Abstract
Background
To explore the potential role of m6A methylation modification in Wilms Tumor (WT) by m6A-RNA Methylation (m6A) regulators.
Methodology
The association of m6A modification patterns with immune and prognostic characteristics of tumors was systematically evaluated using 19 m6A regulators extracted from Wilms Tumor’s samples in public databases. A comprehensive model of “m6Ascore” was constructed using principal component analysis, and its prognostic value was evaluated.
Results
Almost all m6A regulators were differentially expressed between WT and normal tissues. Unsupervised clustering identified three distinct m6A clusters that differed in both immune cell infiltration and biological pathways. The m6Ascore was constructed to quantify m6A modifications in individual patients. Our analysis suggests that m6Ascore is an independent prognostic factor for WT and can be used as a novel predictor of WT prognosis.
Conclusions
This study comprehensively explored and systematically characterized m6A modifications in WT. m6A modification patterns play a critical role in the tumor immune microenvironment (TIME) and WT prognosis. m6Ascore provides a more comprehensive understanding of m6A modifications in WT and offers a practical tool for predicting WT prognosis. This study will help clinicians to identify valid indicators of WT to improve the poor prognosis of this disease.
Supplementary Information
The online version contains supplementary material available at https://www.aliyundrive.com/drive/folder/64be739cd6956a741fb24670baeea53422be6024.
Similar content being viewed by others
Introduction
Renal tumors are the fifth most common tumor in children, and Wilms Tumor (WT) is the most common renal malignancy in children [1]. The long-term survival rate of WT has steadily increased over the past decades to 85% and has even reached 90% in developed countries [2,3,4]. However, nearly 15% of patients still experience recurrence and associated complications [5,6,7]. Genes such as WT1, WT2 and MYCN family have been suggested to be involved in the development of WT, and the detection of CTR9, DICER1, REST, TP53, TRIM28 and WT1 have been recommended as effective predictors of WT [3]. In addition, SPRY1, SPIN4, MAP7D3, C10orf71, and SPAG have also been analyzed for WT [8]. Recent studies suggest that cholesterol markers and methylation modifications may potentially influence WT [9, 10]. However, none of these studies could be included in the individualized assessment of specific patients. Newly discovered markers in WT are increasing, and these may eventually play a role in targeted therapies; unfortunately, only one genetic biomarker, LOH at chromosome 1p/16q, has been used in clinical therapy [11]. Thus, there is an urgent need to find effective biomarkers to predict the prognosis of WT and to develop new targets for WT therapy.
RNA modifications are universal post-transcriptional modifications that play a crucial role in biological regulation [12, 13]. N6-methyladenosine (m6A) is the most abundant epigenetic-transcriptomic modification in eukaryotic mRNAs [14]. As a reversible RNA modification, m6A plays an important role in the regulation of biological processes such as RNA degradation and splicing [15]. There is increasing evidence that m6A modifications play an important role in tumorigenesis and tumor regulation. METTL14, a regulator of m6A, has been reported to affect WT progression and prognosis by regulating related gene expression and splicing patterns [16], and three different multicenter case-control studies have suggested that m6A regulators ALKBH5, YTHDF1 and YTHDF2 all affect WT progression and prognosis to some extent [17,18,19]. Recently, m6A alterations have been shown to be present and to influence tumorigenesis and prognosis in a variety of tumors, including colon, lung, pancreatic, cervical, ovarian, nasopharyngeal, and prostate cancers [20,21,22,23,24,25,26].
In the present study, we systematically evaluated m6A modification patterns and the tumor immune microenvironment (TIME) in WT patients. We identified three distinct m6A modification patterns in WT; these clusters differed significantly in prognosis, immune cell infiltration and biological pathways. Based on m6A regulators and related genes, we constructed a model (called “m6Ascore”) to quantify the m6A modification patterns in individual patients. This study also suggests that m6Ascore may be a new practical tool to predict the prognosis of WT.
Materials and methods
Data acquisition and processing
The RNA sequencing data of Wilms Tumor (2018) were obtained from the Cancer Genome Atlas database (TCGA, https://portal.gdc.cancer.gov/). Gene expression data (measured in fragments per kilobase of exon per million fragments mapped or FPKM) were converted to transcripts per kilobase million (TPM).
