Abstract
Background
In moderate acute respiratory distress syndrome (ARDS) several studies support the usage of assisted spontaneous breathing modes. Only limited data, however, focus on the application in systemic sepsis and developing lung injury. The present study examines the effects of immediate initiation of pressure support ventilation (PSV) in a model of sepsis-induced ARDS.
Methods
18 anesthetized pigs received a two-staged continuous lipopolysaccharide infusion to induce lung injury. The animals were randomly assigned to PSV or volume controlled (VCV) lung protective ventilation (tidal volume each 6 ml kg-1, n = 2x9) over six hours. Gas exchange parameters, hemodynamics, systemic inflammation, and ventilation distribution by multiple inert gas elimination and electrical impedance tomography were assessed. The post mortem analysis included histopathological scoring, wet to dry ratio, and alveolar protein content.
Results
Within six hours both groups developed a mild to moderate ARDS with comparable systemic inflammatory response and without signs of improving gas exchange parameters during PSV. The PSV group showed signs of more homogenous ventilation distribution by electrical impedance tomography, but only slightly less hyperinflated lung compartments by multiple inert gas elimination. Post mortem and histopathological assessment yielded no significant intergroup differences.
Conclusions
In a porcine model of sepsis-induced mild ARDS immediate PSV was not superior to VCV. This contrasts with several experimental studies from non-septic mild to moderate ARDS. The present study therefore assumes that not only severity, but also etiology of lung injury considerably influences the response to early initiation of PSV.
Similar content being viewed by others
Background
In the course of non-pulmonary sepsis respiratory failure is a common cause and occurs in about 50% of the patients with severe sepsis [1]. Patients suffering from sepsis often require mechanical ventilation, even if they do not fulfill the criteria of an acute respiratory distress syndrome (ARDS). On the other hand, mechanical ventilation itself can represent the second hit leading to the development of ARDS. Independent from the underlying illness, lung protective strategies that aim to minimize ongoing pulmonary damage by targeting low tidal volumes and limitation of the inspiratory pressure are regarded as the key interventions when the criteria of ARDS are met [2],[3]. In severe ARDS there is evidence that short-term neuromuscular blockade enables the consequent realization of low tidal volume ventilation and increases survival rates [4]. In mild to moderate or post-acute ARDS, however, the admittance of spontaneous breathing is reported to improve gas exchange, reduce diaphragmatic dysfunction and enable a faster weaning [5],[6]. Several experimental models report beneficial effects of spontaneous breathing in various patterns on gas exchange, edema formation, and lung injury [7]-[9]. These findings, though, have not been verified in primary sepsis-related lung injury. Furthermore, some clinical and experimental data also suggest the value of preventive initiation of lung protective ventilation [10]. But currently the appropriate guidelines do not state on the preemptive application of lung protective ventilation or spontaneous breathing in early sepsis-induced lung injury [11].
We hypothesized that in early sepsis immediate application of pressure support ventilation (PSV) targeted to a tidal volume (Vt) of 6 ml kg-1 will improve the pulmonary function in comparison to conventional volume controlled ventilation (VCV, Vt 6 ml kg-1). Hence, we compared the early effects of PSV and VCV on gas exchange, ventilation/perfusion distribution and histopathological lung injury in a porcine model of systemic, lipopolysaccharide (LPS)-induced sepsis subsequently leading to lung injury.
Methods
The study was approved by the State and Institutional Animal Care Committee (Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany; approval number: G10-1-004). 18 juvenile pigs (Sus scrofa domestica, weight 27 ± 2 kg) were examined in a prospective-randomized setting.
Anesthesia and instrumentation
The animals were sedated by an intramuscular injection of ketamine (8 mg kg.1) and midazolam (0.2 mg kg-1). Anesthesia was induced by intravenous application of propofol (4 mg kg-1) and fentanyl (4 μg kg-1). A single shot of atracurium (0.5 mg kg-1) was added to facilitate endotracheal intubation (internal diameter 7.5 mm tube). General anesthesia was maintained by infusion of ketamine (10-20 mg kg-1 h-1) and midazolam (0.5-2 mg kg-1 h-1). Volume controlled ventilation (VCV; AVEA, CareFusion, USA) was used during the preparation period: Vt 6 ml kg-1, positive end-expiratory pressure (PEEP) 7 cmH2O, fraction of inspired oxygen (FiO2) 0.35, variable respiratory rate to guarantee an endtidal CO2 (etCO2) < 8 kPa, and ph >7.2. Vascular catheters were placed ultrasound-guided in Seldinger’s technique by femoral access: a central venous line, a pulmonary arterial catheter and a PiCCO®-System (Pulsion Medical Systems, Germany). Spirometry and hemodynamics were permanently stored (Datex S/5, GE Healthcare, Germany). The esophageal pressure was measured with an esophageal balloon catheter. Body temperature was measured by a rectal probe, while a surface-warming device maintained normothermia.
