Abstract
Salivary gland (SG) dysfunction impairs the life quality of many patients, such as patients with radiation therapy for head and neck cancer and patients with Sjögren’s syndrome. Multiple SG engineering strategies have been considered for SG regeneration, repair, or whole organ replacement. An in-depth understanding of the development and differentiation of epithelial stem and progenitor cells niche during SG branching morphogenesis and signaling pathways involved in cell–cell communication constitute a prerequisite to the development of suitable bioengineering solutions. This review summarizes the essential bioengineering features to be considered to fabricate an engineered functional SG model using various cell types, biomaterials, active agents, and matrix fabrication methods. Furthermore, recent innovative and promising approaches to engineering SG models are described. Finally, this review discusses the different challenges and future perspectives in SG bioengineering.
Similar content being viewed by others
Background
SG fulfills critical roles in oral health, and their dysfunction can result in extensive deterioration of oral function and other health manifestations [1]. The parotid, submandibular, sublingual, and numerous minor glands secrete saliva in response to a wide range of biochemical signals and environmental cues [2]. Saliva contains water, mucus, antibacterial compounds, electrolytes, and various enzymes, which perform various vital functions in digestion, speaking, chewing, swallowing, and maintaining teeth and gingival tissues [3]. Irreparable SG damage causes hyposalivation manifesting itself by dry mouth symptom (xerostomia) in patients suffering from an autoimmune disease such as Sjögren’s syndrome or treated by radiotherapy for head and neck cancers [4]. Sjögren's syndrome, mainly occurring in middle-aged and older women, affect between 400,000 and 3.1 million adults worldwide [5], and radiotherapy for head and neck cancer treatment is given annually to around 1 million new patients worldwide [6, 7]. Considering existing treatments for hyposalivation are palliative and temporarily alleviate xerostomia [8], re-engineering SG will offer permanent and effective solutions to restore salivation [9]. In 1999, Bruce Baum and colleagues indicated three significant ways to re-engineer salivary epithelial cell functions: redesigning secretory function, repairing hypofunctional SG, and developing artificial SG [10]. Since then, various tissue engineering strategies have been conducted to restore salivation, and some have led to clinical trials [11,12,13,14,15,16,17,18,19]. Indeed, clinical trials suggest stem cell therapy [16, 17, 19] combined with special cell culture methods such as spontaneous cell aggregation, hanging drop and rotating culture vessels [1, 20, 21] or scaffold material [22,23,24,25] to produce functional secretory epithelial organoids, and gene therapy [18] may offer new therapeutic options for radiation-induced xerostomia. Due to challenges related to keeping the functionality of SG cells, researchers have examined the potential benefit of using a combination of different biomaterials [26, 27] and cell types to provide the optimal implant material for SG tissue engineering applications [28,29,30]. Nevertheless, fabricating a fully formed functional SG replacement in a controlled manner remains a challenge [11]. This review aims to provide essential features to be considered for the bioengineering of an SG model. It provides an overview of the existing knowledge on SG anatomy, structure, and function; SG diseases and dysfunction; critical elements in the tissue engineering approach for SG regeneration; strategies and fabrication methods in SG bioengineering; as well as the current challenges and perspectives.
Salivary gland anatomy, structure, and function
SG originates from epithelial branching morphogenesis [31], which is characterized by three major phases: firstly, the development of a relative undifferentiated branched structure involving acinar and ductal precursors together with the developing vasculature and nerves; secondly, the epithelial branching morphogenesis induced by neural crest-derived mesenchymal growth factors and other molecular important cues; and thirdly, the maturation process at which stage the glands are fully functional and well-differentiated [32, 33]. The innervation and vascularization of the SG advance in parallel with the formation and maturation of the glands [32, 33]. The intricate morphogenic and differentiation processes are controlled by multiple signaling networks of cell–cell [32]. Mesenchymal cells and epithelial layer provide the stromal ECM and basement membrane (BM). During the branching process, the ECM composition varies from region to region and directly regulates the SG maturation process [31].
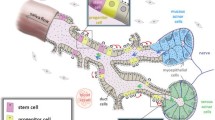
Human SG is made of three pairs of major glands known as parotid (PG), submandibular (SMG), and sublingual gland (SLG), collectively able to synthesize and secrete 90% of the total saliva, as well as comprising around 600–1000 minor glands (MSGs) distributed within the oral cavity that produce the remaining 10% of secreted saliva [34] (Fig. 1). Major glands share similar anatomical architecture: a branching duct with acini at one end and an opening to the oral cavity at the other end [33] (Fig. 1). The serous acini secrete a watery fluid rich in ions and proteins (such as a-amylase), while the mucous cells secrete a more viscous fluid rich in mucins [33]. The SMG and SLG are composed of a mixture of serous and mucous acini, unlike the PG exclusively made of serous acini [33]. Acini are surrounded by ECM, myoepithelial cells, myofibroblasts, immune cells, endothelial cells, stromal cells, and nerve fibers [33]. Myoepithelial cell contractility is regulated by the autonomic nervous system and supports the salivary flow by compressing the parenchymal structure and forcing fluid secretion into the ductal system [34].
Human salivary gland features. A Schematic location of PG, SMG, SLG, and MSG in the oral cavity and the trigeminal nerve spreading postganglionic parasympathetic innervation structures of the head. B Schematic structure of one branch of human SGs, classification of different types of acini, innervation, blood supply (arteries), main excretory duct cells, and contribution of major and minor glands in resting or stimulating saliva flow
Epithelial cell types can be identified using cell markers (Fig. 2). Partitioning-defective 1b (Par-1b) is involved in myoepithelial cell morphogenesis and differentiation during SG development and can be used as a myoepithelial cell marker in addition to SMA [35]. AQP5, a-amylase, AQP3, TMEM16A, and the transcription factor MIST1 are markers of acinar cells [11]. KRT5, KRT14, KRT18, KRT19, NHE1, and SLC26C are markers of ductal cells. Moreover, MUC1, along with the polarity markers Scribble Planar Cell Polarity Protein (SCRIB) and PATJ Crumbs Cell Polarity Complex Component, identify ductal cells in SG and hS/PCs progenitors [36]. Transcription factor P63 is expressed in myoepithelial cells and basal duct cells [32].
Once secreted by acinar cells, the primary saliva passes through an organized network of intercalated ducts, striated ducts, and main excretory ducts (Wharton’s duct for SMG; Stensen’s duct for PG; Bartolini duct for SLG; Rivinis ducts for MSG) [31, 33]. All major SGs ensure the production of daily saliva (0.5-1L per day) [37]. The capillaries diverging from the external carotid artery ensure the vascularizing of the PG, while the submental and sublingual arteries guarantee blood supply to the SMG and SLG [32]. The anatomical and structural features of SG are summarized in Fig. 1.
Saliva secretion is regulated by the interaction of different sensory signals activating afferent fibers of the facial (CNVII), glossopharyngeal (CNIX), and trigeminal (CNV) nerves [38]. The facial and glossopharyngeal nerves send the interneurons to the salivary centers [38]. The glossopharyngeal nerve synapses in the otic ganglion, and then the postganglionic parasympathetic fibers spread to the PG via the facial nerve synapsing in the submandibular ganglion and carrying on to the SLG and SMG [39] (Fig. 1). Sympathetic stimulation mainly results in acinar protein-rich secretion, while parasympathetic stimulation promotes the production of large volumes of saliva [38]. Acetylcholine binding to the muscarinic receptor (M3 receptors in PG and M3 and M1 in SMG) leads to inositol triphosphate and calcium signaling pathway activation and saliva secretion, while noradrenaline binding to beta1 adrenoreceptors leads to cyclic AMP signaling pathway [38]. In addition to these main neurotransmitters, some neuropeptides act as potential co-transmitters, such as substance P and VIP. Purinergic receptors are also involved in the control of SG secretion [40]. Crosstalk between the main neurotransmitter’s signaling pathways amplifies saliva flow and protein secretion under normal reflex conditions [34].
Salivary gland diseases and dysfunction
SG dysfunction can lead to quantitative and/or qualitative changes in saliva composition and flow, leading to hyposalivation (manifesting as xerostomia) or hypersalivation (sialorrhea or hypersialia) [34, 41]. Xerostomia affects at least 10% of the adult population, with women and older people being more affected [42]. In opposition, sialorrhea, also known as drooling, may result from genuine SG alteration (primary sialorrhea), medication side effects, or may be associated with neurological disorders due to an impairment of voluntary oral motor activity or sensory ability (secondary sialorrhea) [34].
Due to its composition, saliva plays major role in food handling, teeth protection, and defense against microorganisms [43]. Therefore, persistent and severe SG hypofunction commonly predisposes patients to mucosal changes, caries, and other viral and bacterial infections [42]. The most common cause of SG hypofunction leading to xerostomia, especially in elderly people, is represented by the side effects of various medications [44, 45]. However, the adverse effects of drugs on salivary secretion are reversible. Numerous diseases and medical conditions can also affect SG function. SG inflammation, referred to as sialadenitis, can result from SG bacterial or viral infection or recurrent inflammatory disorders and is more prone to occur with hyposalivation and/or duct obstruction [46]. An incurable autoimmune disease, Sjögren’s syndrome, and radiation therapy for the treatment of head and neck cancer affect many individuals worldwide. Sjögren’s syndrome is a chronic autoimmune disorder and the second most prevalent rheumatic disease. It results from autoimmune epithelitis leading to partial destruction of exocrine glands due to a lymphoplasmacytic infiltration of the parenchyma [8, 47, 48]. The disease affects 0.1–4.8% of the population, with a female to male ratio of 9:1 [8]. The disease manifests as dry mouth (xerostomia) and dry eye (keratoconjunctivitis sicca) [8]. Currently, no efficient treatment exists for Sjögren’s syndrome, and only a number of tailor-made treatments are available to relieve the symptoms [8]. Radiation therapy is the most common or complementary lifesaving treatment for head and neck tumors, which can lead to irreversibly radiation-induced damage of SG [49]. Head and neck cancers usually begin in the squamous cells that cover the mucosal surfaces of the head and neck (the upper aerodigestive tract, paranasal sinuses, salivary, and thyroid gland) [49]. The most notable side effect of local irradiation is a reduction of saliva secretion due to an alteration of SG functionality. Recent study suggests the retained SG regenerative potential following radiation therapy may offer new avenues for therapeutic intervention [50].
The currently available treatments for xerostomia (stimulant medications or secretagogues, salivary substitutes, or artificial saliva) only provide temporary relief without offering a permanent solution [41]. Therefore, in search for potential and promising ways to permanently restore SG secretory function in patients with SG hypofunction, three main avenues have been considered: gene therapy [18, 51], stem cell therapies [16, 17, 19, 45, 52], and SG bioengineered models [9, 33, 53].
Key features in bioengineering a SG model
A fully functional SG will require complex interactions among multiple cell types such as acinar, ductal, and myoepithelial cells in a branching structure, incorporating with the gland’s microenvironment that supplies it with vascular fluids and neural network. Consequently, an engineered SG must possess unique components and capabilities. An in-depth understanding of the mechanism regulating SG branching, SG cell polarity and secretion, innervation, and vascularization will provide valuable guidelines for engineering an SG model in vitro. Figure 2 shows the essential features that can be considered for proper functionality in SG bioengineered models.
Branching morphogenesis
Branching morphogenesis is a critical process in SG formation [54]. The mechanical force generated by ECM, cell–cell interaction, and different growth factors in cell niche play essential roles in SG branching morphogenesis. These elements could provide structural guidance cues to help the gland keep its desired shape and organization.
Condensation or stretching of bundles of ECM fibrils by mesenchymal cells [55] and mesenchyme-generated traction forces [56] induced mechanical force capable of inducing branching morphogenesis. Although mechanical forces play a vital role in modulating branching structures, the underlying regulating mechanism is unclear. It remains necessary to deepen our understanding of the intracellular and extracellular forces involved in cell shape and morphogenesis [57]. Measuring the precise mechanical forces between cells and their microenvironment niche (ECM and neighbor cells) remains challenging. Biomechanics, mathematical modeling, and microfabricated techniques could provide an opportunity to evaluate cell mechanics in branching morphogenesis [58,59,60]. One of the next generations of advances in SG tissue engineering relies on the complete characterization of these forces to help design hydrogels with the appropriate stiffness and mechanical properties to control morphogenesis. Table 1 shows the ranges of some physical/mechanical parameters which can be applied to foster branching morphogenesis [60, 61].