Analysis of m6A regulators in Wilms Tumor
Based on previous studies, we obtained 19 m6A regulators [27]. These regulators included 7 “Writer” (METTL3, METTL14, WTAP, VIRMA, METTL16, RBM15, and RBM15B), 9 “Reader” (YTHDF1, YTHDF2, YTHDF3,YTHDC1,YTHDC2,IGF2BP1,IGF2BP2,IGF2BP3 and HNRNPA2B1), and 3 “Eraser” (FTO,ALKBH5 and ALKBH1). The expression profiles of these regulators were systematically extracted and analyzed in normal and tumor samples. Somatic mutations of WT were assessed using the R package “maftools”. Tumor mutational burden (TMB) was calculated and the correlation of TMB with clinical features was evaluated. The prognostic value of m6A regulators was assessed using Kaplan-Meier (KM) curves and log-rank tests.
Clustering analysis based on m6A regulators
Based on the expression of m6A regulators, unsupervised clustering was performed using the “ConsensusClusterPlus” R package root to identify different m6A modification patterns in WT patients, and the stability of the clusters was ensured by 1,000 replicates. Survival analysis of different clusters was performed using the KM method. Differences in biological processes between clusters were investigated by gene set variation analysis (GSVA) using the R package “GSVA”. The gene set “c2.cp.kegg. v7.4. symbol” was obtained from the Molecular Signatures Database (MSigDB). An adjusted p-value < 0.05 was considered statistically significant.
Comparison of tumor immune microenvironment between different m6A clusters
Single sample gene set enrichment analysis (ssGSEA) was used to quantify the relative infiltration levels of 29 immune cell types in WT samples. The proportion of immune stromal components in the tumor microenvironment (TME) was measured using the " estimate” R package. Wilcoxon rank sum test was used to analyze differences in TME between clusters. In addition, the “limma” R package was used to examine differences in the expression of molecules such as targeted immune checkpoints between different functional clusters.
Prognostic differential expression of genes between different m6A clusters
Principal component analysis (PCA) was used to investigate the different m6A modification patterns in WT. The empirical Bayesian approach was used to extract the differentially expressed genes (DEGs) between the different m6A clusters. The significance criterion for DEGs was set to adjusted p-value < 0.05. Gene Ontology (GO) biological process analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were used to explore the rich functional annotation of DEGs [28]. Univariate Cox regression analysis was performed to examine the prognostic value of each DEG. Significance criteria were set at p-value < 0.05 and abs (logFC) > 1.
Construction of m6Ascore
A PCA-based scoring system (called “m6Ascore”) was constructed to quantify the m6A modification pattern of individual WT patients. Principal components 1 and 2 and 3 were selected as signature scores. m6Ascore was defined using a method similar to the Genomic Grade Index (GGI) [29, 30]: m6Ascore=∑(PC1i + PC2i + PC3i), where i is the expression of DEG with prognostic efficacy in different m6A clusters. The samples were divided into high and low m6Ascore groups based on the scores. Correlation analysis was performed to investigate the relationship between m6Ascore and a number of relevant biological pathways, including immune correlation analysis, clinical correlation analysis, TMB and targeted immune checkpoint molecules. The prognostic value of m6Ascore was assessed using KM curves. Univariate and multivariate independent prognostic analyses were performed to evaluate whether the model was an independent prognostic factor for WT.
Statistical analysis
All statistical analyses were performed with R software (version 4.1.2). Wilcox test was used to compare m6A regulators expression levels in WT tissues. Patient survival was dichotomized for continuous variables using optimal cutoff values determined by the R package “survminer”. For prognostic analysis, survival curves were constructed using the KM method, and log-rank tests were used to determine the significance of differences. Receiver operating characteristic (ROC) curves (R package “timeROC”) and area under the curve (AUC) values were used to assess the prognostic value of the m6Ascore. Univariate and multivariate independent prognostic analyses were performed to evaluate whether the model was an independent prognostic factor for WT. All statistical p-values were two-sided, and p < 0.05 was considered statistically significant.