Experimental protocol
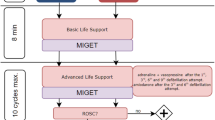
Figure 1 summarizes the experimental protocol. Septic inflammatory response was induced by continuous LPS infusion (E. coli Serotype O111:B4, Sigma-Aldrich, Switzerland). The infusion scheme includes a high-dose induction (100 μg kg-1 h-1) over one hour and a maintenance dosage (10 μg kg-1 h-1) for the entire experiment. Following anesthesia and preparation, but before sepsis induction a non-participant randomized the animals by drawing one of 18 envelopes containing the respective ventilation mode:
PSV-Mode (n = 9): pressure support 15 ± 5 cmH2O, Vt 6 ml kg-1, PEEP 5 cmH2O, FiO2 0.35, trigger = 1.5 l min-1 targeted to an etCO2 < 8 kPa and ph > 7.2
VCV-Mode (n = 9): Vt 6 ml kg-1, PEEP 5 cmH2O, FiO2 0.35, variable respiratory rate targeted to an etCO2 < 8 kPa and ph > 7.2
The VCV group received repeated injections of atracurium (0.5 mg kg-1) to avoid the onset of spontaneous breathing under close monitoring of depth of anesthesia. Measurements were performed at baseline, three and six hours after sepsis induction. To prevent severe hypoxemia or hypercapnia during LPS infusion and developing lung injury that would lead to implausible results, we established an intervention scheme instead of using a fixed setting, which was oriented on the ARDS Network PEEP/FiO2 tables: if the peripheral oxygen saturation dropped under 92% for five minutes, the ventilation parameters were adapted. During the experiment a balanced saline solution (5 ml kg-1 h-1; Sterofundin, B. Braun Germany) was applied continuously. In case of hemodynamic instability (mean arterial pressure < 60 mmHg) the animals received a hydroxylethyl starch infusion (90 ml h-1; Volulyte 6%, Fresenius Kabi, Germany) and an additional bolus once per hour. Persisting instability was treated by continuous noradrenaline infusion. The animals were monitored over six hours following sepsis induction. At the end of the experiments the animals were killed in deep general anesthesia by intravenous injection of propofol (200 mg) and exsanguination.
Electrical impedance tomography and multiple inert gas elimination technique
A 16-electrode electrical impedance tomography device (EIT; Goe-MF II, CareFusion, Germany) recorded relative bioimpedance changes related to pulmonary aeration. To analyze the regional ventilation distribution, the percentage of the respiratory-dependent relative impedance changes was attributed to three regions of interest (non-dependent, central, dependent). Setup, data acquisition and processing were previously described in detail [12],[13]. The ventilation/perfusion (VA/Q) distribution was assessed by means of micropore membrane inlet mass spectrometry - multiple inert gas elimination technique (MMIMS-MIGET, Oscillogy LLC, USA) [14],[15].
Post mortem and histopathological analysis
The lungs were extracted en bloc under continuous positive airway pressure. The upper left lobe was used for bronchoalveolar lavage to determine the alveolar protein content. The lower left lobe was weighted and dried to measure the wet to dry ratio. The right lung was used to quantify the histopathological damage oriented on established scoring systems [16]. The assessment was performed in investigator-blinded manner under supervision of an experienced pathologist. Representative samples of different regions (non-dependent, central, dependent) were extracted and fixed in formalin for paraffin sectioning and hematoxylin/eosin staining. The evaluation included seven different parameters: overdistension, epithelial destruction, inflammatory infiltration, alveolar edema, hemorrhage, interstitial edema, and microatelectasis. Per region each parameter received a severity grade from zero to five points in four non-overlapping fields of view. In a second step the extent of each parameter was assessed in a global overview of the entire region. This results in a summarized score of 175 maximum points per region. Plasma levels of inflammatory cytokines (IL-6, TNF-α) were determined by means of enzyme-linked immunosorbent assays (Porcine IL-6 Quantikine ELISA; Porcine TNF-alpha Quantikine ELISA, R&D System, Germany). Lactate, thrombocytes and leukocytes were analyzed by the Institute of Laboratory Medicine, University Medical Centre Mainz.
