Abstract
Rationale
Bronchiectasis and bronchiolitis are differential diagnoses of asthma; moreover, they are factors associated with worse asthma control.
Objective
We determined clinical courses of bronchiectasis/bronchiolitis-complicated asthma by inflammatory subtypes as well as factors affecting them.
Methods
We conducted a survey of refractory asthma with non-cystic fibrosis bronchiectasis/bronchiolitis in Japan. Cases were classified into three groups, based on the latest fractional exhaled NO (FeNO) level (32 ppb for the threshold) and blood eosinophil counts (320/µL for the threshold): high (type 2-high) or low (type 2-low) FeNO and eosinophil and high FeNO or eosinophil (type 2-intermediate). Clinical courses in groups and factors affecting them were analysed.
Results
In total, 216 cases from 81 facilities were reported, and 142 were stratified: 34, 40 and 68 into the type 2-high, -intermediate and -low groups, respectively. The frequency of bronchopneumonia and exacerbations requiring antibiotics and gram-negative bacteria detection rates were highest in the type 2-low group. Eighty-seven cases had paired latest and oldest available data of FeNO and eosinophil counts; they were analysed for inflammatory transition patterns. Among former type 2-high and -intermediate groups, 32% had recently transitioned to the -low group, to which relatively low FeNO in the past and oral corticosteroid use contributed. Lastly, in cases treated with moderate to high doses of inhaled corticosteroids, the frequencies of exacerbations requiring antibiotics were found to be higher in cases with more severe airway lesions and lower FeNO.
Conclusions
Bronchiectasis/bronchiolitis-complicated refractory asthma is heterogeneous. In patients with sputum symptoms and low FeNO, airway colonisation of pathogenic bacteria and infectious episodes are common; thus, corticosteroids should be carefully used.
Similar content being viewed by others
Introduction
Bronchiectasis is known to be an important comorbidity and differential diagnosis of severe asthma [1, 2]. Previous studies have shown that patients with asthma complicated with bronchiectasis have refractory disease with frequent exacerbations [3], antibiotic use and bronchopneumonia [4]. However, there is no appropriate guide for the management of bronchiectasis-complicated refractory asthma, which may arise from the heterogeneous nature of this condition.
Asthma is typically characterised by eosinophilic, type 2-high inflammation and bronchiectasis in asthma has been commonly associated with allergic bronchopulmonary aspergillosis (ABPA), i.e., eosinophilic bronchiectasis. Even in patients with asthma complicated with bronchiectasis other than ABPA, average blood eosinophil counts are not decreased [1, 5]. Furthermore, studies on bronchiectasis without asthma or ABPA have shown that eosinophilic predominant phenotypes represented approximately 20% of patients with bronchiectasis [6, 7]. Meanwhile, bronchiectasis has been typically characterised by recurrent airway infection and neutrophilic airway inflammation. Low fractional exhaled nitric oxide (FeNO) levels have been described as markers of comorbid bronchiectasis in asthma [4] and infectious exacerbations in patients treated with anti-interleukin (IL)-5 antibody for severe asthma [8]. Collectively, patients with asthma with comorbid bronchiectasis are heterogeneous, including type 2-high and type 2-low phenotypes. However, their characteristics and clinical courses according to inflammatory phenotypes remain unknown.
Furthermore, cases with bronchiectasis are often accompanied by computed tomography (CT) findings suggestive of chronic infectious bronchiolitis [9]. However, the effects of asthma with comorbid bronchiolitis have rarely been discussed, except for eosinophilic bronchiolitis [10]. Thus, the purpose of this nationwide study was to characterise patients with asthma complicated with bronchiectasis/bronchiolitis, clarify their clinical courses by stratifying them through FeNO and blood eosinophil counts, and examine the roles of these two markers in patient management.
Methods
Study design and study population
The BEXAS (bronchiectasis and asthma) study was a nationwide survey conducted at accredited and affiliated facilities of the Japanese Respiratory Society and Japanese Society of Allergology. Patients with refractory asthma complicated by bronchiectasis or bronchiolitis, or both with a history of visits between January 2015 and September 2019 were included. The included cases were resistant to standard management regardless of the doses of inhaled corticosteroid (ICS), and had sputum symptoms. Bronchiectasis was defined as an enlarged bronchoarterial ratio of > 1.1 or lack of tapering of an airway toward the periphery. Detailed assessment of bronchiectasis is shown in Additional file 1. Traction bronchiectasis due to fibrotic interstitial pneumonia, non-tuberculous mycobacterial diseases, cystic fibrosis and acute bronchiolitis cases were excluded.
Questionnaires
The survey asked attending physicians the following information: basic demographic data, medical history, timing and basis of the asthma diagnosis, timing of the diagnosis and morphological and inflammatory patterns of airway lesions, i.e., bronchiectasis and bronchiolitis, the latest and former treatments and their effectiveness, latest and oldest laboratory data in 5 years, latest and oldest (or at the diagnosis) radiological data, frequencies of exacerbations requiring systemic corticosteroids and antibiotics in the latest 2 years and frequencies of bronchopneumonia and hospital admission due to exacerbations in the latest 5 years. Long-term oral corticosteroid (OCS) use was defined as regular current or past OCS use. Cases with ABPA were excluded from this analysis, as it was considered a separate clinical entity based on its response to established treatments. This study was approved by the Kyoto University Medical Ethics Committee (R2168).
