Abstract
Background
There is a relationship between Chronic Obstructive Pulmonary Disease (COPD) and the development of lung cancer (LC). The aim of this study is to analyse several blood markers and compare their concentrations in patients with only COPD and LC + COPD.
Methods
Case-control study with cases presenting combined LC and COPD and two control groups (patients presenting only COPD and patients presenting only LC). We also included LC patients with descriptive purposes. In both groups, peripheral blood analyses of TNF-α, IL-6, IL-8, total leukocyte, lymphocyte and neutrophil counts, neutrophil-to-lymphocyte ratio, total platelet count, mean platelet volume, platelet-to-lymphocyte ratio, alpha 1-antitripsin (A1AT), IgE, C-reactive protein, fibrinogen, cholesterol and bilirubin were performed. We developed univariate and multivariate analyses of these markers, as well as a risk score variable, and we evaluated its performance through ROC curves.
Results
We included 280 patients, 109 cases (LC + COPD), 83 controls (COPD) and 88 LC without COPD. No differences were observed in the distribution by sex, age, BMI, smoking, occupational exposure, lung function, GOLD stage or comorbidity. Patients with LC + COPD had significantly higher levels of neutrophils [OR 1.00 (95%CI 1.00–1.00), p = 0.03] and A1AT [OR 1.02 (95%CI 1.01–1.03), p = 0.003] and lower cholesterol levels [OR 0.98 (95%CI 0.97–0.99), p = 0.009] than COPD controls. We developed a risk score variable combining neutrophils, A1AT and cholesterol, achieving a sensitivity of 80%, a negative predictive value of 90.7% and an area under the curve of 0.78 (95%CI 0.71–0.86).
Conclusions
COPD patients who also have LC have higher levels of neutrophils and A1AT and lower of cholesterol. These parameters could be potentially predicting biomarkers of LC in COPD patients.
Similar content being viewed by others
Background
Lung cancer (LC) is the leading cause of cancer death worldwide, with a 5-year survival of approximately 15%. Chronic Obstructive Pulmonary Disease (COPD) is the fourth cause of death worldwide, with a current prevalence around 10% [1,2,3]. LC mortality is explained by the fact that most diagnoses are made in advanced stages, being able to identify tumors in localized stages only in 16–22% of cases, although new diagnostic techniques and the implementation of rapid diagnostic units are increasing the proportion of patients diagnosed with localized LC [2, 4]. In these cases, survival can reach up to 55.6% at 5 years [5].
Some studies have demonstrated that COPD is a risk factor for LC development, independently of tobacco exposure. In addition, COPD and LC share some common features. Smoking is the main cause of both diseases, COPD affecting 15–20% of smokers, while 80% of LC patients are smokers or ex-smokers. Besides tobacco use, COPD and LC share some genetic backgrounds, environmental exposures, and common underlying inflammatory processes [6, 7].
Airway chronic inflammation is one of the pathophysiological mechanisms that plays a key role in the amplification of the initial mutagenic response of LC. It is possible that persistent airway inflammation in COPD patients induces alterations in the bronchial epithelium which favor carcinogenesis [8]. In patients with COPD and in smokers, the expression of certain cytokines is increased, such as IL-6 and IL-8, which, in turn, through the induction of the enzyme cyclooxygenase-2, promote an inflammatory response in lymphocytes. They can also inhibit apoptosis, interfere with cellular repair mechanisms and promote angiogenesis, contributing to neoproliferative processes [8]. Other cytokines and growth factors such as tumor necrosis factor (TNF)-α have also been shown to participate in the development, tumor growth and metastasis of LC in patients with underlying respiratory conditions [9]. In fact, various blood markers of inflammation have been evaluated separately in patients either with COPD or with LC and other malignant tumors (Additional file 1: Table S1). These markers include C-reactive protein (CRP), platelet, neutrophil, and lymphocyte numbers but especially include neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), mean platelet volume (MPV), alpha-1-antitripsin (A1AT), fibrinogen, cholesterol or bilirubin [10,11,12,13,14]. Therefore, the increased risk of developing LC in patients with COPD could be related to the existence of a previous inflammation, making them more susceptible to the carcinogenic components of tobacco. This inflammation persists even years after having stopped smoking, which may be a cause of LC in ex-smokers [8]. To our knowledge, there are no studies assessing a complete series of inflammatory blood markers in patients with COPD comparing them with LC + COPD patients. To have some predictive markers in COPD patients showing a higher possibility of LC would mean an early diagnosis and therefore improving their clinical results. We have selected 16 biomarkers in order to test the importance of persistent airway inflammation in the development of LC in COPD patients, as these markers have already shown to be high in COPD and related to disease progression, prognosis and response to treatment in LC.