Statistical analysis
Data are reported as mean and standard deviation (SD) or box-plots. Baseline values were compared by t-test or Mann-Whitney-U-Test depending on presence of Gaussian distribution. The effects of group (PSV vs. VCV) and group over time were assessed by two-way analysis of variance (ANOVA) and post-hoc Holm-Sidak-Test. Post mortem data were examined by Mann-Whitney-U-Test and adjusted for multiple comparisons by the Bonferroni method. P values < 0.05 are regarded as significantly different. The statistical software SigmaPlot 12.5 (Systat Software, Germany) was used.
Results
The study protocol was completed in all 18 animals. Table 1 summarizes the ventilatory and hemodynamic data and shows no intergroup differences at baseline.
Gas exchange and respiratory variables
After sepsis induction both groups developed a ratio of the arterial partial pressure of oxygen (PaO2) and FiO2 lower than 300 mmHg (Figure 2). Despite higher PaO2/FiO2 during PSV the values did not reach significance. Tolerable hypercapnia occurred in both groups, which was more pronounced during PSV (p = 0.02) and caused by lower breathing frequencies (p = 0.04). Ventilatory pressures and FiO2 had to be raised over time and respiratory mechanics worsened accordingly without group-related differences.
Regional ventilation distribution and VA/Q ratios
Regional ventilation distribution and VA/Q were assessed by EIT respectively MMIMS-MIGET at baseline, three and six hours. Starting during baseline the EIT data (Figure 3) show a more homogenous distribution of tidal ventilation between the central and non-dependent lung compartments in the PSV group. The regional ventilation in the central compartment is significantly higher in the VCV-group and correspondingly lower in the dependent area. The VA/Q analysis approves healthy baseline conditions. During sepsis increasing shunt and hypoventilated lung areas (low VA/Q) represent the main mode of gas exchange impairment (Figure 4). In the early course (hours 1-3) the VCV group tends to develop higher amounts of shunt and low VA/Q ratios. After six hours measureable, but still non-significant high VA/Q ratios indicating hyperinflation develop in the VCV group.
Regional distribution of the tidal volume [% of the global tidal amplitude] measured by electrical impedance tomography. *indicates p < 0.05 during Baseline. Group effects over time are analyzed by two-way-ANOVA and post-hoc Holm-Sidak procedure. PSV: pressure support ventilation, VCV: volume-controlled ventilation, L1-L3: Level 1-3.
Ventilation/Perfusion Distribution (V A /Q) measured by MMIMS-MIGET. VA/Q ratios were defined as follows: shunt (VA/Q < 0.005), low VA/Q (0.005 < VA/Q < 0.1), normal VA/Q (0.1 < VA/Q < 10), high VA/Q (10 < VA/Q > 100). Group effects over time are analyzed by two-way-ANOVA and post-hoc Holm-Sidak procedure. n.s. = non-significant, PSV: pressure support ventilation, VCV: volume-controlled ventilation.
Hemodynamics and systemic inflammation
Hypotension, decreased cardiac output, and increased pulmonary arterial pressure required noradrenaline infusion to maintain stable conditions without differences between PSV and VCV. Additionally, both groups received hydroxylethyl starch administration of 389 ± 261 ml (PSV) and 458 ± 428 ml (VCV; p = 0.86). Due to high variances the two groups differ in leucocyte levels during baseline. In both groups the systemic LPS exposition resulted in increasing lactate levels and leucopenia. Peak cytokine levels developed within three hours following the initial high-dose LPS administration and persisted at increased levels over six hours (Figure 5).
Post mortem analysis
The histopathological analysis approves the presence of sustained lung injury in both groups without intergroup differences or regional variances (Figure 6). In the pooled data from both groups, however, the extent of lung injury is greater in the dependent lung areas (p = 0.048 vs. non-dependent region). The alveolar protein content and pulmonary wet/dry ratio did not differ between the two groups (Figure 6; p = 0.98 respectively p = 0.67).