Statistical analysis
Analyses were performed using JMP version 15. Two or more groups were compared using the χ2 test, Fisher’s exact test, Wilcoxon rank-sum test and Kruskal–Wallis test, where deemed appropriate. Multiple comparison tests were performed using Steel–Dwass test. The Wilcoxon signed-rank test was used to compare matched samples. Details are shown in the Additional file 1. A p-value of < 0.05 was considered significant. Data are shown as means (SD).
Results
Patient characteristics and stratification by type 2 inflammation
In total, 81 facilities responded, wherein 216 cases were returned, and 35.2% were males and the mean (SD) age was 64.7 (14.6) years. Total of 59% of the patients had severe asthma (details are shown in the Additional file). The mean period from asthma diagnosis to the diagnosis of bronchiectasis/bronchiolitis was 14.4 (17.9) years (n = 180), and the means of current blood eosinophil counts and FeNO levels were 378 (515)/μL (n = 198) and 40 (45) ppb (n = 154), respectively.
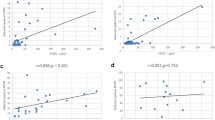
First, current FeNO and blood eosinophil levels that reflected the presence of bronchopneumonia in the last 5 years were determined using a receiver operating characteristic (ROC) curve analysis (Fig. 1). The best thresholds were set at an FeNO level of 32 ppb and blood eosinophil count of 321/μL. Using FeNO level of 32 ppb and blood eosinophil count of 320/μL as thresholds, cases with current data on the two type 2-markers were classified into the following three groups: FeNO and eosinophil high (type 2-high, n = 34), FeNO or eosinophil high (type 2-intermediate, n = 40) and FeNO and eosinophil low (type 2-low, n = 68) groups (Fig. 1). Details on the 142 stratified cases and the remaining 74 cases are presented in Additional file 1: Results and Table S1.
Characteristics and current conditions of the three inflammatory groups
Among the type 2-high, -intermediate and -low groups, no significant differences were noted in terms of age, sex, age at asthma diagnosis, or period from asthma diagnosis to bronchiectasis/bronchiolitis diagnosis (Table 1). The type 2-low group showed the lowest serum total IgE levels and highest serum C-reactive protein levels, and patients’ sputum culture revealed gram-negative bacteria (GNB) and Pseudomonas aeruginosa (P. aeruginosa) most frequently among the three inflammatory groups (Table 1). As expected, the type 2-low group had infectious episodes most frequently (Fig. 2), but the frequencies of asthma exacerbation requiring systemic corticosteroids did not differ among the three groups. The frequencies of other comorbidities did not differ among the groups (Additional file 1: Table S2). Anti-type 2 biologics or regular OCS (≥ 5 mg/day) was administered to 56 cases, and the frequency of administration did not differ among the three groups. The major features of the inflammatory groups did not change when the study was limited to subjects not receiving these medications (Additional file 1: Results and Fig. S1). Impression of the attending physicians is described in the Additional file 1: Results.
Frequencies of A exacerbation requiring systemic corticosteroids (p = 0.30 among the three inflammatory groups by the Kruskal–Wallis test), B exacerbation requiring antibiotics (p = 0.005 among the three groups, p = 0.007 for type 2-low vs type 2-intermedate), C bronchopneumonia (p = 0.01 among the three groups, p = 0.03 for type 2-low vs type 2-high), and D hospitalisation (p = 0.04 among the three groups, p = 0.04 for type 2-low vs type 2-high), in the last 2 years. Boxes and bars indicate upper, lower, and median quartiles
Although the composite of the two type 2-markers described the examined cases well, lower FeNO alone also indicated the presence of GNB and P. aeruginosa in patient’s sputum and more severe airway lesions. The areas under the curves (AUCs) of ROC curves for predicting sputum GNB and P. aeruginosa were 0.74 and 0.74, respectively (Additional file 1: Results and Fig. S2). The AUCs of ROC curves for blood eosinophil counts were 0.54 for GNB and 0.57 for P. aeruginosa in the sputum (data not shown). Lower FeNO alone, but not lower blood eosinophil counts, was also associated with the degree of bronchiectasis and bronchiolitis (Additional file 1: Fig. S3).
Transition pattern of inflammatory types
Subsequently, the transition pattern of inflammatory types was examined in cases that were followed up for ≥ 2 years as well as had paired latest and oldest available data of FeNO and blood eosinophil counts. The mean (SD) period between the latest and oldest data was 3.9 (2.0) years. Former inflammatory types were similarly determined using the thresholds of FeNO (32 ppb) and blood eosinophil (320/μL). Cases who were included in the transition analysis (n = 87) had more items related to asthma diagnosis [3.3 (1.4) vs 2.4 (1.3), p < 0.0001] and less frequently yielded GNB in the past (32% vs 58%, p = 0.004) compared with the remaining cases who were followed for ≥ 2 years but did not have paired data of type 2-markers (n = 90).