The aim of this study is: 1) to assess a panel of different markers (IL-6, IL-8, TNF-α, CRP, PCR, IgE, platelet, neutrophil, and lymphocyte numbers, NLR, PLR, MPV, A1AT, fibrinogen, cholesterol and bilirubin) in three groups of patients (COPD, patients with COPD and LC [LC + COPD] and LC without COPD), focusing on the comparison between COPD and LC + COPD patients and, 2) to select those markers associated with LC + COPD and to create a score to predict the risk of presenting LC based on selected clinical parameters.
Methods
Study design and case and control selection
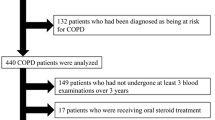
This is a case-control study in which patients with COPD, with LC + COPD and with LC only were included from September 2014 to May 2018 from the Vigo University Hospital. This hospital attends a 450,000-inhabitant area, and the pulmonary department applies practically all pulmonary techniques and procedures. Cases were COPD patients with synchronic LC (LC + COPD) diagnosed in the Lung Cancer Rapid Diagnosis Unit (LCRDU), while controls (patients with COPD and no evidence of LC) were captured in a general pulmonary consultation that was carried out on the same days as the LCRDU, including patients with recently-diagnosed COPD (less than 6 months). We included a second group of controls with LC with normal lung function to make a descriptive comparison of inflammatory marker’s levels between the three groups. The LCRDU permits a diagnostic and staging process of LC and other thoracic neoplasms. This unit assesses around 95% of all LC patients in our area.
Patients with symptoms or evidence by imaging tests of active infection, ischemic or congestive heart disease, thromboembolic disease or other underlying inflammatory processes (outbreak of connective tissue disease, inflammatory bowel disease...), as well as patients with a second synchronous tumor were excluded from the study to avoid false positives when assessing blood markers. In addition, we also excluded all patients with advanced or very symptomatic tumor disease requiring hospital admission (hepatic failure, moderate or massive hemoptysis, superior vena cava syndrome or metastatic disease requiring urgent treatment, such as palliative radiotherapy for bone or brain metastases). Therefore, all patients included in the study were managed on an outpatient basis in the LCRDU. Also, we excluded patients presenting with a microbiological isolation in any of the samples carried out during the process (sputum cultures or cultures of bronchoscopy or surgery samples). However, no cut-off points were established as exclusion criteria in the levels of the biomarkers studied, in order to avoid intervening in the results of the study.
The diagnosis of LC was made after suggestive radiological findings with pathologic confirmation [15]. COPD was defined following the Global Initiative for Obstructive Lung Disease (GOLD) recommendations as the presence of persistent respiratory symptoms and a forced expiratory volume in the first second (FEV1)/ forced vital capacity (FVC) ratio < 0.70 after a bronchodilator test [16]. We excluded patients unwilling to participate or to donate blood samples, those with contraindications or incapable of performing spirometric tests correctly, and patients with any other pulmonary obstructive disease other than COPD.
We assessed a panel of different blood markers in the three groups of patients: IL-6, IL-8, TNF-α, CRP, IgE, platelet, neutrophil, and lymphocyte numbers, NLR, PLR, MPV, A1AT, fibrinogen, cholesterol and bilirubin, and whether patients were receiving growth factors or statins. All biomarkers were tested in a stable phase, in outpatients, without any concomitant infection or inflammatory process, and without any synchronous tumor.