Discussion
The present study features the following main findings: in a porcine model of exclusively sepsis-related lung injury the immediate initiation of low Vt-PSV was feasible, but not superior to low Vt-VCV in terms of gas exchange, respiratory pattern, hemodynamic stability, and did not improve histopathological parameters over six hours.
Sepsis is one of the most frequent risk constellations of ARDS. Systemic LPS exposition triggers inflammation by releasing pro-inflammatory cytokines and leucocyte accumulation. The early response in experimental models is characterized by acute leucopenia and immense cytokine levels. Hemodynamic findings include decreased systemic blood pressure and pulmonary arterial hypertension [17],[18]. Our model adequately reproduces these common early findings. Following central venous LPS infusion the lungs are the first microcirculatory bed to pass. Nevertheless, short-term LPS exposition hardly leads to immediate or persisting ARDS [19],[20]. On the other hand, occurrence of severe hemodynamic failure or septic shock conditions limits the systemic LPS application in experimental models. We therefore chose a two-staged infusion regime that caused significant gas exchange deterioration. The reduced maintenance dosage of LPS may also account for a clinically relevant reduction of bacteremia due to therapy. In contrast, primary pulmonary models like bronchoalveolar lavage or acid aspiration rapidly generate atelectasis, reduced lung compliance and gas exchange impairment directly from the beginning on.
The admittance of assisted spontaneous breathing activity can improve the pulmonary function in comparison to conventional lung protective ventilation [6]. Beneficial effects of PSV from several experimental studies amongst others include improved pulmonary blood flow redistribution and overall gas exchange, as well as attenuation of lung injury and IL-6 levels [8],[21]. Sophisticated variable pressure support ventilation has shown the potential to further increase these effects in several experimental studies [7],[8],[22] and is currently tested for clinical application [23],[24]. The early use of PSV can decrease sedation requirements, improve the cardiopulmonary function and VA/Q matching. The number of days under mechanical ventilation on can also be reduced [25]-[28]. However, more severely lung injured patients tend to respond poorly to PSV [29]. Additionally, it is reported that early PSV increases patient-ventilator asynchrony [6],[30]. This effect may lead to high and harmful tidal volumes even in the early phase of ventilation [31].
Our present findings do not reproduce a significant improvement of gas exchange or lung injury. Ventilation was more homogenously distributed between the central and non-dependent compartment during PSV, but merely a non-significant amount of high VA/Q compartments indicating hyperinflation developed in the VCV group. This is a considerable contrast to the upper mentioned results. However, it is worth to take model dependent characteristics into account: for most experimental studies focusing on various assisted spontaneous breathing modes the bronchoalveolar lavage/surfactant-depletion model was used. Furthermore, the assumed mechanisms that mitigate lung injury through spontaneous ventilation vary between several studies and are not fully elucidated [6]. The lavage model immediately induces atelectasis due to surfactant depletion, which are relative easy to recruit in the early phase [29], whereas LPS-injured lungs tend to respond poorly to recruitment strategies [32]. In a rabbit model of lavage-induced lung injury PSV was beneficial only in mild ARDS, though aggravated a pre-existing severe lung injury [33]. The present data, in this context, assume that not only the severity but also etiology and pathophysiologic considerations may considerably influence the response to early PSV. Furthermore, PSV was started before outright fulfillment of ARDS criteria in our model.
Endotoxemia alone causes a relatively moderate VA/Q impairment in the short run [34]. This is reflected in the moderate VA/Q changes in our data. Interestingly, previous data from a porcine sepsis model combined with non-protective ventilation reported a shunt fraction of 17.3 ± 7.5 [34] without occurrence of low VA/Q units, whereas low VA/Q units represent the predominant pattern of impairment during our low Vt modes (Figure 5). Furthermore, LPS but not bronchoalveolar lavage compromises the hypoxic pulmonary vasoconstriction [35], which should partially compensate the gas exchange deficit. Supporting our results, LPS administration did not significantly affect the ventilation distribution, but only influenced the perfusion pattern [36].