Several ECM proteins accumulating in the clefs, such as collagen, laminin, and perlecan (located at the BM lining the mesenchymal-epithelial junction) are supposed to play a critical role in branching morphogenesis [55, 62]. Fibronectin and proteoglycans were shown to behave respectively as the putative cleft initiator [63] and inducer of branching morphogenesis through the release of fibroblast growth factor 10 (FGF10) [64]. The size and sulfation patterns of heparan sulfate present in proteoglycans modulate the biological activity of FGF10 and thereby epithelial branching morphogenesis [65]. Moreover, the ECM is extensively remodeled by matrix metalloproteinase (MMP) cleavage during branching morphogenesis [66]. Due to the vital role of the ECM in initiating and maintaining branching morphogenesis, numerous research studies have focused on designing hydrogels to grow salivary structures using material composition reproducing native ECM-cell interactions to support branching morphogenesis and cellular polarity [13, 63, 67,68,69], as well as appropriate chemical and physical cues promoting branching and reorganizing salivary structures with the proper orientation in 2D, 2.5D (monolayers on 3D substrates), and 3D-engineered models [15, 25, 69,70,71,72,73].
As mentioned above, changes in structure and cellular organization during branching morphogenesis could be guided by mechanical forces and dynamic interactions between neighboring cells. Epithelial (E)-cadherin plays a crucial role in cell–cell adhesion and increases significantly during SG development [54]. E-cadherin interacts via its C-terminus with various proteins such as β-catenin and forms homotypic interactions with other E-cadherin molecules on neighboring cells to mediate adhesive cell–cell interactions [74]. E-cadherin also plays an essential role in the cell self-organizing process since function-blocking anti-E-cadherin antibodies inhibit the process. Further, this protein is necessary for acinar differentiation and integrity of SG epithelial surfaces [75]. Despite constitutive epithelial E-cadherin expression throughout all stages of SG development, other critical cell–cell junctional proteins such as desmoplakins I/II and zonula occludens-1 (ZO-1) are rapidly lost [76]. It was therefore hypothesized that E-cadherin might act in place of other junctional proteins to stabilize external epithelial surfaces, which is significantly more robust between cell junctions of outer bud epithelial cells than those between the inner bud cells [63, 77]. Though, when a cleft forms to interrupt this layer of outer epithelial cells, E-cadherin localization and expression diminish as cell interactions shift from cell–cell to cell–matrix adhesion [63]. Although the exact role of E-cadherin in SG development remains vague at a mechanistic level, E-cadherin is crucially needed to regulate or mediate SG epithelial self-organization, branching, and acinus formation. Hence, analyzing E-cadherin expression in an SG artificial model can be helpful in evaluating cell–cell interaction for controlling morphogenesis.
Active agents are other cues controlling branching morphogenesis and thereby represent critical elements in tissue engineering approaches. Some growth factors are recognized as regulators for essential functions throughout all phases of embryonic development. The two most crucial growth factors for SG development are the epidermal growth factor (EGF) and the fibroblast growth factor (FGF). EGF promoted bud formation [78], while FGF7 and FGF10 differentially stimulated epithelial bud development and bud/duct elongation, respectively [79]. In addition, ex vivo studies reported that branching morphogenesis involves FGF receptor (FGFR) signaling [78, 80, 81] and EGF receptor (EGFR) signaling [82,83,84]. Other signaling pathways are also essential for SG branching morphogenesis, such as phospholipase Cγ1 (PLCγ1), mitogen-activated protein kinases (ERK-1/2), and phosphatidyl- inositol-3-kinase (PI3K) [79, 82, 85, 86]. These three signaling pathways employ multiple common downstream effectors during SG development that are crossregulated by other growth factor receptor systems. It was suggested that ERK-1/2 (stimulated by EGF and FGF7) is essential for bud formation, while PLCγ1 (stimulated by FGF7 and FGF10) may be important for bud/duct elongation [54]. Upstream regulators of FGFR signaling include the platelet-derived growth factor (PDGF) receptor signaling pathway that leads to the up-regulated FGF expression involved in SG branching morphogenesis [87]. These findings indicated the capability of growth factors to regulate complex arrays of interconnected signaling pathways to control morphogenesis. However, it is essential to continue evaluating how these factors and other different receptor-mediated signal transduction pathways communicate and how the ECM and cell–cell interactions control these signaling pathways and lead to the formation of a functional gland. Physical interactions between growth factors and ECM molecules also need to be considered in establishing morphogen gradients guiding SG branching morphogenesis [88, 89]. As such varying levels of growth factors matrix components might play a basic and unpredictable role in instructively patterning of various cellular functions, including differentiation, growth, and cell death. Moreover, osmotic gradient via ECM swelling and aquaporin water transport activity is another physical features that can induce dome formation to control the branching morphogenesis [89]. Understanding how morphogen gradients are created within ECM and their interplay with cellular and tissue level functions is one of the key gap knowledge limiting advancements in SG bioengineering.
SG cell polarity
SG consists of tightly packed, polarized secretory acinar and absorptive ductal cells surrounded by myoepithelial cells, which are presumed to have contractile forces to ensure the proper unidirectional secretion of saliva. Therefore, obtaining polarized cells with accurate apical-basolateral positioning is essential for engineering a functional SG producing saliva. Epithelial cell polarity depends on different cues such as extracellular signals via BM components, cell–cell binding of asymmetrically distributed cadherins, and tight junctional components on lateral membranes. BM aids in cell sorting by separating epithelial cells from the surrounding stromal cells and managing them into a highly interconnected polarized cell monolayer [66]. In the salivary epithelium, tight junctions (TJs) and anchoring junctions such as adherens junctions and desmosomes control cell–cell interactions. TJs are located in the uppermost region of the lateral plasma membrane and include transmembrane proteins such as claudins, occludins, and anchoring protein ZO-1 [90, 91]. Adherens junctions provide the gland with mechanical support by connecting the actin cytoskeleton of neighboring epithelial cells. These junctions are located below tight TJs on the lateral plasma membrane and contain the transmembrane protein E-cadherin and anchoring proteins catenins, α-actinin, and vinculin. The intermembrane barrier created by TJs provides cell polarity. It prevents the lateral diffusion of membrane proteins between the apical and basolateral membrane domains and maintains the transepithelial ion gradients by prohibiting the free movement of ions through the paracellular space [90,91,92,93].
Cell polarization involves E-cadherin dimerization of two adjacent epithelial cells, followed by the subsequent enrollment of adherens junction, TJ, and cell polarity proteins, such as the partitioning defective (PAR) complex and the activation of Rho GTPases needed for downstream signaling [94, 95]. Cell polarity is regulated and controlled by the PAR complex, Crumbs complex, and scribble complex, irregularly localized to specific cell membrane areas.
BM components stimulate cell polarity by providing binding sites for cell surface receptor β1-integrin, leading to the activation of Rho GTPase RAC1 [96]. The four key components of the BM are laminin, type IV collagen, nidogen/entactin, and heparan sulfate proteoglycan (perlecan/HSPG2) [97]. BM proteins (i.e., laminin, collagen type IV, perlecan, and nidogen) present in Matrigel® are needed to establish acinar polarity of human SMG cells (attested by ZO-1, claudins1-5, and occluding expression), [98]. Similarly, hydrogels containing some BM components activate the expression of one or more TJ proteins [72, 99, 100]. Human stem/progenitor cells encapsulated in HA-based hydrogels containing a peptide from perlecan expressed ZO-1 and differentiated into acini [72, 101]. Further, HA-based hydrogels allowed acinar cells to express aquaporin 5 (AQP5) [72, 101], an apical acinar cell marker [47, 102, 103]. The limited cell polarity of mouse SMG cell clusters encapsulated into fibrin-based hydrogels was improved when laminin peptides were cross-linked to fibrin [25]. Synthetic hydrogels made from poly(ethylene glycol) (PEG) [27, 69, 104] or poly(lactic-co-glycolic acid) (PLGA) are other candidate materials for keeping cell polarity within the hydrogel [105]. When combined with MMP-cleavable sequences, these hydrogels promoted the localization of NKCC1, ZO-1, and AQP5 in mouse SMG cells [27]. Enzymatically degradable materials allow SG cells to undergo dynamic morphogenesis during their growth and proliferation and promote cell polarization by clearing a path in the hydrogel. Accordingly, in vitro models of ECM for SG tissue would provide practical tools to study the effect of different cues on salivary cells' polarity and help to understand the impact of various parameters on cell polarity.
SG cells functionality
Saliva secretion is one of the primary functions of SGs and involves several cell types. As mentioned in previous sections, SGs mainly contain three different cell types: acinar cells, ductal cells, and myoepithelial cells. The acinar cells are specialized secretory cells that can be either serous or mucous [106]. TJ proteins seal acinar cells and form a barrier to the movement of large solutes between the apical/luminal and basal/stromal sides, ensuring no backflow of proteins secreted in saliva [107]. In response to nerve stimulation, the activation of Ca2+-regulated K+ and Cl− channels induces an accumulation of Cl− in the acinar lumen leading to subsequent Na+ movement from the interstitium into the acinar lumen to maintain electroneutrality. As a result of the formation of an osmotic gradient, water move to the apical lumen through the presence of AQP5. After that, saliva composition is modified via the reabsorption of sodium chloride and secretion of potassium bicarbonate in the ductal network. Final hypotonic saliva then enters the mouth [108, 109]. Acinar cells also secrete many proteins, including α-amylase (used as an acinar cell marker), salivary peroxidase, phosphatases, hydrolases, dehydrogenases, arginase, esterases, proline-rich, histatin-rich, and tyrosine-rich proteins playing a role in food breakdown and oral health maintenance [110, 111]. As acinar cells play a crucial role in saliva secretion, the repopulation and regeneration of SG acinar cells represent significant challenges for tissue engineering. Studies suggest that acinar cell markers are downregulated when acinar cells undergo cellular stress or injury [73]. Work is ongoing to identify cues and molecular pathways that encourage acinar cells to regenerate [112]. In native tissue, acinar cells tend to be repopulated through self-duplication [113], but ex vivo acinar cell proliferation and maintenance of differentiation have been challenging to achieve to date [14, 114]. The modification of substrate or hydrogels in which acinar cells are grown or encapsulated could provide some solutions to this problem. Understanding the role played by ECM in acinar cell proliferation, and differentiation allowed researchers to partially overcome this challenge [68, 115]. Results indicated that MMP-cleavable hydrogels are the most successful material in maintaining acinar cells phenotype, proliferation, and function [27]. However, acinar cells often lose their polarity and phenotype in long-term culture, which is indicated by a reduction in the expression of acinar cell markers such as MIST1 and AQP5 [104]. The expression of SOX2 is required to replace secretory cells in mouse models [116]. In addition, the proliferation of SOX2-positive cells could be promoted by acetylcholine, suggesting that acinar cell proliferation can be controlled by incorporating neural signals [112]. A detailed understanding of these interactions requires the development of biomimetic model hydrogel systems in which various cell types, including acinar cells, ductal cells, myoepithelial, and neuronal cells, can be co-cultured.
Many hydrogel formulations such as Matrigel® [98, 117], hyaluronic acid (HA)-based hydrogels [13, 111], and enzymatically degradable PEG-based hydrogels [27] have been reported as suitable candidate materials to maintain α-amylase secretion in acinar cells. Functional responses of acinar cells can also be evaluated by measuring calcium oscillations occurring upon stimulation with β-adrenergic or muscarinic agonists [118, 119]. Such functional response was evaluated in acinar-like cells grown in HA gels [13].
To bioengineer a functional SG producing suitable saliva formulation, it may be necessary to integrate ductal cells as they are essential to change the salivary composition [11, 120] and may overcome protein accumulation within acini lumen that may hinder organoid systems. Engineering an acini-like structure interconnected with a ductal network to transport saliva and secreted proteins to an exterior port represent a major hurdle to overcome in the future.