Results
Differential expression of m6A regulators in Wilms Tumor
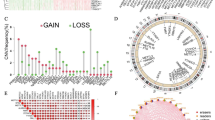
We obtained expression data from the TCGA database for a total of 132 samples, including 6 normal tissues and 126 tumor tissues. The results showed significant differences in the expression of almost all m6A regulators between tumor and normal tissues, with the majority of m6A regulators being upregulated in WT tissues (P < 0.001), while ALKBH5 and YTHDC1 were downregulated in WT expression levels (Fig. 1.A). In addition, our results showed that TMB in WT patients differed with different clinical characteristics, such as younger age group and diffusely anaplastic Wilms Tumor (DAWT) had higher TMB (Fig. 1.B-C).
Univariate COX regression analysis and KM showed that m6A modulators were potential prognostic factors for WT patients, such as RBM15, WTAP and YTHDF2 showed high tumorigenicity (Fig. 2.A-E). There was also a significant positive correlation between each m6A modulator (Fig. 2.F). In conclusion, m6A regulators showed significant heterogeneity and differential expression in WT tissues compared with normal tissues, and m6A regulators may play a critical role in the development and progression of WT.
m6A modification patterns based on m6A regulators
Based on the expression of 19 m6A regulators, unsupervised cluster analysis was performed and three different m6A modification patterns (clusters 1–3) were identified, with 39 cases in cluster1, 25 cases in cluster2 and 62 cases in cluster3 (Fig. 3.A). Survival analysis showed some differences in prognosis between the different modification patterns (Fig. 3.B). Further analysis showed differences in regulator expression in the 3 different m6A alteration patterns (Fig. 3.C). In addition, we grouped the three different modification patterns into two vehicles for GSVA analysis, and the results showed significant differences in biological behavior between the different modification patterns (Fig. 3.D-F).
Immunological characteristics of different m6A modification patterns
The infiltration of 29 immune cells was analyzed by ssGSEA in different m6A modification patterns. The results showed that the immune infiltration was significantly different between the different clusters (Fig. 4.A). The results obtained by the ESTIMATE algorithm show a significant difference in StromalScore between cluster3 and cluster1, with Cluster3 having a higher StromalScore. (Fig. 4.B-D). In addition, we analyzed the expression levels of immune checkpoints, immune cell markers and WT metabolites in different clusters [3]. Among the immune checkpoints, cluster1 had higher expression of CD274 and PDCD1, while cluster3 had higher expression of LAG3; the star molecule in WT metabolites, GPC3, was expressed in the 3 clusters, and cluster2 was higher than the other two groups (Fig. 5).
Generation of the m6Ascore model
PCA analysis showed that there were different m6A modification patterns in WT patients (Fig. 6). GO enrichment analysis and KEGG pathway analysis of different m6A clusters using the R package “clusterProfiler” showed that DEGs were enriched in biological processes related to tumorigenesis and tumor progression, such as rRNA metabolic processes and cell cycle (Fig. 7.A-D).
Univariate Cox regression analysis was used to understand the prognostic value of each DEG, and a total of 284 DEGs with prognostic utility were screened to construct m6Ascore, and the scores were grouped into high and low scores based on the best critical value. The association between m6A modification pattern and m6Ascore was analyzed using analysis of variance, and the results showed that cluster1 had a lower score of m6Ascore (Fig. 8.A). The KM method was used to show that the subgroup with low m6Ascore had a worse prognosis (Fig. 8.B). We used univariate and multivariate Cox regression analysis including gender, age, tumor type, m6Ascore and tumor stage to confirm that m6Ascore was an independent prognostic factor for WT (Fig. 8.C-D). Unfortunately, the ROC curves showed that the predictive power of the m6Ascore model for survival outcomes at 1, 2, 3, and 4 years in the WT patients was poor (Fig. 8.E). We further selected a WT data set of m6A methylation modifications, GSE167054, for differential analysis, and the results showed that m6A modifications differed between normal and WT tissues (Fig. 8.F). This validated the reliability of the m6Ascore model to some extent.