The present study has some limitations: the group sizes were adapted to previous publications that showed beneficial effects of PSV in early ARDS without a prior power analysis. Due absence of a clear-cut trend, however, it appears unlikely that the lack of effect is essentially influenced by the group sizes. We applied standard PSV and not promising but sophisticated spontaneous ventilation approaches like variable, proportional or neutrally adjusted ventilation [6],[37]. However, the initiation of early PSV in beginning sepsis should be feasible almost anywhere, not just in specialized intensive care units. Several studies showed that spontaneous ventilation attenuates histopathological lung injury in mild to moderate ARDS models [7],[8],[33]. But inflammatory response was only slightly altered in comparison to conventional lung protective ventilation [8], while gene expression analysis yielded no significant differences in pulmonary mRNA expression of inflammatory marker genes [7]. With regard to the reported model characteristics and ongoing LPS exposition, which is documented in the high plasma cytokine levels (Figure 3), significant variances in tissue contents of inflammatory markers are highly improbable without the presence of an anti-inflammatory agent over six hours. The present study was designed to focus the early phase of sepsis with a developing lung injury. If possible effects over six hours proceed towards improved long-term outcome, is merely speculative. Nevertheless, adequate identification and selection of patients may considerably influence the effectiveness of early PSV.
Conclusion
In a porcine model of early LPS-induced lung injury direct initiation of low Vt-PSV did not improve pulmonary function or affect lung injury in comparison to low Vt-VCV within six hours. This is a contrast to several studies that report beneficial effects of assisted spontaneous breathing modes in non-septic experimental models of mild to moderate ARDS. Early response to PSV in ARDS seems to be determined not exclusively by severity but also by etiology of the developing lung injury.
Abbreviations
- ANOVA:
-
Analysis of variance
- ARDS:
-
Acute respiratory distress syndrome
- EIT:
-
Electrical impedance tomography
- etCO2:
-
Endtidal CO2
- FiO2:
-
Fraction of inspired oxygen
- IQR:
-
Interquartile range
- LPS:
-
Lipopolysaccharide
- MMIMS-MIGET:
-
Micropore membrane inlet mass spectrometry - multiple inert gas elimination technique
- PaO2:
-
Arterial partial pressure of oxygen
- PSV:
-
Pressure support ventilation
- PEEP:
-
Positive end-expiratory pressure
- Vt:
-
Tidal volume
- VA/Q:
-
Ventilation/perfusion distribution
- VCV:
-
Volume controlled ventilation
References
Sevransky JE, Levy MM, Marini JJ: Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Crit Care Med. 2004, 32: S548-S553. 10.1097/01.CCM.0000145947.19077.25.
Fan E, Villar J, Slutsky AS: Novel approaches to minimize ventilator-induced lung injury. BMC Med. 2013, 11: 85-10.1186/1741-7015-11-85.
Matthay MA, Zemans RL: The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011, 6: 147-163. 10.1146/annurev-pathol-011110-130158.
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010, 363: 1107-1116. 10.1056/NEJMoa1005372.
Gama de Abreu M, Guldner A, Pelosi P: Spontaneous breathing activity in acute lung injury and acute respiratory distress syndrome. Curr Opin Anaesthesiol. 2012, 25: 148-155. 10.1097/ACO.0b013e3283504bde.
Guldner A, Pelosi P, Gama de Abreu M: Spontaneous breathing in mild and moderate versus severe acute respiratory distress syndrome. Curr Opin Crit Care. 2014, 20: 69-76. 10.1097/MCC.0000000000000055.
Spieth PM, Guldner A, Beda A, Carvalho N, Nowack T, Krause A, Rentzsch I, Suchantke S, Thal SC, Engelhard K, Kasper M, Koch T, Pelosi P, de Abreu MG: Comparative effects of proportional assist and variable pressure support ventilation on lung function and damage in experimental lung injury. Crit Care Med. 2012, 40: 2654-2661. 10.1097/CCM.0b013e3182592021.
Spieth PM, Carvalho AR, Guldner A, Kasper M, Schubert R, Carvalho NC, Beda A, Dassow C, Uhlig S, Koch T, Pelosi P, Gama de Abreu M: Pressure support improves oxygenation and lung protection compared to pressure-controlled ventilation and is further improved by random variation of pressure support. Crit Care Med. 2011, 39: 746-755. 10.1097/CCM.0b013e318206bda6.
