task Performance in Children with Autism Spectrum disorder and Attention-Deficit/Hyperactivity disorder. J Child Adolesc Psychopharmacol. 2020;30(7):414–26.
Leufkens TR, Vermeeren A, Smink BE, van Ruitenbeek P, Ramaekers JG. Cognitive, psychomotor and actual driving performance in healthy volunteers after immediate and extended release formulations of alprazolam 1 mg. Psychopharmacology. 2007;191(4):951–9.
O’Neal W, Hur EE, Liranso T, Patel B. Real-world assessment of treatment with extended-release topiramate (trokendi XR((R))) and comparison with previous immediate-release topiramate treatment. J Comp Eff Res. 2018;7(11):1095–105.
Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–7.
Verschoor CP, Tamim H. Frailty is inversely related to age at menopause and elevated in women who have had a hysterectomy: an analysis of the canadian longitudinal study on aging. J Gerontol A Biol Sci Med Sci. 2019;74(5):675–82.
Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40.
Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–805.
Aggarwal N, Singla A, Mathieu C, Montanya E, Pfeiffer AFH, Johnsson E, Zhao J, Iqbal N, Bailey C. Metformin extended-release versus immediate-release: an international, randomized, double-blind, head-to-head trial in pharmacotherapy-naive patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):463–7.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. Classification and diagnosis of diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):19–S40.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–57.
Trimarco V, Izzo R, Morisco C, Mone P, Maria Virginia M, Falco A, Pacella D, Gallo P, Lembo M, Santulli G et al. High HDL (High-Density Lipoprotein) Cholesterol Increases Cardiovascular Risk in Hypertensive Patients.Hypertension2022:101161HYPERTENSIONAHA12219912.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156.
Kang JM, Cho YS, Park S, Lee BH, Sohn BK, Choi CH, Choi JS, Jeong HY, Cho SJ, Lee JH, et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. 2018;18(1):261.
Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379–88.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Gallucci M, Mazzuco S, Ongaro F, Di Giorgi E, Mecocci P, Cesari M, Albani D, Forloni GL, Durante E, Gajo GB, et al. Body mass index, lifestyles, physical performance and cognitive decline: the “Treviso Longeva (TRELONG)” study. J Nutr Health Aging. 2013;17(4):378–84.
Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70(5):360–7.
Kim S, Kim Y, Park SM. Body Mass Index and decline of cognitive function. PLoS ONE. 2016;11(2):e0148908.
Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty.Nutrients2020, 12(11).
Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension. 2023;80(1):22–34.
Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17(10):639–54.
Daniel GD, Chen H, Bertoni AG, Hughes TM, Hayden KM. High visit-to-visit blood pressure variability predicts global cognitive decline: the multi-ethnic study of atherosclerosis. Alzheimers Dement (N Y). 2022;8(1):e12342.
de Montgolfier O, Pincon A, Pouliot P, Gillis MA, Bishop J, Sled JG, Villeneuve L, Ferland G, Levy BI, Lesage F, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and Cognitive decline in mice. Hypertension. 2019;73(1):217–28.
Takeuchi H, Kawashima R. Effects of Diastolic Blood Pressure on Brain Structures and Cognitive Functions in Middle and Old Ages: Longitudinal Analyses.Nutrients2022, 14(12).
Peters R, Xu Y, Eramudugolla R, Sachdev PS, Cherbuin N, Tully PJ, Mortby ME, Anstey KJ. Diastolic blood pressure variability in later life may be a key risk marker for Cognitive decline. Hypertension. 2022;79(5):1037–44.
Sanchez Hoffmann S, Winkler A, Weimar C, Muller-Gerards D, Abramowski J, Moebus S, Jockel KH, Erbel R, Jokisch M. Blood pressure and cognitive decline - the impact of hypertension over one decade. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2021;28(4):528–42.
Ma Y, Hua R, Yang Z, Zhong B, Yan L, Xie W. Different hypertension thresholds and cognitive decline: a pooled analysis of three ageing cohorts. BMC Med. 2021;19(1):287.
Chu NM, Hong J, Harasemiw O, Chen X, Fowler KJ, Dasgupta I, Bohm C, Segev DL, McAdams-DeMarco MA. Global Renal Exercise N: chronic kidney disease, physical activity and cognitive function in older adults-results from the National Health and Nutrition Examination Survey (2011–2014). Nephrol Dial Transplant. 2022;37(11):2180–9.