To further understand the potential biological mechanisms of m6Ascore, we analyzed the correlation between m6Ascore and some biological processes. m6Ascore was closely associated with the infiltration of immune cells, such as a significant positive correlation with dendritic cells and T cells, and a significant negative correlation with neutrophils (Fig. 9.A). However, there was no significant correlation between TMB and m6Ascore (Fig. 9.B). In addition, our results showed that patients with low m6Ascore had a higher percentage of death (Fig. 9.C). Our results also showed significant differences in the expression of immune checkpoints, immune cell markers, and tumor metabolic markers in high and low m6Ascore groups, such as CCL2, CD8A, CD68, CTLA4, HAVCR2, and PDCD1LG2 were highly expressed in the high m6Ascore group, while two immune checkpoints, CD276 and PDCD1, were highly expressed in the low m6Ascore group (Fig. 10). This suggests that m6Ascore may become a new evaluation index in immune-targeted therapy.
Discussion
Currently, an increasing number of studies have shown that m6A modifications play an important role in tumorigenesis and progression [31,32,33]. In general, m6A plays an important role in tumorigenesis and progression by regulating mRNA stability, expression and translation [34]. For example, METTL3 can accelerate the metastasis and radiation resistance of Glioblastoma by enhancing the stability of SOX2. For example, METTL3 can accelerate the metastasis and radiation resistance of Glioblastoma by enhancing the stability of SOX2 leading to malignant events [35]; decreased m6A methylation attenuates the expression of the AKT negative regulator PHLPP2, while increasing the expression of the AKT positive regulator mTORC2 [36]. The pathogenesis of Wilms Tumor is still not very clear, and recent several multicenter case studies have suggested that m6A modification patterns may contribute to the development and progression of WT [17,18,19]. However, most of these studies were limited to single or intergenic studies and did not systematically analyze the impact of m6A regulator-related modification patterns on tumor progression and prognosis.
For the first time, we constructed a model based on m6A regulators to quantify the m6A modification patterns of individual patients, further revealing the potential prognostic value and therapeutic guidance of m6A modification and m6A regulators in WT. To investigate the role of m6A modifications in WT, we systematically analyzed the m6A modification patterns of WT samples extracted from public databases. Three different m6A modification patterns in WT were finally identified by an unsupervised clustering approach [37]. The different modification patterns showed significant differences in immune cell infiltration, biological pathways, and prognosis. To further quantify m6A modifications in individual patients, we constructed a model (‘’m6Ascore”) and demonstrated that this model is an independent prognostic factor for WT. Furthermore, our results suggest that m6A modifications are different in different patients and that related modulators may be a novel prognostic marker.
We found that almost all m6A regulators were expressed at significantly higher levels in WT tissues than in normal tissues, and a previous study suggested that the pathogenesis of WT may be related to changes in RNA methylation [9]. This suggests that altered m6A modifications are associated with the pathogenesis of WT. The three different modification patterns obtained by clustering analysis differed in terms of survival outcomes. m6A regulator expression was low but with poor survival gains in cluster2, and m6A regulator expression was higher and with better survival gains in cluster3. These results suggest that the expression levels of m6A regulators are closely related to tumor progression in WT. Considering the heterogeneity among m6A modifications, the m6Ascore model was constructed to quantify the m6A modification patterns of individual WT patients. Our results indicate that m6Ascore is an independent prognostic factor for WT patients. In addition, we found that m6Ascore was strongly associated with immune cell infiltration. m6Ascore was significantly positively correlated with dendritic cells and T cells and significantly negatively correlated with neutrophils.
A large number of existing studies have shown that the tumor microenvironment can not only influence tumor cell growth and metastasis, but also has great importance in therapy [38,39,40], in particular, the association between TME and methylation modifications and the influence of TME on tumor progression in WT has been reported [41, 42]. We quantified the infiltration of 29 different immune cell types in WT samples by ssGSEA analysis. The results showed a large difference in infiltration between different clusters. Among them, cluster2 had much lower immune infiltration than cluster1 and worse survival. Previous studies have also shown that a high degree of immune infiltration plays an anti-tumor role to some extent [43, 44].
Immune checkpoint inhibitor (ICI) therapies targeting pathways such as PD-1 and PD-L1 have been widely used in the treatment of tumors [45]. In particular, ICI therapies have revolutionized the way cancer is treated, offering new hope to a wide range of patients [46]. Our study found that the expression levels of these molecules were different in different WT patients. A similar situation was also seen in the m6Ascore model.