Figure 3 shows the transition pattern of 87 cases. Among the 22 cases (25%) of the former type 2-low group, 20 remained in the type 2-low group based on the latest available data (defined as low-to-low) and 2 transitioned to the type-2 intermediate group. Among the 65 cases (75%) of former type 2-high and -intermediate groups, 21 transitioned to the current type 2-low group (high’-to-low) and 44 remained in the original category (high’-to-high’); in this transition analysis, the high’ group included type 2-high and -intermediate groups. In the high’-to-high’ group, the frequencies of exacerbations requiring antibiotics and bronchopneumonia in the last 2 years (Fig. 4 and Additional file 1: Fig. S4) were found to be the lowest, whereas the current daily doses of ICS were the highest (Table 2). The three transition groups had exacerbations similarly requiring systemic corticosteroids (63% in the low-to-low group, 65% in the high’-to-low and 53% in high’-to-high’ group, p = 0.60). In the high’-to-high’ group, the ICS dose was significantly increased over time (p = 0.001) (Additional file 1: Fig. S5).
Frequencies of A exacerbation requiring systemic corticosteroids (p = 0.51 among the three transition patterns by the Kruskal–Wallis test), B exacerbation requiring antibiotics (p = 0.02 among the three patterns and p = 0.06 for low-to-low vs high’-to-high’), C bronchopneumonia (p = 0.03 among the three patterns and p = 0.02 for low-to-low vs high’-to-high’), and D hospitalisation (p = 0.08), in the last 2 years. Boxes and bars indicate upper, lower, and median quartiles
The high’-to-low group showed an approximately equal number of positive items associated with asthma diagnosis as the high’-to-high’ group (Additional file 1: Table S4) and a P. aeruginosa detection rate similar to that of the low-to-low group (Table 2). Furthermore, in the high’-to-low group, the rate of long-term OCS use was the highest, and the period between the diagnosis of asthma and bronchiectasis/bronchiolitis was the shortest, among the three groups.
To identify the factors associated with high’-to-low transition, multivariate logistic regression analysis was performed in patients of former type 2-high or -intermediate groups. Long-term OCS use, lower FeNO in the past, and a shorter period between the diagnosis of asthma and airway lesions were independent contributing factors to the high’-to-low transition (Table 3). Details of airway lesions and their transition patterns are presented in the Additional file 1: Results.
Additionally, among cases treated with moderate to high ICS doses, the frequencies of exacerbations requiring antibiotics in the last 2 years were higher in cases with mReiff scores ≥ 2 and bronchiolitis in ≥ 2 lobes on the latest CT, which was also observed when stratified by a recent FeNO of 32 ppb (Fig. 5). These differences were not observed in cases with zero to low ICS doses.
Frequency of exacerbation requiring antibiotics in the last 2 years in cases treated with moderate to high doses of inhaled corticosteroids according to the current degree of A bronchiectasis, i.e., modified Reiff (mReiff) score, B bronchiolitis, and C FeNO level. Boxes and bars indicate upper, lower, and median quartiles
Discussion
This is the first nationwide survey clarifying the characteristics and clinical courses of refractory asthma complicated with bronchiectasis/bronchiolitis by stratifying patients according to FeNO and blood eosinophil levels. As expected, the current type 2-low inflammatory group, which accounted for approximately half of the studied cases, was found to have infectious episodes most frequently among the three inflammatory groups, with the detection of GNB and P. aeruginosa in the sputum. Meanwhile, the current type 2-high group did not suffer from infectious episodes, despite the presence of bronchiectasis and using the highest ICS dose. However, nearly one-third of cases of former type 2-high or -intermediate groups transitioned to the current type 2-low group (high’-to-low), and this transition was contributed by relatively low FeNO levels in the past and long-term OCS use.
Stratification using FeNO and blood eosinophils provided a clear picture of the characteristics and transition patterns of patients with refractory asthma and bronchiectasis/bronchiolitis. Patients in the type 2-low group had the highest number of infectious episodes and hospitalisations, but they required systemic corticosteroids due to exacerbations similar to type 2-high and -intermediate groups. Importantly, nearly half of the current type 2-low group cases were from former type 2-high or -intermediate groups. This high’-to-low group was indistinguishable from the low-to-low group as per current laboratory data and CT findings, but its blood eosinophil counts in the past were as high as those of the high’-to-high’ group.
Low FeNO levels and blood eosinophil counts may indicate type 2-low inflammation. In this study, FeNO performed better than blood eosinophil counts in the detection of GNB and P. aeruginosa in the sputum, reflecting more severe airway lesions. FeNO was shown to be reduced and negatively correlated with the number of lobes affected by bronchiectasis in non-asthmatic patients with P. aeruginosa in the sputum [11]. Low FeNO is also suggestive of infectious exacerbations under anti-IL-5 antibody (mepolizumab) treatment in severe asthma [8]. The risk of bronchiectasis comorbidity can be predicted by lower levels of FeNO, a previous history of Pneumonia, the presence of chronic Expectoration, and the Severity of asthma (NOPES score) [4]. For eosinophil counts, a negative correlation between tissue eosinophil counts and the DNA abundance of the phylum Proteobacteria in the airways of severe asthma is also shown [12] and eosinophils can activate innate and adaptive immune responses against microbials [13,14,15], including the eradication of P. aeruginosa biofilms through their granules [16]. Nonetheless, the roles of blood eosinophil counts in predicting bacterial colonisation or infectious episodes in clinical settings remain to be unknown. The mechanisms underlying the decrease in FeNO in chronic suppurative conditions are not clearly demonstrated, but are likely due to poor NO diffusion across the viscous and increased airway secretions and the removal of NO by reacting with reactive oxygen species [17]. Decreases in FeNO may reflect the impaired antimicrobial activity in the damaged airways, considering that NO attacks viruses and bacteria to prevent infection [18,19,20]. Inducible NO synthase is induced by not only type 2-cytokines, but also antimicrobial cytokines, such as tumour necrosis factor-α, interferon-γ and IL-1β [21]. Clinically, it is difficult to determine whether low FeNO reflects optimally treated asthma or bacterial airway colonisation and the development of bronchiectasis/bronchiolitis, but it is better to consider the possibility of bronchiectasis/bronchiolitis when patients with optimally treated asthma only had purulent sputum and showed low FeNO. Further prospective studies are warranted.
