Abstract
Background
Familial exudative vitreoretinopathy (FEVR) is a complex form of blindness-causing retinal degeneration. This study investigated the potential disease-causing variants in 20 Chinese families with FEVR.
Methods
All available family members underwent detailed ophthalmological examinations, including best-corrected visual acuity and fundus examination. All probands and most family members underwent fluorescein fundus angiography. Twenty probands underwent whole exome sequencing; 16 of them also underwent copy number variant and mitochondrial genome analysis. Bioinformatics analysis and Sanger sequencing of available family members were used to confirm the disease-causing gene variant.
Results
Twenty families were diagnosed with FEVR based on clinical symptoms, fundus manifestations, and fundus fluorescein angiography. Whole exome sequencing revealed 14 variants in NDP, FZD4, LRP5, and TSPAN12 genes among the 13 families. These variants were predicted to be damaging or deleterious according to multiple lines of prediction algorithms; they were not frequently found in multiple population databases. Seven variants had not previously been reported to cause FEVR: c.1039T>G p.(Phe347Val) in the FZD4 gene; c.1612C>T p.(Arg538Trp) and c.3237-2A>C in the LRP5 gene; and c.77T>A p.(Ile26Asn), c.170dupT p.(Leu57Phe fsTer60), c.236T>G p.(Met79Arg) and c.550dupA p.(Arg184Lys fsTer16) in the TSPAN12 gene. We did not detect any variants in the remaining seven families.
Conclusions
These results expand the spectrum of variants in the NDP, FZD4, LRP5, and TSPAN12 genes and provide insights regarding accurate diagnosis, family genetic counseling, and future gene therapy for FEVR.
Similar content being viewed by others
Background
Familial exudative vitreoretinopathy (FEVR, OMIM: 133780) is a clinically and genetically heterogeneous inherited ophthalmic disorder [1, 2]. It is characterized by incomplete retinal vascular development and pathological neovascularization [3]. Patients usually complain of reduced visual acuity or blindness in early childhood. The fundus can exhibit peripheral retinal avascularization, falciform retinal folds, macular ectopia, retinal exudate, retinal neovascularization, and retinal detachment [4]. However, some patients may not complain of any visual impairment; they may only exhibit peripheral avascularization [5]. The reported prevalence is approximately 0.11% in newborns [6], but, the actual prevalence may be underestimated because some patients are asymptomatic and demonstrate peripheral retinal involvement only [7].
FEVR can be inherited in autosomal dominant, autosomal recessive, or X-linked manners; the most common mode of inheritance is autosomal dominant [8]. Thus far, the following eleven genes have been reported to cause FEVR: norrin (NDP, OMIM, 300658) [9], frizzled 4 (FZD4, OMIM, 604579) [10], low density lipoprotein receptor-related protein 5 (LRP5, OMIM, 603506) [11], tetraspanin 12 (TSPAN12, OMIM, 613310) [12], catenin beta 1 (CTNNB1, OMIM, 116806) [13], zinc finger protein 408 (ZNF408, OMIM, 616454) [14], atonal homolog 7 (ATOH7, OMIM, 609875) [15], kinesin family member 11 (KIF11, OMIM, 148760) [16], RCC1 and BTB domain containing protein 1 (RCBTB1, OMIM, 607867) [17], jagged 1 (JAG1, OMIM, 601920) [18], and α-catenin (CTNNA1, OMIM) [19]. Moreover, one locus, EVR3, which maps to 11p13-p12, can also lead to FEVR; its causative gene has not been fully identified [20]. Among these pathogenic genes, FZD4, LRP5, and TSPAN12 are the most common disease-causing genes related to FEVR [21]. The first five genes are involved in the Norrin or Wnt/β-catenin signaling pathway and have functions in cell adhesion, migration, and signaling [22].
Although increasing numbers of gene variants have been identified using next generation sequencing technology, these reported gene variants are responsible for only 50–60% of FEVR cases. Moreover, some patients may exhibit rapid progression without correct diagnosis and intervention. Thus, it is imperative to ascertain genetic etiology and achieve accurate diagnosis for affected patients, especially patients who are asymptomatic and exhibit peripheral retinal involvement alone. In this study, 20 families were diagnosed with FEVR based on clinical manifestations. We performed whole exome sequencing of probands and Sanger sequencing of available family members to elaborate the underling disease-causing gene variant.