Innervation and vascularization
Parasympathetic and sympathetic innervation plays a role in the control of saliva secretion by inducing fluid-rich and protein-rich secretion [109]. Both β-adrenergic and muscarinic agonists can modulate the epithelial structures and function in engineered SG microstructure [13]. Further, neurotrophic factors such as Neurturin (NRTN) can control parasympathetic submandibular ganglion (PSG) function, promote neurite outgrowth and viability, and influence SG branching morphogenesis [121–123]. On the other hand, vascularization in SG requires integrated sympathetic, parasympathetic, and sensory input [118]. Accordingly, artificially engineered implants must provide the capacity to involve local innervation, either by direct secretion of neurotrophic growth factors or by supplementing 3D scaffolds with selected neurotrophins. Recently, transplantation of 3D culture of SG functional organoids and human dental pulp stem cells generated by magnetic 3D bioassembly promoted epithelial and neuronal growth in damaged irradiated mouse SG [22]. The combination of neuronal signaling cues, nanomaterials, and advanced microfabrication methods will open new avenues in SG tissue engineering.
SGs are also surrounded by a dense network of blood vessels that delivers oxygen to salivary cells, removes cellular waste, and provides the gland with an ample supply of fluid needed for saliva formation [118]. SGs are vascularized through the proliferation and migration of endothelial cells from preformed arteries [124]. Several soluble/paracrine strategies have been used to promote angiogenesis: release of vascular endothelial growth factor (VEGF) and platelet-derived growth factor-BB (PDGF-BB) from biodegradable PLGA scaffold [125], immobilization of ephrin A1 conjugated to PEG diacrylate hydrogels [126], covalent immobilization of PDGF-BB and FGF-2 to scaffolds [127–129]. Mesenchymal stem cells (MSCs) also improved tissue vascularization by forming spheroid aggregates that increased VEGF and FGF-2 [130, 131]. Layer-by-layer (LbL) cell coating method allowed the preparation of in vitro oral mucosa models in which the blood vessels were made from human umbilical vein endothelial cells [132]. However, the ability to construct large vasculature networks to develop more complex SGs requires proper ECM proteins [133]. Incorporating these vascular networks into existing or peptide-modified hydrogels might be beneficial for SG tissue engineering [133]. Furthermore, the nervous and vascular systems are two critical features in regulating outcomes of transferring approach for any artificial model in SG regeneration [134]. Despite advancing knowledge regarding the cues and mechanisms involved in SG innervation and vascularization, the development of innervated and vascularized SG remains an open challenge.
Tissue engineering approach in salivary gland regeneration
Cells, bioactive factors, and biomaterials are three critical cues that need to be used in an optimized combination to engineer whole organs and tissues. The combination of these different elements for preparing a SG bioengineered model is summarized in Fig. 3. All utilized cells, biochemical factors, and materials reported for SG tissue engineering applications are outlined below. Different scaffold design and fabrication strategies are discussed in "Strategies and fabrication methods in SG bioengineering".
Cell sources
Cells represent the central aspects of SG regeneration via cell therapy approaches or the tissue engineering paradigm. Single cells [135] or cells retaining their 3D in vivo spatial organization can be obtained following SG dissociation [136]. Single cells can be sorted into specific parenchymal and stromal subpopulations using flow cytometry and/or selective enhancement during in vitro culture [53]. The challenge lies in assembling implantable and secretory tissue models by mixing stem/progenitor cells from adult SG with a biocompatible and biodegradable 3D scaffold. Also, establishing the efficacy of cell sheets or cell-seeded matrix in SG regeneration requires demonstration that the cells survive and are functional for a clinically-relevant duration, which still is a long-standing challenge in SG tissue bioengineering [137].
Salivary gland progenitor and adult stem cells
Defining and distinguishing the difference between progenitor and stem cells is critical since, despite the ambiguous definitions, they are not equivalent and show distinct properties. Progenitor cells can divide and differentiate only into specific types of cells for a limited number of times and cannot self-renew, while stem cells can replicate indefinitely, giving rise to both undifferentiated and differentiated progeny [138]. Both the embryonic and adult SG show multiple progenitor populations characterized by the expression of nuclear, cytoplasmic, and cell surface markers [122]. SG progenitor cells express the receptor tyrosine kinase c-Kit as a selective marker for regenerating damaged SMG in a mouse model [139]. A combination of four surface markers (Lin-CD24 + c-Kit + Sca1+) characterizes a subset of SMG progenitor cells that showed the highest spheroid-forming efficiency in culture and a robust multilineage regenerative ability when transplanted into irradiated mouse SMG [140]. The peripheral epithelial endbud cells express Keratin 14 (K14) in addition to c-Kit. The Kit + K14 + cells are involved in ductal morphogenesis. They establish a communication with the surrounding neuronal niche and proximal keratin 5 (K5) + epithelial progenitors thanks to the release of NRTN promoting parasympathetic nerve survival and axon extension that support the K5 + progenitors and sustains their ductal differentiation together with EGFR signaling [122]. Genetic lineage tracing has shown that K14+ cells are a multipotent epithelial progenitor population within SMG as they give rise to acinar, myoepithelial, and ductal cells, as well as K5 + -expressing cells [20, 122].
Sox-2 is an essential transcription factor for the maintenance of cell self-renewal and pluripotency and is involved in the formation of several tissues during development. Sox-2 + cells are stem/progenitor cells in the adult sublingual gland [116].
AcsI3 is another transcription factor expressed by adult progenitor cells. Genetic lineage tracing studies showed that AcsI3 + cells could generate a subset of the adult ductal and acinar cell descendants [122, 141]. While K14, K5, and c-Kit trace the lineage-restricted progenitors and can be expressed by different cell types within the ductal compartment, lineage-tracing studies have identified Acta2 (alpha-smooth muscle actin; also abbreviated as SMA) as myoepithelial cells marker proving the maintaining through self-duplication [142].
Different data are present in the scientific literature concerning the reproducibility of cell characteristics in engineered SG tissue. SG cells can form epithelial-like isolated clusters on plastic. On Matrigel-coated surfaces, SG can self-assemble into 3D acinar-like structures, expressing TJ proteins (i.e., occludin, claudin proteins, JAM-A, and ZO-1) as well as AQP5 [71, 143]. SG cell culture on microwell culture systems (hydrogel micropatterning and nanofibrous scaffold) led to acinar-like spheroids formation that showed a high expression of the acinar, ductal cell, and TJ markers, the ability to secrete α-amylase and response to adrenergic or cholinergic agonists (increase in intracellular calcium) [1, 144]. Furthermore, the myoepithelial cells had the functional ability to respond to neuronal signals, spread throughout collagen hydrogels and contract the surrounding hydrogel, and ensure the maintenance of spheroid organization to wrap around the spheroids [11, 145].
Despite slow SG cell turnover (> 60 days) and the lack of proof of the existence of a multipotent SG stem cell, studies have shown a regenerative potential of the gland [146]. Moreover, SG is characterized by a potential “absence” of a single and spatially segregated quiescent multipotent stem cell population, otherwise exhibiting the existence of multiple populations of proliferative stem/progenitor-like cells. In adult SG, MSC expressing surface antigens such as CD44, CD49f (integrin), CD90, and CD105 have been identified. SG stem cells can generate acinar, duct, and myoepithelial cell types. Potentially, stem cell populations can change their phenotype properties in response to the surrounding microenvironment exhibiting the plasticity to transition to intermediate, dedifferentiated, or alternated cell types [138]. MSC culture in Matrigel generates branched and aggregated structures resembling native SG acini and ducts [138, 147, 148].
Non salivary gland cells
Different cell therapy approaches involve using non-SG and/or non-epithelial cells to activate regenerative mechanisms in irradiated SGs. These reports include Bone Marrow (BM)-derived MSCs [149], BM-derived cells [150], human adipose-derived MSCs [151], SG-derived MSC-like cells [152], induced pluripotent stem cells (iPSC) [153], amniotic cells [154], embryonic stem cells (ESC) [155]. Among them, adipose-derived MSCs reduced cell apoptosis and tissue fibrosis, and both BM-MSC and SG-derived mesenchymal-like cells exerted immunosuppressive actions. In addition, BM-derived cells improved saliva secretion and microvessel density by inducing epithelial repair. Their positive effects may be mediated via paracrine pro-survival/proliferative actions on surrounding stem/progenitor cells. The paracrine effects of adipose and BM-derived cells are ensured by the released bioactive components (e.g., KGF, VEGF, IL6, and IGF1), also called “soup” [156–161], supporting anti-apoptotic and pro-proliferative actions. The presence of neurotrophic factors in the “soup” remains unclear. In addition, intravenous administration of “soup” may improve saliva production in rodent irradiated SG, whose clinical success directly depends on the remaining cells responding to paracrine action [162].
Pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have been used for SG regeneration. Successful SG regeneration has been achieved through orthotopic transplantation of a self-organized organ rudiment generated from PSCs in mice with defective parotids, in which some transcription factors (i.e., Sox9 and Foxc1) played some key roles in SG development [30]. Our finding that coculture of salivary glands cells with iPS cells formed differentiated salivary glands is significant, and future elucidation of the mechanism could lead to viable regeneration therapy of functional organs using iPS cells. Our study provides new insights for future research into the regeneration of organs, such as salivary glands.
iPS cells have also proved to be a promising treatment for SG cancer in vitro and in vivo in rats [163]. Co-culture of embryonic SG cells could form differentiated SG in vitro and in vivo in mouse SG, suggesting the iPS cell niche affects SG development and regeneration [153]. Furthermore, human SMG stem/progenitor cells (hSMGepiS/PCs)- formed SG organoids in vitro in response to FGF10 and generated an SG in vivo by responding to the mouse mesenchyme niche [164].
Bioactive factors
Tissue-engineered scaffolds are intended to create and preserve differentiated cell phenotype, ensure basic functional units, and induce branching [71]. These functions rely on the use of specific bioactive factors added to the media or scaffolds. Cell culture media supplements include fetal bovine serum, glutamine, and antibiotics. In addition, conditioned media (containing released bioactive molecules) can increase the long-term expansion of salivary stem cells in vitro, showing increased population doubling and sphere-form efficiency. In fact, MSC-conditioned media induced an increment of acinar-like structures and AQP5 and K14 expression in the presence of laminin-111 [165]. As detailed above, soluble growth factors are fundamental in SG development, branching morphogenesis and end bud formation, cell proliferation, cell differentiation, initiation of innervation, and angiogenesis [166]. Chemical agents modulating specific signaling pathways can promote cell proliferation, motility, secretion, and proper cell shape. For example, ROCK inhibitors enhance cell growth, survival, proliferation, and α-amylase and Met protooncogene (c-Met) expression in SG culture [53, 167]. Moreover, soluble ECM proteins have primarily been used as supporting scaffolds to SG cells, promoting cell-ECM and cell–cell interactions. Fibronectin induced branching and ductal elongation. SG ECM extracts induced 3-D sphere-shaped structures and acinar markers, such as AQP5 and Muc-1 expression (53). Figure 4 summarizes the most common soluble cues for improving cultured cells’ morphogenesis, functionality, polarity, and promoting innervation in SG tissue engineering.
Scaffolds materials
The nature of scaffold material plays a key role in mimicking natural tissues' mechanical, physiochemical, and biological features. Hence, in recent years, various scaffold materials have been investigated for SG tissue engineering, which can be sorted into three categories: naturally-derived biomaterials, synthetic polymers, and hybrid scaffolds materials.
Naturally derived biomaterials
Matrigel is one of the frequently used biomaterials for SG tissue engineering applications [98, 143], promoting cell attachment and differentiation in vitro [168, 169]. However, it is not an appropriate material for clinical translation due to its animal origin, and it is not easy to study the effect of its specific protein component on cell behavior. Therefore, using limited numbers of precisely defined BM components helps to identify a relationship between the activity of cells and a particular ECM constituent. For instance, Laminin, the major component of Matrigel, plays a critical role in SG development and morphogenesis [165], but the entire Laminin-1 sequence can cause unwanted side effects, such as tumorigenesis, degradation, and immune reactions, making it unsuitable for clinical applications [170]. Thus, peptides derived from ECM components are other natural components that can be incorporated into scaffolds to support cell–matrix attachment and cell–cell adhesion. Some laminin-derived peptides can promote human SG spheroids formation, branching morphogenesis, and SG functionality [25, 171]. Primary human salivary stem/progenitor cells grown on a scaffold containing a peptide from domain IV of perlecan/ heparan sulfate proteoglycan (HSPG2) formed acini-like spheroids [100, 172]. In addition, human-derived hydrogels based on fibronectin and placenta BM extracts stimulated morphological and functional differentiation of primary human SG epithelial cells to create polarized salivary acinar-like structures [173]. Also, fibronectin-derived RGD peptides and collagen I-derived MMP-sensitive (PQ) peptides are commonly used as cleavable crosslinkers that can be integrated into a synthetic polymer network to aid in thiol-ene polymerizations of PEG hydrogels [104].