Graham MR, Gulati H, Kha L, Girling LG, Goertzen A, Mutch WA: Resolution of pulmonary edema with variable mechanical ventilation in a porcine model of acute lung injury. Can J Anaesth. 2011, 58: 740-750. 10.1007/s12630-011-9517-3.
Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, Gatto LA, Lin X, Dean DA, Vodovotz Y, Nieman G: Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock. 2013, 39: 28-38.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R: Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39: 165-228. 10.1007/s00134-012-2769-8.
Bodenstein M, Wang H, Boehme S, Vogt A, Kwiecien R, David M, Markstaller K: Influence of crystalloid and colloid fluid infusion and blood withdrawal on pulmonary bioimpedance in an animal model of mechanical ventilation. Physiol Meas. 2012, 33: 1225-1236. 10.1088/0967-3334/33/7/1225.
Bodenstein M, David M, Markstaller K: Principles of electrical impedance tomography and its clinical application. Crit Care Med. 2009, 37: 713-724. 10.1097/CCM.0b013e3181958d2f.
Hartmann EK, Boehme S, Bentley A, Duenges B, Klein KU, Elsaesser A, Baumgardner JE, David M, Markstaller K: Influence of respiratory rate and end-expiratory pressure variation on cyclic alveolar recruitment in an experimental lung injury model. Crit Care. 2012, 16: R8-10.1186/cc11147.
Hartmann EK, Duenges B, Baumgardner JE, Markstaller K, David M: Correlation of thermodilution-derived extravascular lung water and ventilation/perfusion-compartments in a porcine model. Intensive Care Med. 2013, 39: 1313-1317. 10.1007/s00134-013-2915-y.
Spieth PM, Knels L, Kasper M, Domingues Quelhas A, Wiedemann B, Lupp A, Hubler M, Neto AG, Koch T, Gama de Abreu M: Effects of vaporized perfluorohexane and partial liquid ventilation on regional distribution of alveolar damage in experimental lung injury. Intensive Care Med. 2007, 33: 308-314. 10.1007/s00134-006-0428-7.
Matute-Bello G, Frevert CW, Martin TR: Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008, 295: L379-L399. 10.1152/ajplung.00010.2008.
Hancock RE, Scott MG: The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. 2000, 97: 8856-8861. 10.1073/pnas.97.16.8856.
Muller-Leisse C, Klosterhalfen B, Hauptmann S, Simon HB, Kashefi A, Andreopoulos D, Kirkpatrick CJ, Gunther RW: Computed tomography and histologic results in the early stages of endotoxin-injured pig lungs as a model for adult respiratory distress syndrome. Invest Radiol. 1993, 28: 39-45. 10.1097/00004424-199301000-00012.
Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M: A comparison among animal models of acute lung injury. Crit Care Med. 1998, 26: 912-916. 10.1097/00003246-199805000-00027.
Carvalho AR, Spieth PM, Pelosi P, Beda A, Lopes AJ, Neykova B, Heller AR, Koch T, Gama de Abreu M: Pressure support ventilation and biphasic positive airway pressure improve oxygenation by redistribution of pulmonary blood flow. Anesth Analg. 2009, 109: 856-865. 10.1213/ane.0b013e3181aff245.
Spieth PM, Carvalho AR, Guldner A, Pelosi P, Kirichuk O, Koch T, de Abreu MG: Effects of different levels of pressure support variability in experimental lung injury. Anesthesiology. 2009, 110: 342-350.
Spieth PM, Guldner A, Huhle R, Beda A, Bluth T, Schreiter D, Ragaller M, Gottschlich B, Kiss T, Jaber S, Pelosi P, Koch T, Gama de Abreu M: Short-term effects of noisy pressure support ventilation in patients with acute hypoxemic respiratory failure. Crit Care. 2013, 17: R261-10.1186/cc13091.
Kiss T, Guldner A, Bluth T, Uhlig C, Spieth PM, Markstaller K, Ullrich R, Jaber S, Santos JA, Mancebo J, Camporota L, Beale R, Schettino G, Saddy F, Vallverdú I, Wiedemann B, Koch T, Schultz MJ, Pelosi P, de Abreu MG: Rationale and study design of ViPS - variable pressure support for weaning from mechanical ventilation: study protocol for an international multicenter randomized controlled open trial. Trials. 2013, 14: 363-10.1186/1745-6215-14-363.