Methods
Clinical examinations
Detailed premature delivery history, oxygen uptake history, family history, and birth weight information were acquired for the probands. Exhaustive ophthalmological examinations were completed, including best-corrected visual acuity, intraocular pressure, slit-lamp microscopy, ophthalmoscopy, fundus photography, and fundus fluorescein angiography (FFA). All participants underwent pupillary dilation with a mixture of 0.5% phenylephrine hydrochloride and 0.5% tropicamide eye drops (Santen Pharmaceutical, Osaka, Japan). Fundus photography was performed with a VISUCAM 200 digital fundus camera (Carl Zeiss Meditec AG, Jena, Thuringia, Germany) or Optos Daytona ultrawide field system (Optos PLC, Dunfermline, United Kingdom). FFA was acquired using SPECTRALIS Engineering systems (Heidelberg Engineering Ltd, Hertfordshire, United Kingdom) or Optos Daytona ultrawide field system (Optos PLC) with 20% fluorescein solution (Guangzhou Pharmaceutical Holdings Limited, Guangzhou, China). FEVR was diagnosed based on previously reported criteria [23].
Whole exome sequencing
Genomic DNA samples were prepared from peripheral blood of all participants using a TIANamp Genomic DNA Kit (TIANGEN Biotech, Beijing, China). Library construction was performed from proband samples using xGen Exome Research Panel (Integrated DNA Technologies, Coralville, Iowa, United States). Samples were sequenced on a HiSeq platform (Illumina, San Diego, California, United States) or MGISEQ-T7 platform (BGI Genomics, Shenzhen, Guangdong, China) using a whole exome sequencing protocol, in accordance with the manufacturer’s instructions. Sequence data were analyzed for corresponding ophthalmologic inherited genes, especially inherited retinal disease genes; sequences were aligned using Burrows-Wheeler Aligner (http://bio-bwa.sourceforge.net/). Variant calling and nomenclature complied with the recommendations of the Human Genome Variation Society (http://www.hgvs.org/). Variant annotation was performed in accordance with American College of Medical Genetics (ACMG, https://www.acmg.net/) guidelines.
Raw reads filtering
First, quality control was carried out for the enriched genes to filter out reads that did not meet quality control criteria. After whole exome sequencing, raw reads were filtered to remove duplicates, then aligned to the hg19 (GRCh37) human genome reference sequence. Quality control was recalibrated by Picard Mark Duplicates (http://sourceforge.net/projects/picard/), Genome Analysis Toolkit (https://gatk.broadinstitute.org/hc/en-us), and SAM tools (http://samtools.sourceforge.net/). Variants were validated and analyzed preferentially if they met the following previously reported criteria [24,25,26]: (1) minor allele frequency of the variant < 0.01 in the 1000 Genomes Project database (http://www.internationalgenome.org/), Exome Aggregation Consortium database (http://exac.broadinstitute.org/), Genome Aggregation database (http://gnomad.broadinstitute.org/), and an in-house Chinese individuals database; (2) variant location in an exon region or canonical splicing intron region that affected transcription splicing; (3) damaging or deleterious variant prediction using Sorting Intolerant From Tolerant (http://sift.jcvi.org/), Protein Variation Effect Analyzer (http://provean.jcvi.org), Polymorphism Phenotyping (http://genetics.bwh.harvard.edu/pph2/), ClinPred (https://sites.google.com/site/clinpred/), Likelihood Ratio Test (http://www.genetics.wustl.edu/jflab/lrt), Mutation Taster (http://www.mutationtaster.org/), Mutation Assessor (http://mutationassessor.org/r3), and Functional Analysis Through Hidden Markov Models (http://fathmm.biocompute.org.uk/); (4) highly conserved variant prediction using Genomic Evolutionary Rate Profiling (http://mendel.stanford.edu/SidowLab/downloads/gerp); (5) other reported pathogenic variant that did not meet the above criteria (e.g., high minor allele frequency variant, deep-intronic variant, or synonymous single nucleotide variant).