The whole decellularized SG ECM can also be used as another naturally-based biomaterial. Indeed, repopulation of decellularized rat SMG with SG cells indicated significant cellular adhesion, differentiation, and gland-like tissue formation in vitro [174]. Decellularized ECM prepared from human SMG biopsies is also used as a substrate for seeding and growing human epithelial cells and fibroblasts [175]. However, biomaterials derived from animal or human tissues lack tunability and reproducibility and increase the risk of tumorigenic or immunogenic reactions [71].
Scaffolds can also be made up of biologically-derived polysaccharides and proteins [176]. HA is one of the popular hydrogel biomaterials in tissue engineering. Recently, HA-based hydrogels allowed a 3D culture of SG spheroids using primary salivary human stem/progenitor cells [26]. Moreover, arginine-glycine-aspartic acid-modified alginate-based hydrogels and chitin/chitosan-based scaffolds provided a suitable environment for enhancing SMG bud expansion and cleft formation as well as SG branch formation to produce essential ECM [177–181]. Collagen type I, fibrin, fibronectin, and silk fibroin proteins are other standard biomaterials that have been used for SG tissue engineering applications and reported to promote SG epithelial cell growth, aggregate formation, and differentiation as well as matrix formation [11, 71, 182] . Recently, a combination of egg components such as egg white and egg yolk plasma proved to be a biocompatible and cost-effective material for 3D SG mesenchymal and epithelial cell survival [183]. Nevertheless, for all natural polymers, due to their solubility in water and poor mechanical properties, some chemical modification and crosslinking strategies are necessary for their use as scaffold biomaterials [183].
Synthetic polymers
Compared with natural polymers, synthetic biomaterials provide adjustable physicochemical and mechanical properties, two crucial environmental cues for cell growth and differentiation [71], and can be produced in large amounts without any limitation of scalability and extraction process. Moreover, synthetic materials can provide a chemically defined, xenogenic-free environment that can be modified for desired outcomes and provide reproducible results, which can be an alternative to Matrigel and other natural source materials [184]. The most common synthetic polymers for SG tissue engineering applications are PLGA [185] and PEG [27, 104]. Most commercially available synthetic materials are produced by ring-opening polymerization. The use of methacrylate-based polymerization of PEG-based hydrogels induces a significant loss in SMG cell viability. In contrast, the use of thiol–ene polymerization of PEG-based hydrogels is more favorable to submandibular cell encapsulation [104]. Nevertheless, the encapsulated single cells in both hydrogels failed to form organized SG structures [104]. Encapsulation of pre-assembled multicellular spheroids in these types of hydrogels could enhance cell viability stimulate cell proliferation, as well as promote and preserve cell–cell contacts [104]. In addition, polyacrylamide gels, utilized to assess the effect of the substrate modulus on SMG regeneration, are physiologically compliant gels (0.48 kPa) as they allow higher SG branching morphogenesis than stiffer gels (20 kPa) [186]. Also, it was reported that PVDF compare to different synthetic biomaterials, including polyvinyl alcohol (PVA), ethylene vinyl alcohol (EVAL), polycarbonate (PC), promoted branching morphogenesis in serum-free in vitro culture [187, 188].
Hybrid materials
Hybrid scaffold biomaterials containing synthetic and natural polymers have been developed to improve the bioactivity of the polymer surface to enhance cell attachment and differentiation. When comparing the effects of several biodegradable polymer-based substrates (i.e., poly-l-lactic acid (PLLA), polyglycolic acid (PGA)) and their copolymers (PLGA) coated with different ECM proteins (i.e., collagen I, collagen IV, laminin, fibronectin, and gelatin), SG cells only attached to polymer disks with preabsorbed proteins and behaved similarly on PLLA and PGA [189]. Another study revealed that coating of PEG-terephthalate/poly (butylene terephthalate) scaffolds with Matrigel improved SG epithelial cell growth and morphology [190]. Also, it was reported that chitosan and laminin-coated PLGA nanofibers enhanced the proliferation of SG acinar and ductal cell lines [191].
Strategies and fabrication methods in SG bioengineering
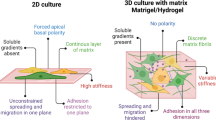
Generally, the bioengineering approach to restoring SG function can be divided into two main categories: cell-based techniques and cell-material-based strategies [192]. Recently, due to the importance of ECM and BM in SG structure and function, such as branching morphogenesis, polarity, and secretion, most recent studies have focused on using the second approach for SG tissue engineering purposes [71]. Indeed, biomaterials act as an ECM to prepare a suitable niche for cell attachment, proliferation, and differentiation [193]. Another classification in SG bioengineering is based on the in vitro cell culture dimensional model, which can be classified into two main groups. First, the 2D model when cells are seeded on the flat tissue culture plate surface (TCPS) or biocompatible polymers to create a polarized epithelial cell monolayer with the capability of unidirectional fluid secretion [71, 189]. Second, the 3D model, when cells form spheroid or are encapsulated and cultured in 3D matrices to proliferate, reconstitute the polarization and secretory acinar structures [13]. Studies related to these two model strategies are summarized in the following sections.
2D models
Several efforts have been made to develop suitable biomaterial scaffolds promoting the proliferation and differentiation of SG progenitor cells [71]. Porous membranes or scaffolds better support cell growth [194] and phenotype retention [182] than flat substrates. Human PG and SMG cells seeded onto Matrigel®-coated or uncoated TCPS promoted expressing α-amylase and AQP5 in 3D acinar-like units and forming ductal cell monolayers with TJs, respectively [98]. Primary human SG cells seeded on TCPS coated with perlecan domain IV peptide (PlnDIV) or Matrigel formed 3D acini-like salivary units expressing α-amylase [101]. Mimicking ECM and BM nanostructure showed that immortalized adult mouse or rat SG cell lines (SIMS, ductal; Par-C10, acinar) had more rounded and clustered morphology, as well as a reduced and more diffuse expression of focal adhesion proteins on fibrous scaffolds [185]. In addition, cell proliferation and polarization strongly depended on the surface coating of the nanofiber scaffolds [191]. To further mimic the architecture of the BM in SG epithelial cell niche, arrays of “craters” in polydimethylsiloxane (PDMS) lined with electrospun PLGA nanofibers were fabricated [195]. Increased crater curvature enhanced the average height of the SIMS cell monolayer, cell polarization, AQP5 expression (in both SIMS and Par-C10 cells), and TJ protein expression (occludin) in Par-C10 cells [195]. When human parotid epithelial cells were cultured on Matrigel, PEG, and micropatterned PEG hydrogel in the presence of an electrospun poly-caprolactone (PCL) nanofibrous microwells, the expression of salivary epithelial markers, TJ proteins, E-cadherin, and F-actin was increased in micropatterned hydrogel [196]. Recent studies related to the fabrication of fibrous and hydrogel scaffolds to evaluate some critical features in 2D salivary bioengineered models and related markers are summarized in Table 2.
Although 2D cell models have been considered a reliable method for studying the in vitro characteristics of various cells, they cannot practically simulate the cell niche to provide appropriate cellular functions such as cell differentiation, proliferation, motility, and metabolism. Indeed, the 2D model cannot reproduce the in vivo interactions of cells or cells with the ECM.
3D models
Considering the inherent limitations of 2D cell models, 3D cell models have become predominant in mimicking physiological conditions and maintain cellular and tissue function by establishing proper cell signaling pathways and ECM interactions [204]. Various 3D culture methods can be used to form cell spheroids, such as spontaneous cell aggregation, hanging drop, magnetic 3D bioassembly, and rotating culture vessels [183, 205]. These 3D culture methods allow cell–cell interactions, cell polarity, and differentiation and recapitulate the ECM properties. Though, in these culture methods, there is no control in spheroid size which causes cell necrosis to occur in the core of the cell aggregates [1]. Following partial SG digestion, cells grown in a serum-free culture media form functional spheroids, with cells retaining their native ECM and expressing markers of cell polarization, acinar cell, and TJ [136]. However, after ten days, cell apoptosis started due to the uncontrollable increase in spheroid size, limitation in nutrients, and oxygen diffusion rate, an possible toxic effect of accumulated proteins within the core of the formed acini units [137]. But, culturing salivary spheroids in a 3D ECM-derived matrix can preserve their structural integrity over ten days [136, 175]. When organoids were produced by seeding cells into BM substrate like hydrogel matrix, they displayed better size uniformity, cell polarization, and cell–cell interactions than the abovementioned method. [206]. However, the main limitations of this method are the presence of xenogeneic components, uncontrollable degradation of the biomaterials, and a lack of mechanical stimulation [183, 207]. Therefore, human fibronectin and BM extracts, utilized as alternative hydrogel materials, promoted the differentiation of human SG cells into acinar-like structures [173, 208]. Further, recycled human tissue ECM (obtained by collecting the residual connective tissue that remained like a gelatinous mass) promoted the growth of epithelial cells exhibiting comparable morphology and proteins composition to the native SG tissue [175].
Recently, evaluating different physicochemical and mechanical effects of biomaterials on cells’ behavior opened a new 3D culture modality as a scaffold-based culture method [1]. In this method, the SG cells are seeded in the gel and form spheroids that can differentiate into acinar-like structures expressing TJ proteins (like occludin) and AQP5 [71, 98, 143]. Common hydrogel materials used in this method are tissue-extracted proteins like collagen, fibrin, and Matrigel [71]. Purified acinar cells seeded in a 2.5D HA-based hydrogel containing PlnDIV showed self-assembling into acini-like structures with cell junctions and a central lumen [72]. Similar results were reported with a 3D HA-based hydrogel culture system but fostered and maintained the growth and differentiation of functional, neurotransmitter-responsive acini-like spheroids for over 100 days [13]. Egg white-alginate blend 2.5D hydrogel is a cost-effective, and suitable material for SG tissue engineering applications as SG spheroid-like structure formation could be controlled by regulating alginate concentrations in the blend polymer solution [209].
Microwells of micropatterning and nanofibrous hydrogel scaffold have been used to promote more uniformed acinar-like spheroids [196]. Compared to 2D and 3D culture systems, the spheroids obtained with these microwell culture systems showed higher expression of acinar, ductal, and TJ markers as well as a significant amount of α-amylase secretion and intracellular calcium levels in response to adrenergic or cholinergic agonists [144]. It was shown that the formation of uniform spheroids needed a niche independent culture system within a serum-free culture medium [144, 196]. Recently, the 3D co-culture of NIH 3T3 fibroblasts and SIMS ductal SG epithelial cells in alginate microtubes via needle-to-needle microfluidic technique also represent a promising co-culture method for further understanding of epithelial and mesenchymal interaction during tissue morphogenesis and future practical applications in regenerative medicine [210].
Bioprinting technologies combined with other technology such as magnetic nanomaterials are alternative methods for adjusting the physical structure of the 3D culture model [211, 212]. There are two strategies for bioprinting via using magnetic forces. The first method is a label-free cell approach in which cells are suspended in paramagnetic liquid containing gadolinium (Gd3 +). The magnetized fluid is stimulated by applying a magnetic field and displacing cells toward regions with a low magnetic gradient [211]. This process can control the cell patterning and is a nozzle-free method that provides a rapid print of multicellular spheroids. But the major concern over this technology is the usage of cytotoxic paramagnetic suspending media and high concentrations of Gd3 + that could be toxic for tissue spheroids and increase the risk of imbalance osmotic pressure due to excessive use of ions in the paramagnetic medium [213]. The second magnetic-based bioprinting technology requires cell labeling with magnetic nanoparticles [6, 214]. In this method, the cells can be easily directed using mild magnetic forces, and spatial patterning of the 3D cell assembly into the desired morphological structure can be regulated by altering the magnetic field's shape or configuration. Besides, the size of the spheroids can be adjusted by tuning magnetic nanoparticle concentration, the number of cells, and magnet size [23, 214]. However, the requirement for specialized equipment and the risk of toxicity for cells limit its application [23]. Accordingly, another group proposed using gel egg yolk plasma (GEYP) as a more abundant, biocompatible, cost-effective material for tissue engineering applications. They showed that GEYP was successfully 3D printed with controlled geometrics [215]. However, they mentioned that bioprinting of SG cells is still in progress and needs more optimizations.