Information retrieval
Collected data included basic demographics: age, gender, tobacco history, functional variables (comorbidity assessed by the Charlson Comorbidity Index [17], FEV1, DLCO and body mass index (BMI). The histological type of LC was also included by reviewing the pathology report, as well as the stage at diagnosis according to the TNM eight edition’s descriptors [15] after complete staging processes. Other data necessary for COPD characterization were included, such as the GOLD and Spanish Guideline for COPD (GesEPOC) classifications valid at the study onset [16, 18], COPD assessment test (CAT) [19] and BODEx index [20].
Smokers were defined as participants who had smoked 100 or more cigarettes in their lifetime. Current smokers were those who smoked more than one cigarette in the month prior to enrollment or quit within one year of enrollment. The remaining smokers were classified as ex-smokers. Never smokers were defined as having smoked less than 100 cigarettes in their lifetime [21].
Spirometry was performed at the time of inclusion in the study by a technician specialized in respiratory functional tests. It was carried out with a Masterlab pneumatic-type spirometer (Jaeger AG, Wuezburg, Germany), using acceptability and reproducibility criteria from SEPAR and ERS [22] guidelines, with Quanjer Gli reference values ( [23]). A bronchodilator test was performed in all cases, by administrating 400 μg of salbutamol in 4 puffs (100 μg per puff) at 30 s intervals.
Emphysema was determined through computed tomography (CT) assessment by experimented radiologists. The CT studies were performed in two devices: Lightspeed VCT of 64 rows of detectors (GE Medical Systems, Milwaukee, Wisconsin) and Somatom Emotion of 16 rows of detectors (Siemens Medical Solutions, Enlargen, Germany).
Peripheral venous blood was collected from all patients into Vacutainer tubes in the morning. Serum was obtained by centrifugation of whole blood at 3000 g for 10 min. Plasma (CITRATE as anticoagulant) was obtained by centrifugation at 3500 g for 15 min at a temperature of 4 °C. Serum samples used to measure IL-6, IL-8 and TNF-α levels were stored at − 80 °C until they were analyzed. IL-6, IL-8 and TNF-α serum concentrations were determined by validated immunoassays (IMMULITE ONE, Siemens, Germany), full blood counts were carried out using ADVIA 2120 (Siemens, Germany); serum CRP, cholesterol and bilirubin were measured using ADVIA 2400 (Siemens, Germany); A1AT was analyzed by nephelometric assay (IMMAGE, Beckman Coulter, USA); IgE levels were measured by fluorometric immunoassay (PHADIA 250, Thermo Scientific, USA) and fibrinogen was calculated in ACL TOP 700 instrument (Werfen Company, Spain) Limits of detection (LOD) for IL-6, IL-8 and TNF-α were 2 pg/ml, 5 pg/ml and 4 pg/ml. Biomarker concentrations were below the LOD in some individuals. To avoid a downward bias of the population data, a nominal level of half of the LOD value was used in the analysis in individuals with values below the LOD [24].
Statistical analysis
The design and statistics of the study were reviewed by a professor of epidemiology who is a co-author of the manuscript, and who has an extensive experience in case-control studies. We first carried out a descriptive analysis of levels of all markers in the three groups of patients through the use of boxplots. Then we developed a univariate analysis to evaluate differences between cases (patients with LC + COPD) and controls (COPD) for all assessed variables. The t-student test was used for quantitative variables and Chi2 test was used to compare percentages for qualitative variables. Our limit of significance was p < 0.05. We included variables with a p < 0.10 in the multivariate models (performed through a forward conditional method), developing interaction analyses for all of them. For the final significant variables, we performed two multivariate logistic regression models, the first adjusting for age and sex and the second also including the remaining variables. To do so, the significant quantitative variables were stratified into terciles for inclusion in the logistic regression models. Application of this multivariate analyses led to the design of a risk score variable for each given patient based on the results of the multivariate logistic regression. Points for a given patient were obtained by summing all the points for each predictor variable, adjusted for sex and age. Then we developed a ROC curve and assessed sensitivity, specificity and predictive values for the risk score variable, taking into account a prevalence of LC in patients with COPD of 25% [7]. The analysis was performed with SPSS 21.0 (IBM Corporation, Armonk, New York).