In silico analysis
Preferentially selected variants were validated and cosegregated by Sanger sequencing, performed using an 3500xL Dx Genetic Analyser (Applied Biosystems, Foster City, California, United States) with ABI BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). Primers were designed with Primer3 (http://primer3.ut.ee/) to amplify NDP, FZD4, LRP5, and TSPAN12 gene fragments. The primer information is provided in Additional file 1: Table S1. Consensus sequences corresponding to proband sequences were downloaded from national center for biotechnology information (https://www.ncbi.nlm.nih.gov/). All sequences were analyzed using SeqMan II software in the Lasergene software package (DNASTAR, Madison, Wisconsin, United States). Evolutionary conservation among different species for single nucleotide variants were analyzed using MegAlign software in Lasergene software package (DNASTAR). Genomic and protein structures were schematically represented using IBS 1.0 software (http://ibs.biocuckoo.org).
Results
Clinical manifestations
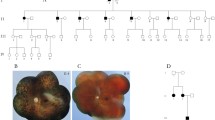
All participants had no history of premature delivery, problems with oxygen uptake, or low birth weight. They had no systematic complaints or extraophthalmic abnormalities that could be identified through conventional examinations (e.g., short stature or microcephaly). All probands complained of reduced visual acuity. Fundus examination of both eyes showed increased numbers of peripheral retinal vessels with willow-like retinal vessels. FFA demonstrated non-perfusion areas, retinal leakage, retinal avascularization, straightened vessels, and increased numbers of vessels. FFA manifestations of probands with potential disease-causing variants are presented in Fig. 1. The ophthalmic features of all probands with potential disease-causing variants are summarized in Table 1. Based on clinical manifestations, they were diagnosed with FEVR.
Sequencing and in silico analysis results
After filtering, the cleaned data of all probands met the quality control criteria. The quality control data for the probands are summarized in Additional file 1: Table S2. In total, 20 probands underwent whole exome sequencing; 16 probands also underwent copy number variation and mitochondrial genome analysis. None of the 16 probands harbored any copy number variant or mitochondrial genome variant. Through bioinformatics analysis and Sanger sequencing, we detected 14 variants in the NDP, FZD4, LRP5, and TSPAN12 genes among the 13 families; seven variants had not previously been reported to cause FEVR. We did not detect any variants in the remaining seven families. Variant c.981G>A in the FZD4 gene did not cosegregate with the disease in family 3, possibly because it was a de novo variant or the proband’s biological parents exhibited chimerism. Because peripheral blood samples from family 5 were unavailable, we could not complete the cosegregation analysis. Sanger sequencing of the remaining 11 families revealed that the variants cosegregated with the FEVR phenotypes in these families. Sanger sequencing chromatographs of the variants are shown in Fig. 2. The pedigrees and cosegregation of families are presented in Additional file 1: Figure S1. Variant information for the NDP, FZD4, LRP5, and TSPAN12 genes is summarized in Table 2. The variants were located in different exons or intron of the NDP, FZD4, LRP5, and TSPAN12 genes; they were predicted to affect the various domains of the NDP, FZD4, LRP5, and TSPAN12 proteins. Schematic representations of the genomic and protein structures of all variants are shown in Additional file 1: Figure S2A and S2B.
These variants were not frequently found in ethnically matched populations in multiple population databases; they were predicted to be damaging or deleterious, using multiple lines of prediction algorithms. Population distribution frequencies and predictive functional effects are summarized in Additional file 1: Table S3. Evolutionary conservation alignment of missense variants showed that they were highly conserved among four different species, except variants c.1612C>T p.(Arg538Trp) and c.4084A>G p.(Ile1362Val) in the LRP5 gene (Additional file 1: Figure S3). Based on Sanger sequencing and bioinformatics analysis, we inferred that these 14 variants in the NDP, FZD4, LRP5, and TSPAN12 genes were potential disease-causing variants in 13 families with FEVR.
Discussion
In this study, we enrolled 20 probands with reduced vision who were diagnosed with FEVR based on clinical symptoms, as well as fundus and FFA examinations. We performed whole exome sequencing, Sanger sequencing validation, cosegregation analysis, functional prediction, population distribution analysis, and evolutionary conservation alignment. Our results suggested that 14 variants in the NDP, FZD4, LRP5 and TSPAN12 gene were potential disease-causing variants in 13 probands. Seven variants had not been reported to cause FEVR. The remaining seven families did not harbor any variant.