Recent studies related to the fabrication of a 3D scaffold-based model for salivary tissue engineering and evaluated markers are summarized in Table 3. Moreover, the pros and cons of 2D and 3D scaffold fabrication methods are listed in Table 4.
Current challenges and future perspective
Despite the recent progress in SG tissue engineering, there are some challenges in bioengineered strategies related to the appropriate secretory function, vascularization, and innervation of the SG tissue models, as well as the risk of adverse host tissue response to the transferred biomaterials [33, 221]. In recent decades, advances in cellular and molecular biology opened a new vista to identify which type of cells, genes, and signaling pathways are playing critical roles in the morphogenesis and functionality of SG to develop new biomaterials and fabrication methods [222–225]. However, many questions related to understanding mechanotransduction mechanisms, branching patterns, and biochemical signaling during SG development still remain [226].
Cell-based strategies, especially using stem cells and cell-biomaterial-based techniques to prepare in vitro SG culture models, have revealed some new opportunities in SG bioengineering [1, 9, 71]. Nevertheless, it has not been clear how long the applied cells can maintain their viability and functionality after transferring them in vivo. It is also ambiguous how long it takes for some of these biomaterials to degrade in vivo and how they react in contact with blood flow, biochemical cues, and immune system, especially in patients with Sjögren’s Syndrome [11]. Although personalized medicine recently could help reduce immune cell penetration in implanted biomaterials, a multidisciplinary approach to engineer the desirable scaffold biomaterials for SG regeneration with optimal cell responses is still required. Moreover, one of the significant challenges in the translation of experimental therapies to clinical implementation is the selection of appropriate animal models for preclinical testing. In most cases, a large animal model is required to mimic as closely as possible the load and weight-bearing characteristics of the human body [227].
Further, despite the suitable polarization maintained by matrix mimetics, the secretory function of SG cells is limited [11]. Hence, incorporating combinatory approaches and increasing the complexity of models by introducing different cell types will offer proper SG cell functionality and secretion in artificial models. Few groups have currently tried to prepare a more complex SG model, but optimization of combined culture media and matrix in these models has not yet been achieved [53]. For example, the use of additive manufacturing methods such as bioprinting and microfluidic proposed that a combination of specific cell lines and growth factors could be a suitable method for achieving innervation and vascularization [6, 53, 207, 228, 229]. However, bioprinted tissue models are still restricted to millimeter size and constructed with only immature vascular networks that cannot support epithelial tissues' long-term culture and morphogenesis. Microfluidic chips are also complicated to fabricate and operate for cell culture and are not yet standardized [228, 229].
So, combining different additive manufacturing methods such as bioprinting and microfluidic with employing endothelial cells, neural cells, growth factors, and multifunctional biomaterials with suitable mechanical and biological properties to promote selective differentiation and organization of multiple cell types will be an objective for future studies [71]. It seems that tunable stimuli-responsive materials with dynamic mechanical properties may provide opportunities to manipulate the properties of engineered ECMs over appropriate time and length scales in future works [230–233]. Furthermore, DNA-cross-linked biomaterials with reversible control of matrix stiffness may offer an opportunity to understand the role of dynamic stiffness changes in epithelial morphogenesis [234]. Also, it seems that a combination of stem cell/gene therapy with 3D organotypic cell-based strategies will become the next generation of biomedical therapies to either restore a damaged SG or develop an in vitro SG model for transplantation in humans suffering from xerostomia [6, 235,236,237]. For instance, SG organoids derived from genetically modified induced pluripotent stem cells (iPSC) can be considered for fabricating SG models [2]. And all cell types present in the gland can be generated via programmed differentiation [115, 238, 239].
Conclusion
Although significant progress in SG regenerative medicine, such as stem cell/gene therapy, has been reported over the past decade [41, 45, 240, 241], a conclusive approach to build a fully functional SG model based on cell-material-based strategies as a substitute for this exocrine organ remains a challenge [11, 242]. Future strategies will have to overcome the challenge of obtaining acinar-like structures surrounded by myoepithelial cells, interconnected in 3D with a network of ducts that will transport saliva to an exterior port, innervated and vascularized. Recently, different material and fabrication methods have been evaluated to prepare 2D and 3D SG models; however, the performance of these materials in vivo and the long-term functionality of the prepared models need to be studied further. Moreover, it remains unclear how long it will take for some of these materials to degrade and how stiff they need to stay intact long enough to support gland regeneration. Even though micro/nanofabrication techniques have provided an opportunity to simulate the cell microenvironment, the lack of fully understating the biomechanics and biochemistry of the natural SG tissue is one of the limitations to forming a complete practical SG model. Hence, developing a fully functional gland requires combined multidisciplinary efforts from various fields such as biology, genetics, biomaterials, engineering, and medicine to identify and mimic microenvironmental signals that are responsible for cell plasticity and functionality in SG tissue [240]. Translating ex vivo and in vitro findings obtained in rodents to human organoids derived from human progenitors remains a major hurdle due to the lower level of knowledge of the human SG morphogenesis and difficulties related to the availability of human cell sources. As SG development involves the interaction of various cells and neuronal signaling plays a vital role in its morphogenesis and functionality, innervation and vascularization, as well as co/multi-culture strategies in preparing 3D SG models, are anticipated to become promising approaches in SG tissue engineering.
Availability of data and materials
Not applicable.
Abbreviations
- A:
-
Acinar
- ABS:
-
Acute bacterial sialadenitis
- AC:
-
Acinar cell
- AL:
-
Alginate
- aPKC:
-
Atypical protein kinase C
- AQP5:
-
Aquaporin 5
- BM:
-
Basement membrane
- CA:
-
Cell attachment
- cAMP:
-
Cyclic AMP
- CBS:
-
Chronic bacterial sialadenitis
- Cdc42:
-
Cell division control protein 42
- CH:
-
Chitosan
- CL:
-
Crosslinking
- c-Met:
-
Met proto oncogene
- CP:
-
Cell polarization
- D:
-
Ductal
- DC:
-
Ductal cell
- dECM:
-
Decellularized extracellular matrix
- E:
-
Epithelial
- EnC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EGFR:
-
EGF Receptor
- EHS:
-
Engelbreth–Holm–Swarm
- ELS:
-
Electrospinning
- ER:
-
Endoplasmic reticulum
- ERK-1/2:
-
Mitogen-activated protein kinases
- ESC:
-
Embryonic stem cells
- EVAL:
-
Ethylene vinyl alcohol
- FGF:
-
Fibroblast growth factor
- FGFR:
-
FGF receptor
- G4RGDS:
-
Gly-Gly-Gly-Gly-Arg-Gly-Asp
- GEYP:
-
Gel egg yolk plasma
- GFRMG:
-
Growth factor reduced Matrigel
- HA:
-
Hyaluronic acid
- hDFs:
-
Human dermal fibroblasts
- hDPSc:
-
Human dental pulp stem cell
- hPECs:
-
Human parotid epithelial cells
- hPG:
-
Human parotid gland
- hPGAC:
-
Human primary parotid gland acinar cells
- hS/PCs:
-
Primary salivary human stem/progenitor cells
- hSG:
-
Human submandibular gland ductal epithelial cell line
- hSGSC:
-
Human single clonal salivary gland stem cells
- HSPG:
-
Heparan sulfate proteoglycan
- iPSC:
-
Induced pluripotent stem cells
- KRT18:
-
Keratin 18
- KRT7:
-
Keratin 7
- LbL:
-
Layer-by-layer
- M:
-
Myoepithelial
- MC:
-
Myoepithelial cell
- MMP:
-
Matrix metalloproteinase
- mPG:
-
Mouse primary parotid gland cells
- MSCs:
-
Mesenchymal stem cells
- MSGs:
-
Minor salivary glands
- mSMG:
-
Ex vivo mouse submandibular gland cells
- N:
-
Neuronal
- NC:
-
Neuronal cell
- NIH3T3:
-
NIH 3T3 fibroblasts
- NP:
-
Nanoparticles
- NRTN:
-
Neurturin
- NS-SV-AC:
-
Immortalized acinar cell from human salivary gland
- PA:
-
Polyacrylamide
- PAR:
-
Partitioning defective
- Par-C10:
-
Immortalized rat parotid gland acinar epithelial cell line
- PATJ:
-
PALS1-associated tight junction
- PC:
-
Polycarbonate
- PCL:
-
Polycaprolactone
- PDGF:
-
Platelet-derived growth factor
- PDMS:
-
Polydimethylsiloxane
- PEG:
-
Poly(ethylene glycol)
- PG:
-
Parotid gland
- PGA:
-
Polyglycolic acid
- PGS:
-
Poly glycerol sebacate
- PI3K:
-
Phosphatidyl-inositol-3-kinase
- PLCγ1:
-
Phospholipase Cγ1
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PLL:
-
Poly l-lysin
- PLLA:
-
Poly-l-lactic acid
- PlnDIV:
-
Peptide derived from domain IV of perlecan
- PNS:
-
Parasympathetic nervous system
- PSG:
-
Parasympathetic submandibular ganglion
- pSGECs:
-
Primary salivary gland epithelial cells
- PVA:
-
Polyvinyl alcohol
- PVDF:
-
Polyvinylidene fluoride
- RGDSP:
-
Integrin-binding peptide
- ROCK-1:
-
Rho-associated coiled-coil-containing kinase 1
- rSGECs:
-
Rat primary salivary gland epithelial cells
- rSGSCs:
-
Rat SG stem/progenitor cells
- SC:
-
Stem cell
- SF:
-
Silk fibroin
- SG:
-
Salivary gland
- SGSC:
-
Human single clonal salivary gland stem cells
- SIMS:
-
Immortalized adult mouse submandibular salivary gland ductal epithelial cell line
- SLG:
-
Sublingual gland
- SMA:
-
Smooth muscle α-actin
- SMG:
-
Submandibular gland
- SMG-C10:
-
Immortalized rat submandibular gland acinar epithelial cell line
- SNS:
-
Sympathetic nervous system
- SVEC4-10:
-
Immortalized mouse lymphoid endothelial cells
- TCPS:
-
Tissue culture plate surface
- TJs:
-
Tight Junctions
- V:
-
Vascular
- VEGF:
-
Vascular endothelial growth factor
- ZO-1:
-
Zonula occludens-1
References
Almansoori AA, Kim B, Lee JH, Tran SD. Tissue engineering of oral mucosa and salivary gland: disease modeling and clinical applications. Micromachines. 2020;11(12):1066.
Tanaka J, Mishima K. In vitro three-dimensional culture systems of salivary glands. Pathol Int. 2020;70(8):493–501.
Edgar WM, O’Mullane DM, Dawes C. Saliva and oral health. Vol. 146. British Dental Association London; 2004.
von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2007;103:S57–61.
Carsons SE, Patel BC. Sjogren syndrome. StatPearls Internet. 2021.
Ferreira JN, Rungarunlert S, Urkasemsin G, Adine C, Souza GR. Three-dimensional bioprinting nanotechnologies towards clinical application of stem cells and their secretome in salivary gland regeneration. Stem Cells Int. 2016.
Jensen SB, Pedersen AML, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18(8):1039–60.
Parisis D, Chivasso C, Perret J, Soyfoo MS, Delporte C. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J Clin Med. 2020;9(7):2299.
Nelson J, Manzella K, Baker OJ. Current cell models for bioengineering a salivary gland: a mini-review of emerging technologies. Oral Dis. 2013;19(3):236–44.
Baum BJ, Wang S, Cukierman E, Delporte C, Kagami H, Marmary Y, et al. Re-engineering the functions of a terminally differentiated epithelial cell in vivo. 1999.
Barrows CM, Wu D, Farach-Carson MC, Young S. Building a functional salivary gland for cell-based therapy: more than secretory epithelial acini. Tissue Eng Part A. 2020;26(23–24):1332–48.
Baum BJ, Tran SD. Synergy between genetic and tissue engineering: creating an artificial salivary gland. Periodontol. 2006;41(1):218–23.
Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia X, Witt RL, Farach-Carson MC. Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters. Tissue Eng Part A. 2013;19(13–14):1610–20.
Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11(1–2):172–81.
Wu D, Chapela P, Farach-Carson MC. Reassembly of functional human stem/progenitor cells in 3D culture. In: Epithelial cell culture. Berlin: Springer; 2018. p. 19–32.