The present study still has the following limitations. First, due to various limitations, we could only assess immune cell infiltration based on algorithms and lack of actual experimental data. Second, due to the lack of data, there is a lack of corresponding validation cohorts and an inability to directly examine the actual immunotherapeutic response in the high and low m6Ascore groups. Similarly, we do not have enough clinical cohorts to validate the prognostic value of m6Ascore in WT, and prospective studies of large cohorts are lacking.
In this study, we systematically analyzed the expression characteristics of m6A-related regulators in WT. m6A modification patterns play an important role in the mechanism and prognosis of WT. Our study provides practical tools for predicting the prognosis of WT, and this study may help clinical practitioners to identify valid indicators of Wilms Tumor for the poor prognosis of this disease.
Data Availability
The raw data of this study are derived from the TCGA database (https://portal.gdc.cancer.gov/), the specific sample is TARGET-WT, which are provided on public databases.
References
Jemal A, et al. Cancer Stat 2006 CA Cancer J Clin. 2006;56:106–30.
Irtan S, Ehrlich PF, Pritchard-Jones K. Wilms tumor: state-of-the-art update, 2016. Semin Pediatr Surg. 2016;25:250–6.
Han C, Fu YX. β-Catenin regulates tumor-derived PD-L. 3.
Groenendijk A, et al. Prognostic factors for Wilms Tumor recurrence: a review of the literature. Cancers. 2021;13:3142.
Kalapurakal JA, et al. Management of Wilms’ tumour: current practice and future goals. Lancet Oncol. 2004;5:37–46.
Brok J, Treger TD, Gooskens SL, van den Heuvel-Eibrink MM, Pritchard-Jones K. Biology and treatment of renal tumours in childhood. Eur J Cancer. 2016;68:179–95.
Davidoff AM. Wilms Tumor. Adv Pediatr. 2012;59:247–67.
Lin X, et al. Identification of a five-mRNA signature as a novel potential prognostic biomarker in pediatric Wilms tumor. Mol Genet Genomic Med. 2020;8.
Jablonowski CM, et al. TERT expression in Wilms Tumor is regulated by promoter mutation or hypermethylation, WT1, and N-MYC. Cancers. 2022;14:1655.
He Y, et al. Using elevated cholesterol synthesis as a prognostic marker in Wilms’ Tumor: a bioinformatic analysis. BioMed Res Int. 2021;2021:1–9.
Theilen T-M, et al. Multidisciplinary treatment strategies for Wilms Tumor: recent advances, technical innovations and future directions. Front Pediatr. 2022;10:852185.
Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in Gene expression regulation. Cell. 2017;169:1187–200.
Boccaletto P, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–7.
He L, et al. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176.
Fazi F, Fatica A. Interplay between N6-Methyladenosine (m6A) and non-coding RNAs in Cell Development and Cancer. Front Cell Dev Biol. 2019;7:116.
Zhuo Z, et al. METTL14 gene polymorphisms decrease Wilms tumor susceptibility in chinese children. BMC Cancer. 2021;21:1294.
Liu Y, et al. Impact of YTHDF1 gene polymorphisms on Wilms tumor susceptibility: a five-center case‐control study. J Clin Lab Anal. 2021;35.
Hua R, et al. ALKBH5 gene polymorphisms and Wilms tumor risk in chinese children: a five-center case‐control study. J Clin Lab Anal. 2020;34.
Wang Z, et al. The contribution of YTHDF2 gene rs3738067 A > G to the Wilms tumor susceptibility. J Cancer. 2021;12:6165–9.
Liu Z, et al. Characterization of the m6A-Associated Tumor Immune Microenvironment in prostate Cancer to Aid Immunotherapy. Front Immunol. 2021;12:735170.
Liu Z, et al. m6A regulators mediated methylation modification patterns and Tumor Microenvironment Infiltration characterization in nasopharyngeal carcinoma. Front Immunol. 2022;12:762243.
Chang L-L, et al. Emerging role of m6A methylation modification in ovarian cancer. Cancer Cell Int. 2021;21:663.