Clinical symptoms and fundus appearances can vary distinctly among patients and genetic backgrounds in patients with FEVR; in some instances, disease presentation can vary between eyes in a single patient [27]. For example, the proband F12 exhibited different appearances between eyes, such that the right eye demonstrated normal vision and mild fundus abnormality, while the left eye demonstrated mild reduced vision and moderate fundus abnormality. Moreover, the progress of disease was asynchronous between eyes: the right eye showed minimal progression, while the left eye showed progression with falciform retinal folds and peripheral retinal exudates at the 2-year follow-up. Distinct fundus findings were also present in proband F6, such that the right eye showed severe retinal detachment, while the left eye showed mild abnormality. Although the two probands has similar disease courses and were of similar age, their symptom severities and fundus appearances were different.
NDP, FZD4, LRP5, and TSPAN12 gene variants can impair the Norrin or Wnt/β-catenin signaling pathways, which are responsible for angiopoiesis during retinal development [28]. In the canonical Wnt/β-catenin pathway, FZD4 and LRP5 form a ternary complex as a coreceptor; Wnt binds to the coreceptor and activates downstream β-catenin signaling [29]. In the Norrin/β-catenin pathway, NDP binds to the coreceptor and activates downstream β-catenin signaling with the TSPAN12 auxiliary component [30]. When these signaling pathways are activated, β-catenin translocates to the nucleus and interacts with the T-cell factor/lymphoid enhancing factor family of transcription factors, thus initiating RNA transcription and elongation [31, 32].
The NDP gene encodes the Norrin protein, which contains a signal peptide that directs its localization and a typical motif of six cysteines that forms a cysteine knot-like domain [33]. The variant c.118A>G p.(Met40Val) in the NDP gene is located in the highly conserved amino acid motif; it may affect the high affinity between Norrin and the FZD4 transmembrane protein [34].
The FZD4 gene encodes the FZD4 protein, which contains an extracellular cysteine-rich domain, seven-pass transmembrane domains, and a frizzled domain in the extracellular region [35]. The variants c.757C>T p.(Arg253Cys), c.981G>A, p.(Trp327Ter), and c.1039T>G, p.(Phe347Val) in the FZD4 gene are located in the transmembrane domains of the FZD4 protein. The c.981G>A p.(Trp327Ter) variant leads to premature termination of protein translation. The other two missense variants may disturb the highly conserved region of the transmembrane domain of the FZD4 protein, leading to aberrant downstream signaling [36].
The LRP5 gene encodes the LRP5 protein, which contains a putative signal peptide, four β-propeller motifs at the amino terminal that alternate with four epidermal growth factor-like repeats, three low-density lipoprotein receptor-like repeats, a single transmembrane domain, and a cytoplasmic domain [37]. Although the exact functions of these domains are unknown thus far, studies of LRP6 (with strong homology and similar function to LRP5) showed that the first and second β-propeller motifs, the third and fourth β-propeller motifs formed tandems to function respectively [38]. Variants located in the β-propeller motif may destroy the stable structure of β-propellers and interrupt their interactions with NDP or FZD4. The c.685C>T p.(Arg229Trp), c.1210G>A p.(Gly404Arg), c.1612C>T p.(Arg538Trp), and c.3232C>T p.(Arg1078Ter) variants in the LRP5 gene are located in the β-propeller motif of the LRP5 protein; therefore, these variants may cause β-propeller motifs tandems to become inactive. The c.3237-2A>C variant may affect the splice mode and form a new transcript. The c.4084A>G p.(Ile1362Val) variant is located in the transmembrane domain of the LRP5 protein and may disturb the low-density lipoprotein receptor-like ligand binding domains.