The risks associated with long-term OCS use are well-known. As this was a retrospective study over two time points and details of treatments over the two time points were unavailable, the interpretations and conclusions should be made with caution. Nonetheless, the present study showed its potential risk of infection in refractory asthma complicated with bronchiectasis/bronchiolitis, by showing that long-term OCS use was associated with the transition from former type 2-high or -intermediate groups to the current type 2-low group, independent of lower FeNO in the past. Meanwhile, ICS did not affect the above transition. ICS efficiently suppresses airway inflammation, reduces mucus obstruction and protects against bacterial airway invasion [22]. Additionally, unlike chronic obstructive pulmonary disease [23] or bronchiectasis, the risks of pneumonia in ICS for asthma can vary among studies [24,25,26]. Nonetheless, ICS may be a double-edged sword, particularly at high doses, as is alerted elsewhere [27, 28]. This was likely especially in type 2-low and structurally damaged airways, as seen in the increased exacerbations requiring antibiotics in cases treated with moderate to high ICS doses in this study. Several studies have showed a loss of diversity in microbiota composition [29] and increased pathogenic Proteobacteria, particularly Haemophilus influenzae and P. aeruginosa, in the airways of patients with asthma receiving ICS either with or without OCS compared to those without any corticosteroid use [30, 31]. This may be associated with an increased risk of lower respiratory tract infection in susceptible patients in the later years. Guidelines for bronchiectasis recommend the non-use of ICS, except for in cases of concomitant asthma [32, 33]. However, in patients with asthma complicated by severer bronchiectasis/bronchiolitis with type 2-low inflammation, lower ICS doses may be preferable and physiotherapy, including airway clearance should be implemented, as in bronchiectasis without asthma.
A previous microbiota study on asthma reported that patients with type 2-high asthma had a significantly lower bronchial bacterial burden than those with type 2-low asthma [34]. Consistent with the results of a previous study, the type 2-high group, which represented 24% of the studied population, had least number episodes of infection and GNB and P. aeruginosa in sputum despite the use of the highest dose of ICS. Although bronchiolitis pathology was not examined here, centrilobular micronodules on CT in this group may have included eosinophilic bronchiolitis. Neutrophilic and eosinophilic airways may have different microbiome composition [12, 35], which could be explained by the effect of microbial itself and the selective pressure of airway inflammatory types [29]. Further studies on the mechanisms underlying these differences are thus required.
The comorbidity of chronic infectious bronchiolitis in asthma has been rarely discussed; however, the degree of bronchiolitis was associated with infectious episodes in this study. Furthermore, 30% of former bronchiolitis developed into bronchiectasis. The mechanisms underlying this are yet to be determined, but small airway inflammation may cause the dilatation of larger adjacent airways through protease/elastase secretion from neutrophils [36]. Although not focusing on small airways, studies on children showed that 12% of protracted bacterial bronchitis cases progressed to bronchiectasis [37]. Overall, bronchiolitis may be a possible precursor of bronchiectasis. CT findings suggestive of chronic infectious bronchiolitis should be noted in the management of refractory asthma with sputum symptoms and low FeNO.
Diagnosing asthma with bronchiectasis is always a dilemma, but bronchiectasis has often been considered a consequence of severe, uncontrolled asthma, as bronchial dilatation on CT is known to be more prevalent in patients with asthma than in healthy subjects, and the degree of dilatation is associated with asthma severity [38, 39]. According to a study by Mäntyä et al., when asthma and bronchiectasis overlap, the focus should be on the time of diagnosis of each disease; moreover, an earlier diagnosis of asthma may indicate that bronchiectasis is a consequence of asthma [40]. Similar to their findings, wherein the diagnosis of asthma preceded that of bronchiectasis by an average of 18.5 years, in our study, the diagnosis of asthma preceded that of bronchiectasis by 14 years. Additionally, systemic corticosteroid use was similarly required in the low-to-low group, which may also support the presence of asthma in this group.
This study has some limitations. First, the timing, frequencies, types, procedures of examinations and diagnostic and treatment strategies were not standardised among the facilities, due to the multicenter retrospective survey nature of this study. The transition patterns of inflammatory types were determined using two data points. However, the trajectories of blood eosinophil counts from patients with ≥ 3 data points appeared to show believable differences among the three transition patterns (Additional file 1: Fig. S7). In this study, the definition of bronchiectasis did not satisfy the latest recommendation in clinical trials [41]; however, we enroled cases with radiological diagnosis of bronchiectasis and sputum symptoms. In addition, the aetiology of exacerbations may not be sufficiently accurate, but the frequency of exacerbations requiring antibiotics was strongly associated with that of bronchopneumonia (rho = 0.78, p < 0.0001). Lastly the cases stratified by FeNO and blood eosinophil counts were part of the collected cases, but the stratified cases showed more items related to asthma diagnosis (Additional file 1: Table S1) than those which were not stratified. We believe that the stratified cases were a proper population for this analysis of refractory asthma.