Grønhøj C, Jensen DH, Vester-Glowinski P, Jensen SB, Bardow A, Oliveri RS, et al. Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled phase 1/2 trial (MESRIX). Int J Radiat Oncol Biol Phys. 2018;101(3):581–92.
Grønhøj C, Jensen DH, Glovinski PV, Jensen SB, Bardow A, Oliveri RS, et al. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): study protocol for a randomized controlled trial. Trials. 2017;18(1):1–10.
Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci. 2012;109(47):19403–7.
Blitzer GC, Rogus-Pulia NM, Mattison RJ, Varghese T, Ganz O, Chappell R, et al. Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction (MARSH): Study protocol for a phase 1 dose-escalation trial of patients with xerostomia after radiation therapy for head and neck cancer: MARSH: Marrow-Derived Autologous Stromal Cells for the Restoration of Salivary Hypofunction. Cytotherapy. 2022;24:534.
Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3(4): e2063.
Nanduri LS, Lombaert IM, Van Der Zwaag M, Faber H, Brunsting JF, Van Os RP, et al. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013;108(3):458–63.
Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66.
Ferreira JN, Hasan R, Urkasemsin G, Ng KK, Adine C, Muthumariappan S, et al. A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J Tissue Eng Regen Med. 2019;13(3):495–508.
Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8(10):1940–9.
Nam K, Wang CS, Maruyama CLM, Lei P, Andreadis ST, Baker OJ. L1 peptide–conjugated fibrin hydrogels promote salivary gland regeneration. J Dent Res. 2017;96(7):798–806.
Ozdemir T, Fowler EW, Liu S, Harrington DA, Witt RL, Farach-Carson MC, et al. Tuning hydrogel properties to promote the assembly of salivary gland spheroids in 3D. ACS Biomater Sci Eng. 2016;2(12):2217–30.
Shubin AD, Felong TJ, Schutrum BE, Joe DS, Ovitt CE, Benoit DS. Encapsulation of primary salivary gland cells in enzymatically degradable poly (ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017;50:437–49.
Lee HW, Hsiao YC, Chen YC, Young TH, Yang TL. Salispheres from different major salivary glands for glandular regeneration. J Dent Res. 2019;98(7):786–94.
Szlávik V, Vág J, Markó K, Demeter K, Madarász E, Oláh I, et al. Matrigel-induced acinar differentiation is followed by apoptosis in HSG cells. J Cell Biochem. 2008;103(1):284–95.
Tanaka J, Ogawa M, Hojo H, Kawashima Y, Mabuchi Y, Hata K, et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat Commun. 2018;9(1):1–13.
Porcheri C, Mitsiadis TA. Physiology, pathology and regeneration of salivary glands. Cells. 2019;8(9):976.
Emmerson E, Knox SM. Salivary gland stem cells: a review of development, regeneration and cancer. Genesis. 2018;56(5):e23211.
Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Saliva Secret Funct. 2014;24:1–13.
Pedersen AML, Sørensen CE, Proctor GB, Carpenter GH, Ekström J. Salivary secretion in health and disease. J Oral Rehabil. 2018;45(9):730–46.
Gervais EM, Sequeira SJ, Wang W, Abraham S, Kim JH, Leonard D, et al. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis. 2016;12(4):194–216.
Wu D, Chapela PJ, Barrows CML, Harrington DA, Carson DD, Witt RL, et al. MUC1 and Polarity markers INADL and SCRIB identify salivary ductal cells. J Dent Res. 2022;00220345221076122.
Brazen B, Dyer J. Histology. Salivary Glands. 2019.
Proctor GB, Carpenter GH. Salivary secretion: mechanism and neural regulation. Saliva Secret Funct. 2014;24:14–29.
Whelton H. Introduction: the anatomy and physiology of salivary glands. Saliva Oral Health. 1996;1–9.
Khalafalla MG, Woods LT, Jasmer KJ, Forti KM, Camden JM, Jensen JL, et al. P2 receptors as therapeutic targets in the salivary gland: from physiology to dysfunction. Front Pharmacol. 2020;11:222.
Rocchi C, Emmerson E. Mouth-watering results: clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol Med. 2020;26(7):649–69.
Shinohara C, Ito K, Takamatsu K, Ogawa M, Kajii Y, Nohno K, et al. Factors associated with xerostomia in perimenopausal women. J Obstet Gynaecol Res. 2021;47(10):3661–8.
Brosky ME. The role of saliva in oral health: strategies for prevention and management of xerostomia. J Support Oncol. 2007;5(5):215–25.
Smith CH, Boland B, Daureeawoo Y, Donaldson E, Small K, Tuomainen J. Effect of aging on stimulated salivary flow in adults. J Am Geriatr Soc. 2013;61(5):805–8.
Tanaka J, Mishima K. Application of regenerative medicine to salivary gland hypofunction. Jpn Dent Sci Rev. 2021;57:54–9.
Bolk K, Mueller K, Phalke N, Walvekar RR. Management of Benign salivary gland conditions. Surg Clin. 2022;102(2):209–31.
D’Agostino C, Elkashty OA, Chivasso C, Perret J, Tran SD, Delporte C. Insight into salivary gland aquaporins. Cells. 2020;9(6):1547.
Tzioufas AG, Voulgarelis M. Update on Sjögren’s syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol. 2007;21(6):989–1010.
Crozier E, Sumer BD. Head and neck cancer. Med Clin. 2010;94(5):1031–46.
Luitje ME, Israel AK, Cummings MA, Giampoli EJ, Allen PD, Newlands SD, et al. Long-term maintenance of acinar cells in human submandibular glands after radiation therapy. Int J Radiat Oncol Biol Phys. 2021;109(4):1028–39.
Baum BJ, Alevizos I, Chiorini JA, Cotrim AP, Zheng C. Advances in salivary gland gene therapy–oral and systemic implications. Expert Opin Biol Ther. 2015;15(10):1443–54.
Chihaby N, Orliaguet M, Le Pottier L, Pers JO, Boisramé S. Treatment of Sjögren’s syndrome with mesenchymal stem cells: a systematic review. Int J Mol Sci. 2021;22(19):10474.
Piraino LR, Benoit DS, DeLouise LA. Salivary gland tissue engineering approaches: state of the art and future directions. Cells. 2021;10(7):1723.
Hsu JC-F, Yamada KM. Salivary gland branching morphogenesis—recent progress and future opportunities. Int J Oral Sci. 2010;2(3):117–26.
Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104(1):51–9.
Wan X, Li Z, Lubkin SR. Mechanics of mesenchymal contribution to clefting force in branching morphogenesis. Biomech Model Mechanobiol. 2008;7(5):417–26.
Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8(8):633–44.
Nerger BA, Siedlik MJ, Nelson CM. Microfabricated tissues for investigating traction forces involved in cell migration and tissue morphogenesis. Cell Mol Life Sci. 2017;74(10):1819–34.
Sutlive J, Xiu H, Chen Y, Gou K, Xiong F, Guo M, et al. Generation, transmission, and regulation of mechanical forces in embryonic morphogenesis. Small. 2022;18(6):2103466.
Polacheck WJ, Chen CS. Measuring cell-generated forces: a guide to the available tools. Nat Methods. 2016;13(5):415–23.
Lubkin SR, Li Z. Force and deformation on branching rudiments: cleaving between hypotheses. Biomech Model Mechanobiol. 2002;1(1):5–16.
Hayakawa T, Kishi J, Nakanishi Y. Salivary gland morphogenesis: possible involvement of collagenase. Matrix Stuttg Ger Suppl. 1992;1:344–51.
Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–81.
Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. 2007.
Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, et al. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 2008;283(14):9308–17.
Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, et al. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev Cell. 2009;17(4):482–93.
Neves SC, Pereira RF, Araújo M, Barrias CC. Bioengineered peptide-functionalized hydrogels for tissue regeneration and repair. In: Peptides and proteins as biomaterials for tissue regeneration and repair. Amsterdam: Elsevier; 2018. p. 101–25.
Sequeira SJ, Larsen M, DeVine T. Extracellular matrix and growth factors in salivary gland development. Salivary Glands. 2010;14:48–77.
Song Y, Sharipol A, Uchida H, Ingalls MH, Piraino L, Mereness JA, et al. Encapsulation of primary salivary gland acinar cell clusters and intercalated ducts (AIDUCs) within matrix metalloproteinase (MMP)-degradable hydrogels to maintain tissue structure and function. Adv Healthc Mater. 2022;11:2101948.
Hubka KM, Carson DD, Harrington DA, Farach-Carson MC. Perlecan domain I gradients establish stable biomimetic heparin binding growth factor gradients for cell migration in hydrogels. Acta Biomater. 2019;97:385–98.
Ozdemir T, Fowler EW, Hao Y, Ravikrishnan A, Harrington DA, Witt RL, et al. Biomaterials-based strategies for salivary gland tissue regeneration. Biomater Sci. 2016;4(4):592–604.
Pradhan S, Liu C, Zhang C, Jia X, Farach-Carson MC, Witt RL. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryngol Neck Surg. 2010;142(2):191–5.
Shubin AD, Sharipol A, Felong TJ, Weng PL, Schutrum BE, Joe DS, et al. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res. 2020;380(3):487–97.
Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–34.
Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10(1):21–31.
Hieda Y, Iwai K, Morita T, Nakanishi Y. Mouse embryonic submandibular gland epithelium loses its tissue integrity during early branching morphogenesis. Dev Dyn. 1996;207(4):395–403.
Walker JL, Menko AS, Khalil S, Rebustini I, Hoffman MP, Kreidberg JA, et al. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Dev Dyn Off Publ Am Assoc Anat. 2008;237(11):3128–41.
Morita K, Nogawa H. EGF-dependent lobule formation and FGF7-dependent stalk elongation in branching morphogenesis of mouse salivary epithelium in vitro. Dev Dyn Off Publ Am Assoc Anat. 1999;215(2):148–54.
Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223.
Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, et al. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP-and FGF-dependent mechanisms. Development. 2002;129:5767.
Jaskoll T, Zhou YM, Chai Y, Makarenkova HP, Collinson JM, West JD, et al. Embryonic submandibular gland morphogenesis: stage-specific protein localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and abnormal SMG phenotypes in FgfR2-IIIc+/Δ, BMP7–/–and Pax6–/–Mice. Cells Tissues Organs. 2002;170(2–3):83–98.
Kashimata M, Sayeed S, Ka A, Onetti-Muda A, Sakagami H, Faraggiana T, et al. The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev Biol. 2000;220(2):183–96.
Kashimata M, Gresik EW. Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the α6-integrin subunit. Dev Dyn Off Publ Am Assoc Anat. 1997;208(2):149–61.
Umeda Y, Miyazaki Y, Shiinoki H, Higashiyama S, Nakanishi Y, Hieda Y. Involvement of heparin-binding EGF-like growth factor and its processing by metalloproteinases in early epithelial morphogenesis of the submandibular gland. Dev Biol. 2001;237(1):202–11.
Koyama N, Kashimata M, Sakashita H, Sakagami H, Gresik EW. EGF-stimulated signaling by means of PI3K, PLCγ1, and PKC isozymes regulates branching morphogenesis of the fetal mouse submandibular gland. Dev Dyn Off Publ Am Assoc Anat. 2003;227(2):216–26.
Larsen M, Hoffman MP, Sakai T, Neibaur JC, Mitchell JM, Yamada KM. Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Dev Biol. 2003;255(1):178–91.
Yamamoto S, Fukumoto E, Yoshizaki K, Iwamoto T, Yamada A, Tanaka K, et al. Platelet-derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression. J Biol Chem. 2008;283(34):23139–49.
Jayadev R, Sherwood DR. Morphogenesis: shaping tissues through extracellular force gradients. Curr Biol. 2017;27(17):R850–2.
Ishida-Ishihara S, Akiyama M, Furusawa K, Naguro I, Ryuno H, Sushida T, et al. Osmotic gradients induce stable dome morphogenesis on extracellular matrix. J Cell Sci. 2020;133(14):jcs243865.
Baker OJ. Tight junctions in salivary epithelium. J Biomed Biotechnol. 2010;2010:1–10.
Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35.
Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9(11):846–59.
Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69.
Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13(5):609–22.
Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol. 2007;178(3):517–27.
Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, et al. β1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16(2):433–45.
LeBleu VS, MacDonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med. 2007;232(9):1121–9.
Maria OM, Maria O, Liu Y, Komarova SV, Tran SD. Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol. 2011;43(4):622–31.
Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG). J Cell Sci. 1996;109(8):2013–21.
Nam K, Dean SM, Brown CT, Smith RJ Jr, Lei P, Andreadis ST, et al. Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019;91:186–94.
Pradhan S, Zhang C, Jia X, Carson DD, Witt R, Farach-Carson MC. Perlecan domain IV peptide stimulates salivary gland cell assembly in vitro. Tissue Eng Part A. 2009;15(11):3309–20.
King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–98.
Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270(4):1908–12.
Shubin AD, Felong TJ, Graunke D, Ovitt CE, Benoit DS. Development of poly (ethylene glycol) hydrogels for salivary gland tissue engineering applications. Tissue Eng Part A. 2015;21(11–12):1733–51.
Jean-Gilles R, Soscia D, Sequeira S, Melfi M, Gadre A, Castracane J, et al. Novel modeling approach to generate a polymeric nanofiber scaffold for salivary gland cells. J Nanotechnol Eng Med. 2010;1(3).
Nanci A. Ten Cate’s Oral Histology-e-book: development, structure, and function. USA: Elsevier Health Sciences; 2017.
Baker OJ. Current trends in salivary gland tight junctions. Tissue Barriers. 2016;4(3): e1162348.
Delporte C. Aquaporins in secretory glands and their role in Sjögren’s syndrome. In: Aquaporins. Berlin: Springer; 2009. p. 185–201.
Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol Rev. 2012;92(1):39–74.
Witt RL. Salivary gland diseases: surgical and medical management. Thieme; 2011.
Srinivasan PP, Patel VN, Liu S, Harrington DA, Hoffman MP, Jia X, et al. Primary salivary human stem/progenitor cells undergo microenvironment-driven acinar-like differentiation in hyaluronate hydrogel culture. Stem Cells Transl Med. 2017;6(1):110–20.
Emmerson E, May AJ, Nathan S, Cruz-Pacheco N, Lizama CO, Maliskova L, et al. SOX2 regulates acinar cell development in the salivary gland. Elife. 2017;6: e26620.
Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell. 2015;33(2):231–7.
Jang SI, Ong HL, Gallo A, Liu X, Illei G, Alevizos I. Establishment of functional acinar-like cultures from human salivary glands. J Dent Res. 2015;94(2):304–11.
Oliver C, Waters JF, Tolbert CL, Kleinman HK. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro Cell Dev Biol. 1987;23(7):465–73.
Emmerson E, May AJ, Berthoin L, Cruz-Pacheco N, Nathan S, Mattingly AJ, et al. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med. 2018;10(3): e8051.
McCall AD, Nelson JW, Leigh NJ, Duffey ME, Lei P, Andreadis ST, et al. Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng Part A. 2013;19(19–20):2215–25.
Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3–18.
Segawa A, Takemura H, Yamashina S. Calcium signalling in tissue: diversity and domain-specific integration of individual cell response in salivary glands. J Cell Sci. 2002;115(9):1869–76.
Mosca AC, Chen J. Food-saliva interactions: mechanisms and implications. Trends Food Sci Technol. 2017;66:125–34.
Ferreira JN, Hoffman MP. Interactions between developing nerves and salivary glands. Organogenesis. 2013;9(3):199–205.
Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25:52–60.
Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4(1):1–7.
Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104(4):428–41.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34.
Saik JE, Gould DJ, Keswani AH, Dickinson ME, West JL. Biomimetic hydrogels with immobilized ephrinA1 for therapeutic angiogenesis. Biomacromol. 2011;12(7):2715–22.
Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly (ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20(12):1763–79.
Leslie-Barbick JE, Shen C, Chen C, West JL. Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng Part A. 2011;17(1–2):221–9.
Saik JE, Gould DJ, Watkins EM, Dickinson ME, West JL. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly (ethylene glycol) hydrogels. Acta Biomater. 2011;7(1):133–43.
Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32(11):2734–47.
Melchiorri AJ, Nguyen BNB, Fisher JP. Mesenchymal stem cells: roles and relationships in vascularization. Tissue Eng Part B Rev. 2014;20(3):218–28.
Nishiyama K, Akagi T, Iwai S, Akashi M. Construction of vascularized oral mucosa equivalents using a layer-by-layer cell coating technology. Tissue Eng Part C Methods. 2019;25(5):262–75.
Asano Y, Nishiguchi A, Matsusaki M, Okano D, Saito E, Akashi M, et al. Ultrastructure of blood and lymphatic vascular networks in three-dimensional cultured tissues fabricated by extracellular matrix nanofilm-based cell accumulation technique. Microscopy. 2014;63(3):219–26.
Zhang X, Yang N, Liu X, Su J, Cong X, Wu L, et al. Autonomic reinnervation and functional regeneration in autologous transplanted submandibular glands in patients with severe keratoconjunctivitis sicca. Int J Oral Sci. 2018;10(2):1–7.
Pringle S, Maimets M, van der Zwaag M, Stokman MA, van Gosliga D, Zwart E, et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells. 2016;34(3):640–52.
Seo YJ, Lilliu MA, Abu Elghanam G, Nguyen TT, Liu Y, Lee JC, et al. Cell culture of differentiated human salivary epithelial cells in a serum-free and scalable suspension system: the salivary functional units model. J Tissue Eng Regen Med. 2019;13(9):1559–70.
Dos Santos HT, Kim K, Okano T, Camden JM, Weisman GA, Baker OJ, et al. Cell sheets restore secretory function in wounded mouse submandibular glands. Cells. 2020;9(12):2645.
Weng PL, Aure MH, Ovitt CE. Concise review: a critical evaluation of criteria used to define salivary gland stem cells. Stem Cells. 2019;37(9):1144–50.
Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17(5):445–9.
Kwak M, Ninche N, Klein S, Saur D, Ghazizadeh S. c-Kit+ cells in adult salivary glands do not function as tissue stem cells. Sci Rep. 2018;8(1):1–11.
Bullard T, Koek L, Roztocil E, Kingsley PD, Mirels L, Ovitt CE. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol. 2008;320(1):72–8.
May AJ, Cruz-Pacheco N, Emmerson E, Gaylord EA, Seidel K, Nathan S, et al. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development. 2018;145(21):dev166363.
Maria OM, Zeitouni A, Gologan O, Tran SD. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A. 2011;17(9–10):1229–38.
Shin HS, Hong HJ, Koh WG, Lim JY. Organotypic 3D culture in nanoscaffold microwells supports salivary gland stem-cell-based organization. ACS Biomater Sci Eng. 2018;4(12):4311–20.
Ozdemir T, Srinivasan PP, Zakheim DR, Harrington DA, Witt RL, Farach-Carson MC, et al. Bottom-up assembly of salivary gland microtissues for assessing myoepithelial cell function. Biomaterials. 2017;142:124–35.
Rocchi C, Barazzuol L, Coppes RP. The evolving definition of salivary gland stem cells. Npj Regen Med. 2021;6(1):1–8.
Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92(3):466–71.
Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, et al. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells. 2007;9(2):191–205.
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim HJ, et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013;49(2):136–43.
Lombaert IM, Wierenga PK, Kok T, Kampinga HH, Dehaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12(6):1804–12.
Kojima T, Kanemaru SI, Hirano S, Tateya I, Ohno S, Nakamura T, et al. Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope. 2011;121(9):1864–9.
Lim JY, Yi T, Lee S, Kim J, Kim S, Song SU, et al. Establishment and characterization of mesenchymal stem cell-like clonal stem cells from mouse salivary glands. Tissue Eng Part C Methods. 2015;21(5):447–57.
Ono H, Obana A, Usami Y, Sakai M, Nohara K, Egusa H, et al. Regenerating salivary glands in the microenvironment of induced pluripotent stem cells. BioMed Res Int. 2015;2015.
Zhang NN, Huang GL, Han QB, Hu X, Yi J, Yao L, et al. Functional regeneration of irradiated salivary glands with human amniotic epithelial cells transplantation. Int J Clin Exp Pathol. 2013;6(10):2039.
Kawakami M, Ishikawa H, Tachibana T, Tanaka A, Mataga I. Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro. Hum Cell. 2013;26(2):80–90.
Fang D, Hu S, Liu Y, Quan VH, Seuntjens J, Tran SD. Identification of the active components in Bone Marrow Soup: a mitigator against irradiation-injury to salivary glands. Sci Rep. 2015;5(1):1–12.
Fang D, Shang S, Liu Y, Bakkar M, Sumita Y, Seuntjens J, et al. Optimal timing and frequency of bone marrow soup therapy for functional restoration of salivary glands injured by single-dose or fractionated irradiation. J Tissue Eng Regen Med. 2018;12(2):e1195–205.
Fang D, Su X, Liu Y, Lee JC, Seuntjens J, Tran SD. Cell extracts from spleen and adipose tissues restore function to irradiation-injured salivary glands. J Tissue Eng Regen Med. 2018;12(2):e1289–96.
Misuno K, Tran SD, Khalili S, Huang J, Liu Y, Hu S. Quantitative analysis of protein and gene expression in salivary glands of Sjogren’s-like disease NOD mice treated by bone marrow soup. PLoS ONE. 2014;9(1): e87158.
Su X, Fang D, Liu Y, Ruan G, Seuntjens J, Kinsella JM, et al. Lyophilized bone marrow cell extract functionally restores irradiation-injured salivary glands. Oral Dis. 2018;24(1–2):202–6.
Tran SD, Liu Y, Xia D, Maria OM, Khalili S, Wang RWJ, et al. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS ONE. 2013;8(4): e61632.
Lombaert I, Movahednia MM, Adine C, Ferreira JN. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells. 2017;35(1):97–105.
Alaa El-Din Y, Sabry D, Abdelrahman AH, Fathy S. Potential therapeutic effects of induced pluripotent stem cells on induced salivary gland cancer in experimental rats. Biotech Histochem. 2019;94(2):92–9.
Sui Y, Zhang S, Li Y, Zhang X, Hu W, Feng Y, et al. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res Ther. 2020;11(1):1–13.
Maruyama CLM, Leigh NJ, Nelson JW, McCall AD, Mellas RE, Lei P, et al. Stem cell–soluble signals enhance multilumen formation in SMG cell clusters. J Dent Res. 2015;94(11):1610–7.
Moskwa N, Mahmood A, Nelson DA, Altrieth AL, Forni PE, Larsen M. Single-cell RNA sequencing reveals PDFGRα+ stromal cell subpopulations that promote proacinar cell differentiation in embryonic salivary gland organoids. Development. 2022;149(6):dev200167.
Tao X, Chen Q, Li N, Xiang H, Pan Y, Qu Y, et al. Serotonin-RhoA/ROCK axis promotes acinar-to-ductal metaplasia in caerulein-induced chronic pancreatitis. Biomed Pharmacother. 2020;125: 109999.
Baker OJ, Schulz DJ, Camden JM, Liao Z, Peterson TS, Seye CI, et al. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng Part C Methods. 2010;16(5):1135–44.
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86.
Borkent D, Moharamzadeh K. Tissue engineering of salivary glands. In: Biomaterials for oral and dental tissue engineering. Amsterdam: Elsevier; 2017. p. 337–51.
Nam K, Jones JP, Lei P, Andreadis ST, Baker OJ. Laminin-111 peptides conjugated to fibrin hydrogels promote formation of lumen containing parotid gland cell clusters. Biomacromol. 2016;17(6):2293–301.
Farach-Carson MC, Brown AJ, Lynam M, Safran JB, Carson DD. A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol. 2008;27(2):150–60.
Maria OM, Liu Y, El-Hakim M, Zeitouni A, Tran SD. The role of human fibronectin-or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J Tissue Eng Regen Med. 2017;11(9):2643–57.
Gao Z, Wu T, Xu J, Liu G, Xie Y, Zhang C, et al. Generation of bioartificial salivary gland using whole-organ decellularized bioscaffold. Cells Tissues Organs. 2014;200(3–4):171–80.
Lilliu MA, Seo YJ, Isola M, Charbonneau AM, Zeitouni A, El-Hakim M, et al. Natural extracellular matrix scaffolds recycled from human salivary digests: a morphometric study. Oral Dis. 2016;22(4):313–23.
Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med. 2010;5(6):961–70.
Patil SV, Nanduri LS. Interaction of chitin/chitosan with salivary and other epithelial cells—An overview. Int J Biol Macromol. 2017;104:1398–406.