The TSPAN12 gene encodes the TSPAN12 protein, which contains four-pass transmembrane domains and four cysteines in the second extracellular region, forming two extracellular loops and an intracellular loop. Variants in transmembrane domains and extracellular regions can severely impair function, variants in the C-terminal end can moderately impair function, and variants in the N-terminal end can slightly impair function [36]. The transmembrane domains provide a scaffold for extracellular loops to change conformation and interact with FZD4 for allosteric modulation. The variants c.77T>A p.(Ile26Asn) and c.236T>G p.(Met79Arg) in the TSPAN12 gene are located in the transmembrane domain and may disrupt the domain structure of the TSPAN12 protein, potentially preventing TSPAN12 incorporation into the receptor complex and destabilizing the NDP/FZD4/LRP5 interaction [39]. The frameshift variants c.170dupT p.(Leu57Phe fsTer60) and c.550dupA p.(Arg184Lys fsTer16) in the TSPAN12 gene are predicted to create a premature stop codon and a truncated TSPAN12 protein.
Although we found 14 disease-causing variants, including seven novel variants, in 13 FEVR families, there were some limitations in this study. First, we only speculated that variants were potential disease-causing based on clinical manifestations, whole exome sequencing, and bioinformatics analysis. Second, the study collected a small group of samples and thus cannot expand the overall understanding of the pathogenic mechanisms by which these genes cause FEVR. We plan to validate the pathogenicity of these variants by in vivo and in vitro analyses, and we will attempt to enroll more families in a future study.
Conclusions
In conclusion, through whole exome sequencing and bioinformatics analysis, we identified 14 variants in the NDP, FZD4, LRP5, and TSPAN12 gene in 13 families with FEVR. To our knowledge, this is the first report regarding c.1039T>G p.(Phe347Val) in the FZD4 gene; c.1612C>T p.(Arg538Trp) and c.3237-2A>C in the LRP5 gene; and c.77T>A p.(Ile26Asn), c.170dupT p.(Leu57Phe fsTer60), c.236T>G p.(Met79Arg), and c.550dupA p.(Arg184Lys fsTer16) in the TSPAN12 gene as potential disease-causing variants in patients with FEVR. These results expand the spectra of variants in the NDP, FZD4, LRP5, and TSPAN12 genes. We presume that these findings will provide insights regarding accurate diagnosis, family genetic counseling, and future gene therapy for FEVR.
Availability of data and materials
The sequencing data used and/or analyzed during the current study are available at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA752456.
Abbreviations
- FEVR:
-
Familial exudative vitreoretinopathy
- NDP:
-
Norrin
- FZD4:
-
Frizzled class receptor 4
- LRP5:
-
Low density lipoprotein receptor-related protein 5
- TSPAN12:
-
Tetraspanin 12
- FFA:
-
Fundus fluorescein angiography
- BCVA:
-
Best-corrected visual acuity
- ACMG:
-
American College of Medical Genetics
References
Robitaille JM, Gillett RM, LeBlanc MA, Gaston D, Nightingale M, Mackley MP, Parkash S, Hathaway J, Thomas A, Ells A, et al. Phenotypic overlap between familial exudative vitreoretinopathy and microcephaly, lymphedema, and chorioretinal dysplasia caused by KIF11 mutations. JAMA Ophthalmol. 2014;132(12):1393–9.
Wu JH, Liu JH, Ko YC, Wang CT, Chung YC, Chu KC, Liu TT, Chao HM, Jiang YJ, Chen SJ, et al. Haploinsufficiency of RCBTB1 is associated with Coats disease and familial exudative vitreoretinopathy. Hum Mol Genet. 2016;25(8):1637–47.
Lin Y, Gao H, Chen C, Zhu Y, Li T, Liu B, Ma C, Jiang H, Li Y, Huang Y, et al. Clinical and next-generation sequencing findings in a Chinese family exhibiting severe familial exudative vitreoretinopathy. Int J Mol Med. 2018;41(2):773–82.
Ranchod TM, Ho LY, Drenser KA, Capone A Jr, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070–5.
Kashani AH, Learned D, Nudleman E, Drenser KA, Capone A, Trese MT. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(1):262–8.
Tang H, Li N, Li Z, Zhang M, Wei M, Huang C, Wang J, Li F, Wang H, Liu Z, et al. Fundus examination of 199 851 newborns by digital imaging in China: a multicentre cross-sectional study. Br J Ophthalmol. 2018;102(12):1742–6.
Sun W, Xiao X, Li S, Jia X, Wang P, Zhang Q. Pathogenic variants and associated phenotypic spectrum of TSPAN12 based on data from a large cohort. Graefes Arch Clin Exp Ophthalmol. 2021;259:2929–39.