Schadler D, Elke G, Engel C, Bogatsch H, Frerichs I, Kuhlen R, Rossaint R, Quintel M, Scholz J, Brunkhorst FM, Loeffler M, Reinhart K, Weiler N: Ventilatory strategies in septic patients. Results from a nationwide observational trial. Anaesthesist. 2013, 62: 27-33. 10.1007/s00101-012-2121-2.
Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, Mutz N: Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001, 164: 43-49. 10.1164/ajrccm.164.1.2001078.
Brander L, Slutsky AS: Assisted spontaneous breathing during early acute lung injury. Crit Care. 2006, 10: 102-10.1186/cc3953.
Gilstrap D, Macintyre N: Patient-ventilator interactions. Implications for clinical management. Am J Respir Crit Care Med. 2013, 188: 1058-1068. 10.1164/rccm.201212-2214CI.
Cereda M, Foti G, Marcora B, Gili M, Giacomini M, Sparacino ME, Pesenti A: Pressure support ventilation in patients with acute lung injury. Crit Care Med. 2000, 28: 1269-1275. 10.1097/00003246-200005000-00002.
Chanques G, Kress JP, Pohlman A, Patel S, Poston J, Jaber S, Hall JB: Impact of ventilator adjustment and sedation-analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med. 2013, 41: 2177-2187. 10.1097/CCM.0b013e31828c2d7a.
Richard JC, Lyazidi A, Akoumianaki E, Mortaza S, Cordioli RL, Lefebvre JC, Rey N, Piquilloud L, Sferrazza Papa GF, Mercat A, Brochard L: Potentially harmful effects of inspiratory synchronization during pressure preset ventilation. Intensive Care Med. 2013, 39: 2003-2010. 10.1007/s00134-013-3032-7.
Fernandez-Bustamante A, Easley RB, Fuld M, Mulreany D, Hoffman EA, Simon BA: Regional aeration and perfusion distribution in a sheep model of endotoxemic acute lung injury characterized by functional computed tomography imaging. Crit Care Med. 2009, 37: 2402-2411. 10.1097/CCM.0b013e3181a02354.
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y: The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med. 2013, 41: 536-545. 10.1097/CCM.0b013e3182711972.
Neumann P, Hedenstierna G: Ventilation-perfusion distributions in different porcine lung injury models. Acta Anaesthesiol Scand. 2001, 45: 78-86. 10.1034/j.1399-6576.2001.450113.x.
Easley RB, Mulreany DG, Lancaster CT, Custer JW, Fernandez-Bustamante A, Colantuoni E, Simon BA: Redistribution of pulmonary blood flow impacts thermodilution-based extravascular lung water measurements in a model of acute lung injury. Anesthesiology. 2009, 111: 1065-1074. 10.1097/ALN.0b013e3181bc99cf.
Fagerberg A, Sondergaard S, Karason S, Aneman A: Electrical impedance tomography and heterogeneity of pulmonary perfusion and ventilation in porcine acute lung injury. Acta Anaesthesiol Scand. 2009, 53: 1300-1309. 10.1111/j.1399-6576.2009.02103.x.
Cordioli RL, Akoumianaki E, Brochard L: Nonconventional ventilation techniques. Curr Opin Crit Care. 2013, 19: 31-37. 10.1097/MCC.0b013e32835c517d.
Acknowledgements
The authors thank Dagmar Dirvonskis, Dana Pieter and Wieslawa Bobkiewicz for logistics and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The study was funded in part by the German Research Council PAK 415 and a Stage 1 grant of the Johannes-Gutenberg University Mainz to EKH. The funders had no influence on study design, data collection, analysis, or manuscript preparation. None of the authors reports a conflict of interest.
Authors’ contributions
AZ, EKH, RT, TL, and BD conducted the experiments. AZ, EKH, AS, MB, and SCT performed the data analysis. AZ and EKH drafted the manuscript. MD participated in the study design, supervision of laboratory, data interpretation and revision of the manuscript. All authors edited and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ziebart, A., Hartmann, E.K., Thomas, R. et al. Low tidal volume pressure support versus controlled ventilation in early experimental sepsis in pigs. Respir Res 15, 101 (2014). https://doi.org/10.1186/s12931-014-0101-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-014-0101-6