Results
We included 280 patients: 109 cases (LC + COPD), 83 controls (COPD) and 88 LC patients. A descriptive and univariate analysis comparing baseline characteristics and marker levels of cases and controls is included in Table 1. As shown in the table, baseline characteristics of both groups were very homogeneous, with no relevant differences between groups, also in terms of baseline treatments. There were no patients undertaking any growth factor. One case and two controls had A1AT < 90 mg/dl. Five patients had cachexia, four were cases (one in the group with high cholesterol levels and three with medium cholesterol levels) and one was a control. Baseline characteristics of LC patients without COPD are included in Table 2.
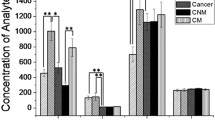
The most frequent histological type was adenocarcinoma, in 84 cases (44.9%), followed by squamous (26.7%), undifferentiated (18.2%), small-cell LC (SCLC) (7%) and carcinoid (3.2%). Regarding tumour characteristics, when comparing LC + COPD with LC patients, we found that patients with LC without COPD had more adenocarcinomas (54.5% vs 38.4%; p = 0.02), whereas patients with LC + COPD had more SCLC (17.8% vs 4.9%; p = 0.006). There were no differences in stage at diagnosis. Figure 1 shows descriptive boxplots for all inflammatory markers in the three groups of patients.
We developed a multivariate logistic regression model comparing cases and controls (Table 3). High neutrophil and A1AT levels and low cholesterol levels were the only significant variables in the multivariate analysis. Therefore, those were the variables chosen for their stratification in terciles and their inclusion in the score. It can be observed that three variables are associated significantly with the probability of being a case: patients with LC + COPD had significantly higher levels of neutrophils [OR 4.90 (95%CI 1.60–14.94 for those in the highest tercile of neutrophils, p = 0.005] and A1AT [OR 3.6 (95%CI 1.23–10.53), for those in the highest tercile of A1AT, p = 0.019] and lower cholesterol levels [OR 2.91 (95%CI 1.08–7.85), for those in the lowest tercile of cholesterol, p = 0.03] than COPD controls. The point scoring system shown in Table 4 was used to measure the magnitude of the association of each of the significant factors in the multivariate analysis with the odds of being a case, thus leading to the development of a risk score. Performance and ROC curves of this risk score are presented on Fig. 2 and Table 5. Based on our model and assuming a prevalence of 25% among COPD patients, we reached a sensitivity of 80%, with an optimal negative predictive value (NPV) of 90.7% [7].
We repeated the univariate and multivariate analyses excluding patients with advanced LC stage, to minimize the effect of higher inflammation levels in this kind of tumours. We found that in local LC + COPD, A1AT was significantly higher than in COPD patients: OR 1.02 (1.00–1.03); p = 0.03, with an AUC of 66.4 (Table 6).
Discussion
Our results suggest that a panel of 3 biomarkers out of a panel of 16, which are easy to assess, might be able to detect LC in patients presenting COPD. As we have previously stated, evidence suggests that COPD is a risk factor for developing LC [7], and one of the underlying mechanisms described is inflammation. Chronic inflammation has long been associated with carcinogenesis, contributing to 25% of all human cancers [8]. We have observed that neutrophils, A1AT and cholesterol are associated with the risk of LC in COPD patients, and if they are used combined they might predict LC risk with an AUC close to 80%. If confirmed in other studies, these results could be relevant since LC is frequent among COPD patients and their use might detect the disease in earlier stages predicting a better clinical outcome.
Evidence suggests that COPD is a risk factor for developing LC [7], and one of the underlying mechanisms described is inflammation. Chronic inflammation has long been associated with carcinogenesis, contributing to 25% of all human cancers [8] and systemic inflammation has also been shown to be a relevant manifestation of COPD [25].