Wang S, Zhang X, Hu Y, Fei P, Xu Y, Peng J, Zhao P. Clinical and genetical features of probands and affected family members with familial exudative vitreoretinopathy in a large Chinese cohort. Br J Ophthalmol. 2021;105(1):83–6.
De Silva SR, Arno G, Robson AG, Fakin A, Pontikos N, Mohamed MD, Bird AC, Moore AT, Michaelides M, Webster AR, et al. The X-linked retinopathies: physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog Retin Eye Res. 2021;82:100898.
Fei P, Zhu X, Jiang Z, Ma S, Li J, Zhang Q, Zhou Y, Xu Y, Tai Z, Zhang L, et al. Identification and functional analysis of novel FZD4 mutations in Han Chinese with familial exudative vitreoretinopathy. Sci Rep. 2015;5:16120.
Pefkianaki M, Hasanreisoglu M, Suchy SF, Shields CL. Familial exudative vitreoretinopathy with a novel LRP5 mutation. J Pediatr Ophthalmol Strabismus. 2016;53:e39-42.
Xu Y, Huang L, Li J, Zhang Q, Fei P, Zhu X, Tai Z, Ma S, Gong B, Li Y, et al. Novel mutations in the TSPAN12 gene in Chinese patients with familial exudative vitreoretinopathy. Mol Vis. 2014;20:1296–306.
Panagiotou ES, Sanjurjo Soriano C, Poulter JA, Lord EC, Dzulova D, Kondo H, Hiyoshi A, Chung BH, Chu YW, Lai CHY, et al. Defects in the cell signaling mediator beta-catenin cause the retinal vascular condition FEVR. Am J Hum Genet. 2017;100(6):960–8.
Collin RW, Nikopoulos K, Dona M, Gilissen C, Hoischen A, Boonstra FN, Poulter JA, Kondo H, Berger W, Toomes C, et al. ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature. Proc Natl Acad Sci USA. 2013;110(24):9856–61.
Khan K, Logan CV, McKibbin M, Sheridan E, Elcioglu NH, Yenice O, Parry DA, Fernandez-Fuentes N, Abdelhamed ZI, Al-Maskari A, et al. Next generation sequencing identifies mutations in Atonal homolog 7 (ATOH7) in families with global eye developmental defects. Hum Mol Genet. 2012;21(4):776–83.
Kondo H, Matsushita I, Nagata T, Fujihara E, Hosono K, Uchio E, Hotta Y, Kusaka S. Retinal features of family members with familial exudative vitreoretinopathy caused by mutations in KIF11 gene. Transl Vis Sci Technol. 2021;10(7):18.
Chung MY, Chen SJ, Jiang YJ. Phenotype variability in the patients of familial exudative vitreoretinopathy: the RCBTB1 case. Curr Eye Res. 2021;46:1931.
Zhang L, Zhang X, Xu H, Huang L, Zhang S, Liu W, Yang Y, Fei P, Li S, Yang M, et al. Exome sequencing revealed Notch ligand JAG1 as a novel candidate gene for familial exudative vitreoretinopathy. Genet Med. 2020;22(1):77–84.
Zhu X, Yang M, Zhao P, Li S, Zhang L, Huang L, Huang Y, Fei P, Yang Y, Zhang S, et al. Catenin alpha 1 mutations cause familial exudative vitreoretinopathy by overactivating Norrin/beta-catenin signaling. J Clin Invest. 2021;131(6):e139869.
Downey LM, Keen TJ, Roberts E, Mansfield DC, Bamashmus M, Inglehearn CF. A new locus for autosomal dominant familial exudative vitreoretinopathy maps to chromosome 11p12-13. Am J Hum Genet. 2001;68(3):778–81.
Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye (London). 2015;29(1):1–14.
Serru V, Dessen P, Boucheix C, Rubinstein E. Sequence and expression of seven new tetraspans. Biochim Biophys Acta. 2000;1478(1):159–63.
Kashani AH, Brown KT, Chang E, Drenser KA, Capone A, Trese MT. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(11):2220–7.