Pansini A, Lombardi A, Morgante M, Frullone S, Marro A, Rizzo M, Martinelli G, Boccalone E, De Luca A, Santulli G, et al. Hyperglycemia and physical impairment in Frail Hypertensive older adults. Front Endocrinol (Lausanne). 2022;13:831556.
Maciejczyk M, Zebrowska E, Chabowski A. Insulin Resistance and Oxidative Stress in the Brain: What’s New?Int J Mol Sci2019, 20(4).
Assuncao N, Sudo FK, Drummond C, de Felice FG, Mattos P. Metabolic syndrome and cognitive decline in the elderly: a systematic review. PLoS ONE. 2018;13(3):e0194990.
Vitale C, Mammi C, Gambacciani M, Russo N, Spoletini I, Fini M, Volterrani M, Rosano GMC. Effect of hormone replacement therapy with the anti-mineralocorticoid progestin drospirenone compared to tibolone on endothelial function and central haemodynamics in post-menopausal women. Int J Cardiol. 2017;227:217–21.
Jankie S, Pinto Pereira LM. Targeting insulin resistance with selected antidiabetic agents prevents menopausal associated central obesity, dysglycemia, and cardiometabolic risk. Post Reprod Health. 2021;27(1):45–8.
Riemma G, Schiattarella A, La Verde M, Zarobbi G, Garzon S, Cucinella G, Calagna G, Labriola D, De Franciscis P. Efficacy of Low-Dose Paroxetine for the Treatment of Hot Flushes in Surgical and Physiological Postmenopausal Women: Systematic Review and Meta-Analysis of Randomized Trials.Medicina (Kaunas)2019, 55(9).
Glossmann HH, Lutz OMD. Metformin and Aging: a review. Gerontology. 2019;65(6):581–90.
Mohammed I, Hollenberg MD, Ding H, Triggle CR. A critical review of the evidence that metformin is a putative anti-aging drug that enhances Healthspan and extends Lifespan. Front Endocrinol (Lausanne). 2021;12:718942.
Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging. 2010;2(12):945–58.
Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8–9):685–93.
Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, MacDonald R, Hollenberg MD, Hill MA. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133:155223.
Lv Z, Guo Y. Metformin and its benefits for various Diseases. Front Endocrinol (Lausanne). 2020;11:191.
Au Yeung SL, Luo S, Schooling CM. The impact of GDF-15, a biomarker for metformin, on the risk of coronary artery disease, breast and colorectal cancer, and type 2 diabetes and metabolic traits: a mendelian randomisation study. Diabetologia. 2019;62(9):1638–46.
Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP, Barzilai N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults.Aging Cell2018, 17(2).
Marra PS, Yamanashi T, Crutchley KJ, Wahba NE, Anderson ZM, Modukuri M, Chang G, Tran T, Iwata M, Cho HR, et al. Metformin use history and genome-wide DNA methylation profile: potential molecular mechanism for aging and longevity. Aging. 2023;15(3):601–16.
Kulkarni AS, Gubbi S, Barzilai N. Benefits of Metformin in attenuating the Hallmarks of Aging. Cell Metab. 2020;32(1):15–30.
Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5(1):e8758.
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–66.
Le Couteur DG, Barzilai N. New horizons in life extension, healthspan extension and exceptional longevity.Age Ageing2022, 51(8).
Funding
The Santulli’s Lab is supported in part by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.).
Author information
Authors and Affiliations
Contributions
PM and GS contributed to study design and manuscript writing. GM, AL, SM, SF, and LC made substantial contributions to study design and intellectual direction. PM, GM, ALL, AM, SDG, and DM acquired data and performed data analysis. SF and LC contributed to interpretation of data and revision of the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local Ethical Committee (Campania Nord). All subjects signed an informed consent.
Consent for publication
All authors gave the consent for the publication of the article.
Competing interests
No competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mone, P., Martinelli, G., Lucariello, A. et al. Extended-release metformin improves cognitive impairment in frail older women with hypertension and diabetes: preliminary results from the LEOPARDESS Study. Cardiovasc Diabetol 22, 94 (2023). https://doi.org/10.1186/s12933-023-01817-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01817-4