As exposed in Additional file 1: Table S1, several markers have shown associations with both COPD and LC. Leukocytes, TNF-α, IL-6, IL-8, cholesterol, bilirubin and fibrinogen levels increase mortality in COPD patients, whereas white blood and platelet markers are associated with a risk of COPD exacerbations [10, 11, 26,27,28]. Also, elevated IgE levels can be found in COPD patients [29]. In addition, TNF-α, IL-6, IL-8, NLR, PLR, IgE have been associated with LC risk in healthy subjects, being IL-6, lymphocytes, neutrophils, NLR, platelets, PLR, fibrinogen, A1AT, CRP and bilirubin poor prognostic factors in LC patients [11, 14, 30,31,32]. According to our results (Fig. 1), we found differences in marker levels in patients with LC + COPD, and even in LC patients without COPD, such as IL- 6, leukocytes, PLR, fibrinogen, neutrophils, NLR, platelets, A1AT and CRP. This may indicate that these markers seem to be more related to the existence of LC than to COPD itself.
In the multivariate analysis we found that some markers were statistically associated with LC onset: higher levels of neutrophils and A1AT and lower cholesterol levels.
Lymphocytes play a crucial role in the cell-mediated host immune response to tumors. Infiltration of tumors by lymphocytes correlates with better prognosis in some cancers, although disease progression is associated with high leukocyte and neutrophil count [11]. Neutrophils support angiogenesis by secreting proangiogenic factors or proteolytic activation of such factors. Also, they ensure the collection of epidermal growth factor (EGFR), transforming growth factor-β1 (TGF- β1), platelet-derived growth factors that contribute to tumorigenesis. Neutrophils contain both pro- and anti-tumor subpopulations [11]. Neutrophil counts are known to be an independent indicator of poor prognosis in LC patients, whereas low neutrophil counts are associated with longer survival [33].
Most of the literature available on the relationship between A1AT and COPD or LC focuses on the deficit of this protein [34]. However, its role as an inflammatory marker when it presents high levels has been less studied. Possible carcinogenic mechanisms have been suggested from the excess activity of neutrophil elastase [34], which induces tissue damage at the pulmonary level due to a protease-antiprotease imbalance. More studies are needed to establish if there is an association between the A1AT and LC risk. Nevertheless, there is evidence that A1AT promotes lung adenocarcinoma metastasis [13].
Although hyperlipidemia is a negative prognostic factor in patients with stomach and prostate cancers, very few studies have explored the significance of this in LC. In one trial, HDL, LDL and total cholesterol levels were lower in LC patients when compared with healthy controls, although only HDL levels were prognostically significant [11]. The observed results of cholesterol levels in this study has not shown to be related to statin consumption or to the presence of cachexia.
In this study, we provide a risk score for COPD patients with higher risk of CP, achieving high sensitivity and NPV. In fact, the area under curve is close to 80%, and therefore only 20% of patients using this score would be misclassified. Our approach involves the measurement of A1AT, neutrophils and cholesterol to generate a classification score for each individual to predict LC. Although we did reach high sensitivity and NPV, specificity and positive predictive value were modest. This was expected since the alteration of any of the selected markers is not specific for LC, given that they are markers that show high heterogeneity among patients, that they can be modified by the different comorbidities, and that there is an important variability that it is shown in the size of some of the confidence intervals [35].
We repeated the analyses in patients with COPD and localized LC, in order to minimize biases due to higher inflammation levels in patients with advanced LC, given that patients with local LC would be the objective in the case of an eventual LC screening. In this case, the only parameter which was significantly higher in patients with LC + COPD was A1AT, although the number of patients in the group of cases was considerably reduced (37 patients), which limits the conclusions that can be drawn from this subgroup of patients. It is therefore pending if this panel results are maintained when using LC patients at an early stage presenting COPD.
The use of risk prediction models may inform selection of subjects most likely to benefit from computed tomography screening; and risk markers such as A1AT, neutrophils or cholesterol may provide useful risk information in addition to questionnaire information on tobacco exposure history. Inflammation markers are unlikely to provide enough added risk information on their own, but in combination with other risk markers they may be useful for risk stratification.