Dan H, Li T, Lei X, Huang X, Xing Y, Shen Y. Whole exome sequencing of a family revealed a novel variant in the CHM gene, c.22delG p.(Glu8Serfs*4), which co-segregated with choroideremia. Biosci Rep. 2020;40(5):20200067.
Dan H, Huang X, Xing Y, Shen Y. Application of targeted panel sequencing and whole exome sequencing for 76 Chinese families with retinitis pigmentosa. Mol Genet Genomic Med. 2020;8(3):e1131.
Dan H, Huang X, Xing Y, Shen Y. Application of targeted exome and whole-exome sequencing for Chinese families with Stargardt disease. Ann Hum Genet. 2020;84(2):177–84.
Tian T, Chen C, Zhang X, Zhang Q, Zhao P. Clinical and genetic features of familial exudative vitreoretinopathy with only-unilateral abnormalities in a Chinese cohort. JAMA Ophthalmol. 2019;137(9):1054–8.
Zhu X, Sun K, Huang L, Ma S, Hao F, Yang Z, Sundaresan P, Zhu X. Identification of novel mutations in the FZD4 and NDP genes in patients with familial exudative vitreoretinopathy in South India. Genet Test Mol Biomark. 2020;24(2):92–8.
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23.
Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139(2):299–311.
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205.
Meitinger T, Meindl A, Bork P, Rost B, Sander C, Haasemann M, Murken J. Molecular modelling of the Norrie disease protein predicts a cystine knot growth factor tertiary structure. Nat Genet. 1993;5(4):376–80.
Iarossi G, Bertelli M, Maltese PE, Gusson E, Marchini G, Bruson A, Benedetti S, Volpetti S, Catena G, Buzzonetti L, et al. Genotype-phenotype characterization of novel variants in six Italian patients with familial exudative vitreoretinopathy. J Ophthalmol. 2017;2017:3080245.
Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4(2):60–7.
Xiao H, Tong Y, Zhu Y, Peng M. Familial exudative Vitreoretinopathy-related disease-causing genes and Norrin/beta-catenin signal pathway: structure, function, and mutation spectrums. J Ophthalmol. 2019;2019:5782536.
Hey PJ, Twells RC, Phillips MS, Yusuke N, Brown SD, Kawaguchi Y, Cox R, Guochun X, Dugan V, Hammond H, et al. Cloning of a novel member of the low-density lipoprotein receptor family. Gene. 1998;216(1):103–11.
MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4(12):a007880.
Lai MB, Zhang C, Shi J, Johnson V, Khandan L, McVey J, Klymkowsky MW, Chen Z, Junge HJ. TSPAN12 Is a Norrin co-receptor that amplifies Frizzled4 ligand selectivity and signaling. Cell Rep. 2017;19(13):2809–22.
Acknowledgements
The authors would like to thank all the patients who participated in this study.
Funding
This study was supported by Basic Research Project of Henan Eye Research Institute (21JCQN003), Basic Research Project of Henan Eye Research Institute (20JCZD001), Key Research and Development of Henan Province (192102310075), and Joint Project of Medical Science and Technology of Henan Province (SBGJ2018080).
Author information
Authors and Affiliations
Contributions
Concept and design: ZS; Acquisition, analysis, or interpretation of data: DW, ZH, QS, MZ, YX; Drafting of the manuscript: HD; Critical revision of the manuscript: ZS; Statistical analysis: HD; Administrative, technical, or material support: YX, ZH, ZS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the medical ethics committee of Henan Provincial People's Hospital and conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants or, if participants are under 16, from a parent and/or legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure S1.
Pedigrees and cosegregation of families. Figure S2. Schematic representations of the genomic and protein structures of all variants. Figure S3. Evolutionary conservation of ten missense variants. Table S1. Primers used in Sanger sequencing. Table S2. Quality control information of probands with potential disease-causing variants. Table S3. Population distribution frequencies and predictive functional effects of variants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dan, H., Wang, D., Huang, Z. et al. Whole exome sequencing revealed 14 variants in NDP, FZD4, LRP5, and TSPAN12 genes for 20 families with familial exudative vitreoretinopathy. BMC Med Genomics 15, 54 (2022). https://doi.org/10.1186/s12920-022-01204-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01204-0