In conclusion, this comprehensive nationwide survey suggests that refractory asthma complicated by bronchiectasis/bronchiolitis was heterogeneous. Long-term OCS use may increase the risk of transition from the originally high or intermediate type 2 to type 2-low inflammation with infectious episodes. Low FeNO may serve as a clinical indicator of this transition in patients with sputum symtoms despite optimal asthma treatment. Further prospective studies are needed to confirm the findings.
Availability of data and materials
Not applicable.
References
Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, Senna G, Canonica GW, Network S. The severe asthma network in italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019;7:1462–8.
Crimi C, Ferri S, Campisi R, Crimi N. The link between asthma and bronchiectasis: state of the art. Respiration. 2020;99:463–76.
Kang HR, Choi GS, Park SJ, Song YK, Kim JM, Ha J, Lee YH, Lee BH, Kim SH, Lee JH. The effects of bronchiectasis on asthma exacerbation. Tuberc Respir Dis (Seoul). 2014;77:209–14.
Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, Marco-Galve I, Plata AJ, Alvarez A, Rivas-Ruiz F, Carmona-Olveira A, Cebrian-Gallardo JJ, Martinez-Garcia MA. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19:43.
Matsumoto H. Bronchiectasis in severe asthma and asthmatic components in bronchiectasis. Respir Investig. 2022;60:187–96.
Tsikrika S, Dimakou K, Papaioannou AI, Hillas G, Thanos L, Kostikas K, Loukides S, Papiris S, Koulouris N, Bakakos P. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine. 2017;99:281–6.
Shoemark A, Shteinberg M, De Soyza A, Haworth CS, Richardson H, Gao Y, Perea L, Dicker AJ, Goeminne PC, Cant E, et al. Characterization of eosinophilic bronchiectasis: A European Multicohort Study. Am J Respir Crit Care Med. 2022;205:894–902.
McDowell PJ, Diver S, Yang F, Borg C, Busby J, Brown V, Shrimanker R, Cox C, Brightling CE, Chaudhuri R, et al. The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study. Lancet Respir Med. 2021;9:1174–84.
Winningham PJ, Martínez-Jiménez S, Rosado-de-Christenson ML, Betancourt SL, Restrepo CS, Eraso A. Bronchiolitis: a practical approach for the general radiologist. Radiographics. 2017;37:777–94.
Takayanagi N, Kanazawa M, Kawabata Y, Colby TV. Chronic bronchiolitis with associated eosinophilic lung disease (eosinophilic bronchiolitis). Respiration. 2001;68:319–22.
Tsang KW, Leung R, Fung PC, Chan SL, Tipoe GL, Ooi GC, Lam WK. Exhaled and sputum nitric oxide in bronchiectasis: correlation with clinical parameters. Chest. 2002;121:88–94.
Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–84.
Aoki A, Hirahara K, Kiuchi M, Nakayama T. Eosinophils: cells known for over 140 years with broad and new functions. Allergol Int. 2021;70:3–8.
Kolsum U, Donaldson GC, Singh R, Barker BL, Gupta V, George L, Webb AJ, Thurston S, Brookes AJ, McHugh TD, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18:88.
Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8:464–75.
Pulido D, Prats-Ejarque G, Villalba C, Albacar M, Gonzalez-Lopez JJ, Torrent M, Moussaoui M, Boix E. A novel RNase 3/ECP peptide for Pseudomonas aeruginosa biofilm eradication that combines antimicrobial, lipopolysaccharide binding, and cell-agglutinating activities. Antimicrob Agents Chemother. 2016;60:6313–25.
Ho LP, Innes JA, Greening AP. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J. 1998;12:1290–4.
American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30.
Dressel H, de la Motte D, Reichert J, Ochmann U, Petru R, Angerer P, Holz O, Nowak D, Jorres RA. Exhaled nitric oxide: independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir Med. 2008;102:962–9.
Bayarri MA, Milara J, Estornut C, Cortijo J. Nitric oxide system and bronchial epithelium: more than a barrier. Front Physiol. 2021;12: 687381.
Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–65.
Barbier M, Agusti A, Alberti S. Fluticasone propionate reduces bacterial airway epithelial invasion. Eur Respir J. 2008;32:1283–8.
Malo de Molina R, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJ, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36:751–7.
O’Byrne PM, Pedersen S, Carlsson LG, Radner F, Thoren A, Peterson S, Ernst P, Suissa S. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med. 2011;183:589–95.
Heffler E, Madeira LNG, Ferrando M, Puggioni F, Racca F, Malvezzi L, Passalacqua G, Canonica GW. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018;6:776–81.
McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case-control study. Chest. 2013;144:1788–94.
Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID. Inhaled corticosteroid therapy in adult asthma. Time for a new therapeutic dose terminology. Am J Respir Crit Care Med. 2019;199:1471–7.
Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention., May 17 2021 edition. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf; 2021.
Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(94–103): e115.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5: e8578.
Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, Sperling AI, Solway J, Naureckas ET, Gilbert JA, White SR. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016;137(1398–1405): e1393.
Henkle E, Curtis JR, Chen L, Chan B, Aksamit TR, Daley CL, Griffith DE, Winthrop KL. Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur Respir J. 2019;54:1801896.
Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, Murris M, Canton R, Torres A, Dimakou K, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50:1700629.
Durack J, Lynch SV, Nariya S, Bhakta NR, Beigelman A, Castro M, Dyer AM, Israel E, Kraft M, Martin RJ, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140:63–75.
Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, Kebadze T, Cohen S, Newbold P, Rapley L, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol. 2018;141(2027–2036): e2012.
King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411–9.
Chang AB, Upham JW, Masters IB, Redding GR, Gibson PG, Marchant JM, Grimwood K. Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr Pulmonol. 2016;51:225–42.
Takemura M, Niimi A, Minakuchi M, Matsumoto H, Ueda T, Chin K, Mishima M. Bronchial dilatation in asthma: relation to clinical and sputum indices. Chest. 2004;125:1352–8.
Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, Shteinberg M, Aliberti S, Chalmers JD. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328.
Mäntylä J, Mazur W, Törölä T, Bergman P, Saarinen T, Kauppi P. Asthma as aetiology of bronchiectasis in Finland. Respir Med. 2019;152:105–11.
Aliberti S, Goeminne PC, O’Donnell AE, Aksamit TR, Al-Jahdali H, Barker AF, Blasi F, Boersma WG, Crichton ML, De Soyza A, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10:298–306.
Acknowledgements
The authors would like to thank all the physicians who contributed to the BEXAS study despite the COVID-19 pandemic (listed below). The authors also thank Ms Miho Moriwaki and Mai Morita for their technical assistance.
List of the BEXAS Study Contributors (Alphabetical Order of Facility Names): Miho Ikeda, Kayoko Okamura, Hisashi Ohnishi (Akashi Medical Center), Junko Terada-Hirashima (Center Hospital of the National Center for Global Health and Medicine), Sumito Isogai, Kazuyoshi Imaizumi (Fujita Health University), Ryosuke Hirano, Masaki Fujita (Fukuoka University), Tomoyuki Fujisawa, Takafumi Suda (Hamamatsu University School of Medicine), Yoichi Takaki (Harasanshin Hospital), Naoko Higaki, Shintaro Miyamoto, Taku Nakashima, Hiroshi Iwamoto (Hiroshima University), Koji Mikami, Toshiyuki Minami, Ryo Takahashi, Takashi Kijima (Hyogo Medical University), Kazunori Tobino (Iizuka Hospital), Makoto Hoshino (International University of Health and Welfare Atami Hospital), Shiro Imokawa (Iwata City Hospital), Taisuke Tsuji, Noriya Hiraoka (Japanese Red Cross Kyoto Daiichi Hospital), Tatsuyoshi Ikeue, Takakazu Sugita (Japanese Red Cross Wakayama Medical Center), Naomi Kunichika (Japanese Red Cross Yamaguchi Hospital), Shinya Tomari [Japan Community Health Care Organization (JCHO) Isahaya General Hospital], Yasumi Okochi (JCHO Tokyo Yamate Medical Center), Naoko Mato, Koichi Hagiwara (Jichi Medical University), Kunio Dobashi (Jobu Hospital for Respiratory Diseases), Yasuyuki Taooka (JR Hiroshima Hospital), Kentaro Machida (Kagoshima University), Takae Tanosaki, Katsunori Masaki, Koichi Fukunaga (Keio University School of Medicine), Akiko Sano, Takashi Iwanaga, Yuji Higashimoto (Kindai University), Masataka Matsumoto, Kiyonobu Takatsuki (Kita-Harima Medical Center), Kazuma Nagata, Ryo Tachikawa, Keisuke Tomii (Kobe City Medical Center General Hospital), Masahiro Kaneko, Hiromi Tomioka (Kobe City Medical Center West Hospital), Tatsuya Nagano (Kobe University), Mayuka Yamane (Kochi University), Chieko Yoshida, Takuro Sakagami (Kumamoto University), Yurie Seto, Yoshiko Kaneko, Koichi Takayama (Kyoto Prefectural University of Medicine), Satoru Terada, Kenta Nishi (Kyoto University), Tomoko Tajiri (Nagoya City University), Saya Nakamura, Keiko Wakahara (Nagoya University), Takefumi Ito (Nara Prefecture General Medical Center), Takako Nakano, Takafumi Yamashita, Shohei Takata [National Hospital Organization (NHO) Fukuokahigashi Medical Center], Yoshihiro Seri, Yasuyuki Mizumori, Hiroaki Tsukamoto, Ryogo Kagami, Yasuharu Nakahara (NHO Himeji Medical Center), Yukio Ishii (NHO Ibarakihigashi National Hospital), Toshiyuki Kita (NHO Kanazawa Medical Center), Kouko Hidaka (NHO Kokura Medical Center), Masayoshi Minakuchi, Tomomasa Tsuboi (NHO Minami Kyoto Hospital), Shinji Tamaki (NHO Nara Medical Center), Takanori Matsuki, Hiroshi Kida (NHO Toneyama Medical Center), Katsuyuki Tomita (NHO Yonago Medical Center), Takashi Abe, Joe Shindoh (Ogaki Municipal Hospital), Akihiko Taniguchi (Okayama University), Masato Azuma (Okinawa Prefectural Nanbu Medical Center And Children’s Medical Center), Mikio Kataoka (Onomichi Municipal Hospital), Haruhiko Ogawa (Saiseikai Kanazawa Hospital), Takeshi