Regarding LC characteristics, the most frequent histological type was adenocarcinoma, which goes in line with other studies [5]. SCLC was significantly higher in LC + COPD patients and there was a trend, although not significant for squamous LC in this group of patients. This study found that smoking had a significantly higher effect on the SCLC risk of COPD subjects, compared with non-COPD subjects [36]. Also, COPD status was independently associated with SCLC risk when adjusted for age, gender, and smoking. Squamous LC was also more frequent in smokers and has been associated with the presence of emphysema [37].
Our study shows several limitations, inherent to its case-control design. The number of controls (COPD) is slightly lower than the number of cases (LC + COPD), although we have a second control group of patients with LC without COPD. Furthermore, the score created should be classified as exploratory, though it has relatively high discrimination power. It has to be validated against other cohorts of patients from other settings. On the positive side, study groups are very similar regarding gender and age distribution. In addition, there are limitations derived from the nature of the markers that, as previously discussed, are not very specific and may present a great inter and intraindividual variability.
Our study also presents a series of advantages. This is the first work analyzing a panel of 16 blood markers in a subgroup of patients with underlying COPD, with adjustments by stage, histological type, emphysema and smoking. Also, we added a second control group of patients with LC, to show that some markers are more related to the existence of LC than to COPD itself. Most patients presented with a mild COPD. This is useful, as they represent the group most likely to benefit from more aggressive approaches of a malignancy. On the other hand, a very complete collection of variables was made, and the sample size is very acceptable. The whole Galician population has public health coverage, meaning there was no selection bias for our sample as we recruited more than 95% of all LC cases diagnosed in the referral area during the study period. We must consider that, despite a reasonable but modest sample size, we have included in the logistic regression model five variables of which three remained statistically significant, regardless of sex and age, which is why they are good predictors of the risk of being a patient with LC and COPD, which makes our results relevant. In addition, our risk score comprises only 3 parameters which may be analyzed routinely. This makes our risk score simple and affordable, and it may prove useful at guiding decision-making in clinical practice, such as whether to implement a LC screening system for at-risk patients, or even modify the probability of malignancy scales when evaluating a pulmonary nodule.
Conclusions
Patients with COPD who also suffer from LC have higher levels of A1AT and neutrophils and lower cholesterol. These markers seem to be more related to the presence of LC than to COPD itself, since they are increased in patients with LC without COPD. In patients with LC + COPD at localized stage, A1AT is significantly higher. The combination of A1AT, neutrophils and cholesterol in the risk score variable presents a high sensitivity and NPV, so it can be a useful tool when identifying patients with LC + COPD. However, although sensitive, these markers are not specific of LC, and more studies are needed to inform selection of COPD subjects most likely to benefit from computed tomography screening or selection of nodules at higher risk of being malignant, as risk markers such as A1AT, neutrophils and cholesterol may provide useful risk information in addition to clinical questionnaires.
Abbreviations
- A1AT:
-
Alpha-1 antitripsin
- AUC:
-
Area under the curve
- CAT:
-
COPD assessment test
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C Reactive protein
- CT:
-
Computed tomography
- DLCO:
-
Carbon monoxide diffusion capacity
- FEV1:
-
Forced expiratory volume in the first second
- FVC:
-
Forced vital capacity
- GesEPOC:
-
Guía Española de la EPOC
- GOLD:
-
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- LC:
-
Lung cancer
- LCRDU:
-
Lung cancer rapid diagnosis unit
- LOD:
-
Limit of detection
- MPV:
-
Mean platelet volume
- NLR:
-
Neutrophil/lymphocyte ratio
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PLR:
-
Platelet/lymphocyte ratio
- SCLC:
-
Small cell lung cancer
- TGF-β1:
-
Beta-one transforming growth factor
- TNF:
-
Tumor necrosis factor
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23–34.
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish society of pulmonology and thoracic surgery. Arch Bronconeumol. 2012;48:247–57.
Cancer of the Lung and Bronchus, Cancer Stat Facts. Available from: https://seer.cancer.gov/statfacts/html/lungb.html. [Cited 2018 Oct 10].