Zhang W, et al. m6A Regulator-Mediated tumour infiltration and methylation modification in Cervical Cancer Microenvironment. Front Immunol. 2022;13:888650.
Yang C, Yu T, Li Q, Xie F, Lin Q. A signature based on m6A pattern and tumor microenvironment.
Sun M, et al. m6A methylation modification patterns and Tumor Microenvironment Infiltration characterization in pancreatic Cancer. Front Immunol. 2021;12:739768.
Fan Y, et al. m6A Regulator-Mediated methylation modification patterns and characterisation of Tumour Microenvironment Infiltration in Non-Small Cell Lung Cancer. J Inflamm Res. 2022;15:1969–89.
An Y, Duan H. The role of m6A RNA methylation in cancer metabolism. Mol Cancer. 2022;21:14.
Kanehisa M, Goto SKEGG. Kyoto Encyclopedia of Genes and Genomes.
Zeng D, et al. Tumor Microenvironment characterization in gastric Cancer identifies prognostic and immunotherapeutically relevant Gene Signatures. Cancer Immunol Res. 2019;7:737–50.
Sotiriou C, et al. Gene expression profiling in breast Cancer: understanding the molecular basis of histologic Grade to improve prognosis. JNCI J Natl Cancer Inst. 2006;98:262–72.
Chen Y, Lin Y, Shu Y, He J, Gao W. Interaction between N6-methyladenosine (m6A) modification and noncoding RNAs in cancer. Mol Cancer. 2020;19:94.
Yi L, Wu G, Guo L, Zou X, Huang P. Comprehensive Analysis of the PD-L1 and Immune Infiltrates of m6A RNA methylation regulators in Head and Neck squamous cell carcinoma. Mol Ther - Nucleic Acids. 2020;21:299–314.
Gao Y, et al. m6A modification prevents formation of endogenous double-stranded RNAs and deleterious Innate Immune responses during hematopoietic development. Immunity. 2020;52:1007–1021e8.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88.
Visvanathan A, et al. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–33.
Liu J, et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–83.
Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26:1572–3.
Peña-Romero AC, Orenes-Piñero E. Dual effect of Immune cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and their triggers. Cancers. 2022;14:1681.
Zeng Z, et al. Immune and stromal scoring system associated with tumor microenvironment and prognosis: a gene-based multi-cancer analysis. J Transl Med. 2021;19:330.
Eulberg D, Frömming A, Lapid K, Mangasarian A, Barak A. The prospect of tumor microenvironment-modulating therapeutical strategies. Front Oncol. 2022;12:1070243.
Zhang F, et al. Crosstalk among m6A RNA methylation, hypoxia and metabolic reprogramming in TME: from immunosuppressive microenvironment to clinical application. J Hematol Oncol J Hematol Oncol. 2022;15:84.
Tian X-M, et al. Immune-related gene signature associates with immune landscape and predicts prognosis accurately in patients with Wilms tumour. Front Immunol. 2022;13:920666.
Rossetti RAM, et al. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PLoS ONE. 2018;13:e0199034.
Eosinophils: methods and protocols. vol. 2241 (Springer US, 2021).
Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for Cancer Therapy. Immunity. 2016;44:1069–78.
Xie D, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. 2021;9:652–2.
Acknowledgements
We appreciate the free use of TCGA database.
Funding
Supported by Grant 2019GSF108120 from the Key Research and Development Plan of Shandong Province, and by Grant ZR2020MH076 from Natural Science Foundation of Shandong Province.
Author information
Authors and Affiliations
Contributions
CJ and HG contributed to the design of the study, and CJ drafting the paper. XL and MC contributed to acquisition of data and revision of the paper. WM contributed to analysis and interpretation of data and revising the paper critically. FS contributed to design, acquisition, analysis, interpretation of data and revising the paper critically. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not necessary. The data was from the public database.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jia, C., Gao, H., Ma, W. et al. Identification of the expression patterns and potential prognostic role of m6A-RNA methylation regulators in Wilms Tumor. BMC Med Genomics 16, 222 (2023). https://doi.org/10.1186/s12920-023-01660-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01660-2