Taketa H, Sathi GA, Farahat M, Rahman KA, Sakai T, Hirano Y, et al. Peptide-modified substrate for modulating gland tissue growth and morphology in vitro. Sci Rep. 2015;5(1):1–9.
Yang TL, Hsiao YC. Chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Biomaterials. 2015;66:29–40.
Yang TL, Young TH. The enhancement of submandibular gland branch formation on chitosan membranes. Biomaterials. 2008;29(16):2501–8.
Yang TL, Young TH. Chitosan cooperates with mesenchyme-derived factors inregulating salivary gland epithelial morphogenesis. J Cell Mol Med. 2009;13(9a):2853–63.
Zhang BX, Zhang ZL, Lin AL, Wang H, Pilia M, Ong JL, et al. Silk fibroin scaffolds promote formation of the ex vivo niche for salivary gland epithelial cell growth, matrix formation, and retention of differentiated function. Tissue Eng Part A. 2015;21(9–10):1611–20.
Charbonneau AM, Tran SD. 3D cell culture of human salivary glands using nature-inspired functional biomaterials: the egg yolk plasma and egg white. Materials. 2020;13(21):4807.
Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5(7):539–51.
Sequeira SJ, Soscia DA, Oztan B, Mosier AP, Jean-Gilles R, Gadre A, et al. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials. 2012;33(11):3175–86.
Peters SB, Naim N, Nelson DA, Mosier AP, Cady NC, Larsen M. Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng Part A. 2014;20(11–12):1632–42.
Negrini NC, Volponi AA, Higgins CA, Sharpe PT, Celiz AD. Scaffold-based developmental tissue engineering strategies for ectodermal organ regeneration. Mater Today Bio. 2021;10:100107.
Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, et al. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4(1):1–10.
Aframian DJ, Cukierman E, Nikolovski J, Mooney DJ, Yamada KM, Baum BJ. The growth and morphological behavior of salivary epithelial cells on matrix protein-coated biodegradable substrata. Tissue Eng. 2000;6(3):209–16.
Sun T, Zhu J, Yang X, Wang S. Growth of miniature pig parotid cells on biomaterials in vitro. Arch Oral Biol. 2006;51(5):351–8.
Cantara SI, Soscia DA, Sequeira SJ, Jean-Gilles RP, Castracane J, Larsen M. Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials. 2012;33(33):8372–82.
Khan E, Farooq I, Khabeer A, Ali S, Zafar MS, Khurshid Z. Salivary gland tissue engineering to attain clinical benefits: a special report. Regen Med. 2020;15(3):1455–61.
Mitroulia A, Gavriiloglou M, Athanasiadou P, Bakopoulou A, Poulopoulos A, Panta P, et al. Salivary gland stem cells and tissue regeneration: an update on possible therapeutic application. J Contemp Dent Pr. 2019;20:978–86.
Chen MH, Hsu YH, Lin CP, Chen YJ, Young TH. Interactions of acinar cells on biomaterials with various surface properties. J Biomed Mater Res Part Off J Soc Biomater Jpn Soc Biomater Aust Soc Biomater Korean Soc Biomater. 2005;74(2):254–62.
Soscia DA, Sequeira SJ, Schramm RA, Jayarathanam K, Cantara SI, Larsen M, et al. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials. 2013;34(28):6773–84.
Shin HS, Kook YM, Hong HJ, Kim YM, Koh WG, Lim JY. Functional spheroid organization of human salivary gland cells cultured on hydrogel-micropatterned nanofibrous microwells. Acta Biomater. 2016;45:121–32.
Foraida ZI, Kamaldinov T, Nelson DA, Larsen M, Castracane J. Elastin-PLGA hybrid electrospun nanofiber scaffolds for salivary epithelial cell self-organization and polarization. Acta Biomater. 2017;62:116–27.
Sfakis L, Kamaldinov T, Khmaladze A, Hosseini ZF, Nelson DA, Larsen M, et al. Mesenchymal cells affect salivary epithelial cell morphology on PGS/PLGA core/shell nanofibers. Int J Mol Sci. 2018;19(4):1031.
Miyajima H, Matsumoto T, Sakai T, Yamaguchi S, An SH, Abe M, et al. Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials. 2011;32(28):6754–63.
Chan YH, Huang TW, Chou YS, Hsu SH, Su WF, Lou PJ, et al. Formation of post-confluence structure in human parotid gland acinar cells on PLGA through regulation of E-cadherin. Biomaterials. 2012;33(2):464–72.
Yamada Y, Hozumi K, Aso A, Hotta A, Toma K, Katagiri F, et al. Laminin active peptide/agarose matrices as multifunctional biomaterials for tissue engineering. Biomaterials. 2012;33(16):4118–25.
Dos Santos HT, Nam K, Brown CT, Dean SM, Lewis S, Pfeifer CS, et al. Trimers conjugated to fibrin hydrogels promote salivary gland function. J Dent Res. 2021;100(3):268–75.
Lee S-W, Ryu JH, Do MJ, Namkoong E, Lee H, Park K. NiCHE platform: nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl Mater Interfaces. 2020;12(4):4285–94.
Campbell JJ, Watson CJ. Three-dimensional culture models of mammary gland. Organogenesis. 2009;5(2):43–9.
Charbonneau AM, Al-Samadi A, Salo T, Tran SD. 3D culture histology cryosectioned well insert technology preserves the structural relationship between cells and biomaterials for time-lapse analysis of 3D cultures. Biotechnol J. 2019;14(11):1900105.
Mantha S, Pillai S, Khayambashi P, Upadhyay A, Zhang Y, Tao O, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323.
Adine C, Ferreira J. Bioprinting strategies to engineer functional salivary gland organoids. In: Organ tissue engineering. Cham: Springer; 2020. p. 1–22.
Maria OM, Maria AM, Cai Y, Tran SD. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012;18(2):162–8.
Zhang Y, Pham HM, Munguia-Lopez JG, Kinsella JM, Tran SD. The optimization of a novel hydrogel—egg white-alginate for 2.5 D tissue engineering of salivary spheroid-like structure. Molecules. 2020;25(23):5751.
Jorgensen M, Ramesh P, Toro M, Evans E, Moskwa N, Zhang X, et al. Alginate hydrogel microtubes for salivary gland cell organization and cavitation. Bioengineering. 2022;9(1):38.
Abdel Fattah AR, Meleca E, Mishriki S, Lelic A, Geng F, Sahu RP, et al. In situ 3D label-free contactless bioprinting of cells through diamagnetophoresis. ACS Biomater Sci Eng. 2016;2(12):2133–8.
Turker E, Arslan-Yildiz A. Recent advances in magnetic levitation: a biological approach from diagnostics to tissue engineering. ACS Biomater Sci Eng. 2018;4(3):787–99.
Parfenov VA, Koudan EV, Bulanova EA, Karalkin PA, Pereira FD, Norkin NE, et al. Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication. 2018;10(3): 034104.
Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5(4):291–6.
Charbonneau AM, Kinsella JM, Tran SD. 3D cultures of salivary gland cells in native or gelled egg yolk plasma, combined with egg white and 3D-printing of gelled egg yolk plasma. Materials. 2019;12(21):3480.
Pradhan-Bhatt S, Harrington DA, Duncan RL, Farach-Carson MC, Jia X, Witt RL. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope. 2014;124(2):456–61.
Martinez M, Witt RL, Farach-Carson MC, Harrington DA. Functionalized biomimetic hydrogels enhance salivary stem/progenitor cell organization. bioRxiv. 2021;70:7.
Fowler EW, Venrooy EV, Witt RL, Jia X. TGFβR inhibition represses TGF-β1 initiated keratin-7 expression in human salivary gland progenitor cells. bioRxiv. 2021;4:1–361.
Song Y, Uchida H, Sharipol A, Piraino L, Mereness JA, Ingalls MH, et al. Development of a functional salivary gland tissue chip with potential for high-content drug screening. Commun Biol. 2021;4(1):1–15.
Shin K, Koo KH, Jeong J, Park SJ, Choi DJ, Ko YG, et al. Three-dimensional culture of salivary gland stem cell in orthotropic decellularized extracellular matrix hydrogels. Tissue Eng Part A. 2019;25(19–20):1396–403.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
Song EAC, Min S, Oyelakin A, Smalley K, Bard JE, Liao L, et al. Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci Rep. 2018;8(1):1–15.
Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213).
Liu F, Wang S. Molecular cues for development and regeneration of salivary glands. Histol Histopathol. 2014;29(3):305.
Hauser BR, Aure MH, Kelly MC, Hoffman MP, Chibly AM. Generation of a single-cell RNAseq atlas of murine salivary gland development. Iscience. 2020;23(12): 101838.
Lu P, Sternlicht MD, Werb Z. Comparative mechanisms of branching morphogenesis in diverse systems. J Mammary Gland Biol Neoplasia. 2006;11(3–4):213–28.
Gregory CA, Lee RH, Liu F, Alge D. Approaches that foster a pro-regenerative environment. Front Bioeng Biotechnol. 2022;10.
Konar D, Devarasetty M, Yildiz DV, Atala A, Murphy SV. Lung-on-a-chip technologies for disease modeling and drug development: supplementary issue: image and video acquisition and processing for clinical applications. Biomed Eng Comput Biol. 2016;7:BECB-S34252.
Kim S, Takayama S. Organ-on-a-chip and the kidney. Kidney Res Clin Pract. 2015;34(3):165–9.
Chan HF, Zhao R, Parada GA, Meng H, Leong KW, Griffith LG, et al. Folding artificial mucosa with cell-laden hydrogels guided by mechanics models. Proc Natl Acad Sci. 2018;115(29):7503–8.
Hörner M, Raute K, Hummel B, Madl J, Creusen G, Thomas OS, et al. Phytochrome-based extracellular matrix with reversibly tunable mechanical properties. Adv Mater. 2019;31(12):1806727.
Liu L, Shadish JA, Arakawa CK, Shi K, Davis J, DeForest CA. Cyclic stiffness modulation of cell-laden protein–polymer hydrogels in response to user-specified stimuli including light. Adv Biosyst. 2018;2(12):1800240.
Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L, et al. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun. 2018;9(1):1–10.
Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV. Dynamics of mechanosensitive neural stem cell differentiation. Stem Cells. 2017;35(2):497–506.
Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–29.
Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell. 2018;22(6):929–40.
Sidar B, Jenkins BR, Huang S, Spence JR, Walk ST, Wilking JN. Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip). Lab Chip. 2019;19(20):3552–62.
Pringle S, Van Os R, Coppes RP. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2013;31(4):613–9.
Yoo C, Vines JB, Alexander G, Murdock K, Hwang P, Jun HW. Adult stem cells and tissue engineering strategies for salivary gland regeneration: a review. Biomater Res. 2014;18(1):1–12.
Chibly AM, Aure MH, Patel VN, Hoffman MP. Salivary gland function, development and regeneration. Physiol Rev. 2022.
Wu D, Lombaert IM, DeLeon M, Pradhan-Bhatt S, Witt RL, Harrington DA, et al. Immunosuppressed miniswine as a model for testing cell therapy success: experience with implants of human salivary stem/progenitor cell constructs. Front Mol Biosci. 2021;8.
Zhao C, Meng C, Cui N, Sha J, Sun L, Zhu D. Organoid models for salivary gland biology and regenerative medicine. Stem Cells Int. 2021;2021.
Acknowledgements
The authors gratefully acknowledge Université Libre de Bruxelles, the Fonds de la Recherche Scientifique—FNRS and the Fonds de la Recherche du Québec—Santé for their financial support. Figures were created using BioRender.com.
Funding
This research was funded by Université Libre de Bruxelles, the Fonds de la Recherche Scientifique—FNRS (Fund for Scientific Research), grant number PINT-BILAT-P-R.P006.19, the Fonds de la Recherche du Québec—Santé, grant number 281271.
Author information
Authors and Affiliations
Contributions
M.H was involved in conceptualization, writing of the original draft, constructing figures and tables, and reviewing and editing. C.DA was involved in the writing of the original draft and Figures. J.S was involved in constructing figures and conceptualization. S.D.T, A.S, and C.D. were involved in conceptualization, reviewing, and editing. All authors contributed to the article and approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hajiabbas, M., D’Agostino, C., Simińska-Stanny, J. et al. Bioengineering in salivary gland regeneration. J Biomed Sci 29, 35 (2022). https://doi.org/10.1186/s12929-022-00819-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-022-00819-w