Leiro-Fernández V, Mouronte-Roibás C, Ramos-Hernández C, Botana-Rial M, González-Piñeiro A, García-Rodríguez E, et al. Changes in clinical presentation and staging of lung cancer over two decades. Arch Bronconeumol. 2014;50:417–21.
Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90:121–7.
Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–6.
Álvarez FV, Trueba IM, Sanchis JB, López-Rodó LM, Rodríguez Suárez PM, de Cos Escuín JS, et al. Recommendations of the Spanish Society of Pneumology and Thoracic Surgery on the diagnosis and treatment of non-small-cell lung cancer. Arch Bronconeumol. 2016;52:2–62.
Carpagnano GE, Spanevello A, Curci C, Salerno F, Palladino GP, Resta O, et al. IL-2, TNF-alpha, and leptin: local versus systemic concentrations in NSCLC patients. Oncol Res. 2007;16:375–81.
Agustí A, Edwards LD, Red SI, MacNee W, Tal-Singer R, Miller BE, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483.
Şahin F, Aslan AF. Relationship between inflammatory and biological markers and lung Cancer. J Clin Med. 2018;7.
Kim KH, Park TY, Lee JY, Lee S-M, Yim J-J, Yoo C-G, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung Cancer. J Korean Med Sci. 2014;29:507–11.
Li Y, Miao L, Yu M, Shi M, Wang Y, Yang J, et al. α1-antitrypsin promotes lung adenocarcinoma metastasis through upregulating fibronectin expression. Int J Oncol. 2017;50:1955–64.
Song Y-J, Gao X-H, Hong Y-Q, Wang L-X. Direct bilirubin levels are prognostic in non-small cell lung cancer. Oncotarget. 2017;9:892–900.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung Cancer stage classification. Chest. 2017;151:193–203.
Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53:324–35.
Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline LN. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–54.
Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, Tordera MP, Sánchez PR. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009;103:692–9.
Henschke CI, Yip R, Boffetta P, Markowitz S, Miller A, Hanaoka T, et al. CT screening for lung cancer: importance of emphysema for never smokers and smokers. Lung Cancer. 2015;88:42–7.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43.
Muir K, Gomeni R. Non-compartmental analysis. In: Bonate PL, Howard DR, editors. Pharmacokinetics in drug development: clinical study design and Analyisis. Arlington, VA: AAPS Press; 2004. p. 235–66.
Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, et al. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014;69:666–72.
Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, et al. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691–7.
Moberg M, Vestbo J, Martinez G, Lange P, Ringbaek T. Prognostic value of C-reactive protein, leukocytes, and vitamin d in severe chronic obstructive pulmonary disease. ScientificWorldJournal. 2014;140736.
Tanrıverdi H, Örnek T, Erboy F, Altınsoy B, Uygur F, Atalay F, et al. Comparison of diagnostic values of procalcitonin, C-reactive protein and blood neutrophil/lymphocyte ratio levels in predicting bacterial infection in hospitalized patients with acute exacerbations of COPD. Wien Klin Wochenschr. 2015;127:756–63.
Jin J, Liu X, Sun Y. The prevalence of increased serum IgE and Aspergillus sensitization in patients with COPD and their association with symptoms and lung function. Respir Res. 2014;15:130.
Brenner DR, Fanidi A, Grankvist K, Muller DC, Brennan P, Manjer J, et al. Inflammatory cytokines and lung Cancer risk in 3 prospective studies. Am J Epidemiol. 2017;185:86–95.
Helby J, Bojesen SE, Nielsen SF, Nordestgaard BG. IgE and risk of cancer in 37 747 individuals from the general population. Ann Oncol Off J Eur Soc Med Oncol. 2015;26:1784–90.
Agassandian M, Shurin GV, Ma Y, Shurin MR. C-reactive protein and lung diseases. Int J Biochem Cell Biol. 2014;53:77–88.
Czyżykowski R, Nowak D, Janiak A, Włodarczyk A, Sarniak A, Krakowska M, et al. A retrospective evaluation of associations between chronic obstructive pulmonary disease, smoking, and efficacy of chemotherapy and selected laboratory parameters in patients with advanced non-small cell lung cancer. Contemp Oncol Poznan Pol. 2016;20:407–13.
Köhnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121:3–9.
Agusti A, Sin DD. Biomarkers in COPD. Clin Chest Med. 2014;35:131–41.
Huang R, Wei Y, Hung RJ, Liu G, Su L, Zhang R, et al. Associated links among smoking, chronic obstructive pulmonary disease, and small cell lung Cancer: a pooled analysis in the international lung Cancer consortium. EBioMedicine. 2015;2:1677–85.
Smith BM, Schwartzman K, Kovacina B, Taylor J, Kasymjanova G, Brandao G, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer. 2012;76:61–6.
Barreiro E, Fermoselle C, Mateu-Jimenez M, Sánchez-Font A, Pijuan L, Gea J, et al. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and COPD. Free Radic Biol Med. 2013;65:859–71.
Balla MMS, Desai S, Purwar P, Kumar A, Bhandarkar P, Shejul YK, et al. Differential diagnosis of lung cancer, its metastasis and chronic obstructive pulmonary disease based on serum Vegf, Il-8 and MMP-9. Sci Rep. 2016;04(6):36065.
Stockley JA, Walton GM, Lord JM, Sapey E. Aberrant neutrophil functions in stable chronic obstructive pulmonary disease: the neutrophil as an immunotherapeutic target. Int Immunopharmacol. 2013;17:1211–7.
Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–78.
Agapakis DI, Massa EV, Hantzis I, Maraslis S, Alexiou E, Imprialos KP, et al. The role of mean platelet volume in chronic obstructive pulmonary disease exacerbation. Respir Care. 2016;61:44–9.
Budweiser S, Harlacher M, Pfeifer M, Jörres RA. Co-morbidities and hyperinflation are independent risk factors of all-cause mortality in very severe COPD. COPD. 2014;11:388–400.
Acknowledgements
The authors specially thank Sara Fernández García, Marcos González Fariña, Abel Pallarés Sanmartín, Ana Priegue Carrera, Cristina Represas Represas, Ramón Tubío Pérez and Carlos Vilariño-Pombo for actively recruiting patients, and the radiologists: María Ángel Álvarez Moure, Adriana Carolina Caldera Díaz, Elena Chábarri Ibáñez, Míriam García Vázquez-Noguerol, Paula Rodríguez Fernández and Amara Tilve Gómez for CT assessing. This study is part of the work aimed at the completion of the Ph Degree of Cecilia Mouronte-Roibás, in the Public Health and Epidemiology Department of the Santiago de Compostela University.
Availability of data and material
The materials described in the manuscript, including all relevant data, are freely available to any scientist wishing to use them for non-commercial purposes.
Funding
This work was supported by the project 110/2016 of the Spanish Society of Respiratory Pathology (SEPAR).
Author information
Authors and Affiliations
Contributions
CMR recruited patients, helped in the study design, data collection and analysis, wrote the initial manuscript and took part in the manuscript revision and submission. VLF took part in the study design and in the manuscript revision and submission. ARR helped in the manuscript revision. CRH helped at data collection and analysis. PCR analyzed all inflammatory markers in the laboratory, MBR took part in the data analysis, EGR helped to recruit patients and to obtain blood samples and AFV helped in the study design, in the data analysis and in the revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patient data and blood samples were obtained in full compliance with the clinical and ethical practices of the Spanish Government and the Helsinki Declaration. The study was approved by the Ethics Committee for Clinical Research of Galicia (expedient 2013/439). Informed consent was obtained from all individual participants included in the study. Variables were included in an anonymized database to maintain the principles of confidentiality and data protection.
Consent for publication
This manuscript contains no personal data in any form.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mouronte-Roibás, C., Leiro-Fernández, V., Ruano-Raviña, A. et al. Predictive value of a series of inflammatory markers in COPD for lung cancer diagnosis: a case-control study. Respir Res 20, 198 (2019). https://doi.org/10.1186/s12931-019-1155-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-019-1155-2