Matsumoto, Kensaku Aihara (Saiseikai Noe Hospital), Kazuyuki Nakagome (Saitama Medical University), Satsuki Miyajima (Sapporo Medical University), Kentaro Hashimoto, Tetsuhiro Shiota (Shiga General Hospital), Masafumi Yamaguchi, Yasutaka Nakano (Shiga University of Medical Science), Kojiro Otsuka (Shinko Hospital), Masanori Yasuo, Masayuki Hanaoka (Shinshu University), Takashi Yamada (Shizuoka City Shizuoka Hospital), Toshihiro Shirai (Shizuoka General Hospital), Yoshinobu Iwasaki (Showa General Hospital), Masamichi Mineshita (St. Marianna University School of Medicine), Takahiro Tsuburai, Yuko Komase (St. Marianna University School of Medicine Yokohama Seibu Hospital), Hidefumi Koh (Tachikawa Hospital), Koichi Hasegawa, Hideo Kita (Takatsuki Red Cross Hospital), Koji Murakami, Hisatoshi Sugiura, Masakazu Ichinose (Tohoku University Graduate School of Medicine), Tomoko Kutsuzawa, Tsuyoshi Oguma, Jun Tanaka (Tokai University School of Medicine), Yuta Kono, Shinji Abe (Tokyo Medical University), Morio Nakamura (Tokyo Saiseikai Central Hospital), Mami Orimo, Etsuko Tagaya (Tokyo Women’s Medical University), Toshiaki Matsuda (Tosei General Hospital), Tomoya Harada (Tottori University), Hiroaki Iijima (Tsukuba Medical Center Hospital), Hiroki Kawabata, Kazuhiro Yatera (University of Occupational and Environmental Health, Japan), Hironori Masuko, Yuko Morishima (University of Tsukuba), Masanori Nakanishi, Nobuyuki Yamamoto (Wakayama Medical University), Sumito Inoue (Yamagata University), Kazuki Hamada, Yoshikazu Yamaji, Tsunahiko Hirano, Kazuto Matsunaga (Yamaguchi University).
Funding
This work was supported by the Scientific Assembly of Allergy, Immunology & Inflammation, Japanese Respiratory Society, and Novartis Japan.
Author information
Authors and Affiliations
Consortia
Contributions
NN contributed to the design of the work and acquisition, analysis and interpretation of data and drafted the manuscript. HM contributed to the conception and design of the work, analysis and interpretation of data and critical revision of the manuscript. Ayo, YN, KA, HN and Tho contributed to the design of the work, acquisition of data and critical revision of the manuscript. AN, YT, Nhar, MN, HI, MK, NM, Nhi, MH, Nhat, Nhas, Aya, Tkad, Tki, MM, HT, MT, Tkaw, OM and YS have substantially contributed to data acquisition and critical revision of the manuscript. HS, TN, TO and Thi contributed to the interpretation of data and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Kyoto University Medical Ethics Committee (R2168). Given the retrospective nature of the study and the use of anonymous patient data, requirements for written informed consent were waived.
Consent for publication
Not applicable.
Competing interests
HM received lecturer fees from Novartis Japan, Sanofi K.K., AstraZeneca K.K., GlaxoSmithKline, Kyorin Pharmaceutical Co. and Boehringer Ingelheim received grants from Kyorin Pharmaceutical Co., Boehringer Ingelheim and Teijin Pharma outside the submitted work as well as received support from the Japanese Respiratory Society and Research Grant from Novartis Japan. AY received lecturer fees from AstraZeneca K.K., Novartis Japan, GlaxoSmithKline, Sanofi K.K. and Boehringer Ingelheim. YN received lecturer fees from Novartis Japan, AstraZeneca K.K. and Kyorin Pharmaceutical Co. KA received lecturer fees from Novartis Japan, Sanofi K.K., AstraZeneca K.K., GlaxoSmithKline plc and Boehringer Ingelheim Japan Inc. AN received lecturer fees from AstraZeneca K.K., Kyorin Pharmaceutical Co. and Novartis and received consulting fees from MSD. YT received lecturer fees from Kyorin Pharmaceutical Co., Teijin Pharma, Novartis Japan, Sanofi and AstraZeneca, received consulting fees from GlaxoSmithKline and Kyorin Pharmaceutical Co., and received grants from Kyorin Pharmaceutical Co., Astellas, Taiho, Boehringer Ingelheim and Teijin Pharma. NHar received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K., Novartis Pharma K. K. and Sanofi K.K. and received grants from AstraZeneca K.K., Kyorin Pharmaceutical Co., Daikin (China) Investment Co., Ltd., Kao Corporation, SRL Medisearch Inc. and TOSOH Corporation. MN received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K. and Torii Pharmaceutical Co. Ltd. HI received lecturer fees and/or advisory board from Astellas, AstraZeneca, Boehringer-Ingelheim, Fukuda-Denshi, GlaxoSmithKline, Kracie, Kyorin, Novartis, Omron, Pfizer and Sanofi and received research grants and support to Kagoshima University from Asahi-Kasei Pharma, AstraZeneca, Boehringer-Ingelheim, Chugai, GlaxoSmithKline, Kyorin, Otsuka, Teijin, Taiho and Ono. NM received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K., Novartis Pharma K.K. and Nippon Boehringer Ingelheim Co., Ltd. Nhi received lecturer fees from AstraZeneca, GlaxoSmithKline and Novartis and received grants from GlaxoSmithKline and Novartis. MH received lecturer fees from AstraZeneca, GlaxoSmithKline and Novartis Pharma. Nhat received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis Pharma and Sanofi. Nhas received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis and Boehringer Ingelheim and received a research grant from Boehringer Ingelheim outside the submitted work. TK received lecturer fees from GlaxoSmithKline plc., Sanofi K.K., Nippon Boehringer Ingelheim Co., Ltd., Astra Zeneca K.K, Eli Lilly Japan K.K, Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., Meiji Seika Pharma Co., Ltd., Bristol Myers Squibb, DAIICHI SANKYO COMPANY, LIMITED and TEIJIN PHARMA LIMITED. OM received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis Pharma and Sanofi. TO received lecturer fees from AstraZeneca K.K., GlaxoSmithKline plc., Boehringer Ingelheim Japan Inc., Kyorin Pharmaceutical Co., Novartis Japan and Sanofi K.K. HS belongs to the endowed chair supported by Phillips-Respironics, ResMed, Fukuda Denshi and Fukuda Lifetec Keiji.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Transition patterns of inflammatory types in cases who were not receiving anti-type 2 biologics or regular oral corticosteroids (≥5 mg/day) and were followed up for ≥2 years (n = 51). Red flow indicates former type 2-high group (n = 15), yellow flow indicates former type 2-intermediate group (n = 21) and blue flow indicates former type 2-low inflammatory group (n = 15). Figure S2. Receiver operating characteristic (ROC) curve for current levels of exhaled nitric oxide (FeNO) that reflected the presence of A) gram-negative bacteria (GNB) and B) P. aeruginosa in the sputum. Detection rate of C) GNB and D) P. aeruginosa in the sputum according to the FeNO levels, as determined by ROC curve analysis. Figure S3. A) Modified Reiff score and B) number of lobes affected by bronchiolitis in low and high exhaled nitric oxide (FeNO) groups. Recent indices were analysed. Boxes and bars indicate upper, lower, and median quartiles. Figure S4. Patterns of cases with exacerbations requiring systemic corticosteroids (SCS) and antibiotics and bronchopneumonia, according to the transition patterns of inflammatory groups (p = 0.056 among the three groups). Red bar indicates cases with three types of episodes in the last 2 years, i.e., exacerbations requiring SCS and antibiotics, and bronchopneumonia; orange bar, exacerbations requiring SCS and antibiotics; yellow bar, exacerbations requiring SCS only: blue bar, bronchopneumonia and exacerbations requiring antibiotics; purple bar, bronchopneumonia only; green bar, exacerbations requiring antibiotics only. Complete answers were missing from two cases in the low-to-low group, four in the high’-to-low group, and eight in the high’-to-high’ group. Ratios of cases with exacerbation requiring antibiotics (p = 0.01) and bronchopneumonia (p = 0.006) were significantly different among the three groups. Figure S5. Changes in terms of A) inhaled corticosteroid (ICS) doses (equivalent to fluticasone propionate), B) modified Reiff scores, C) number of lobes affected by bronchiolitis, D) detection rates of gram-negative bacteria (GNB) in sputum and E) detection rates of P. aeruginosa in sputum over time. Blue line indicates low-to-low group, yellow line indicates high’-to-low group and pink line indicates high’-to-high’ group. The numbers of cases in the low-to-low, high’-to-low, and high’-to-high’ groups were 17/19/35 for A), 19/21/40 for B); 15/21/34 for C); 17/15/32 for D) and 17/15/32 for E). Figure S6. Transition patterns of airway lesions, i.e., bronchiectasis, bronchiolitis, and both, in cases followed for 2 years or more. A) In all cases regardless of type 2 inflammation level, purple flow indicates former bronchiectasis only (n = 17); orange flow, former bronchiolitis only (n = 36); green flow, bronchiectasis and bronchiolitis in the past (n = 63); B) current type 2-high group (n = 14), C) current type 2-intermediate group (n = 17) and D) current type 2-low group (n = 41). Figure S7. Trajectories of blood eosinophil counts of patients with ≥3 data points allocated to A) high’-to-high’, B) high’-to-low and C) low-to-low groups. Patients represented by grey and brown lines in A), who received oral corticosteroids or anti-type 2 biologics, or both, had elevated exhaled nitric oxide (>100 ppb), and they were allocated to the high’-to-high group. Table S1. Characteristics of patients who were and were not stratified. Table S2. Features related to asthma and comorbidities according to the current inflammatory types. Table S3. Most effective treatment evaluated by attending physicians. Table S4. Features related to asthma according to the transition patterns of inflammatory types.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nomura, N., Matsumoto, H., Yokoyama, A. et al. Nationwide survey of refractory asthma with bronchiectasis by inflammatory subtypes. Respir Res 23, 365 (2022). https://doi.org/10.1186/s12931-022-02289-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02289-y