Abstract
Rickettsia occurs worldwide and rickettsiosis is recognized as an emerging infection in several parts of the world. Ticks are reservoir hosts for pathogenic Rickettsia species in humans and domestic animals. Most pathogenic Rickettsia species belong to the spotted Fever Group (SFG). This study aimed to identify and diagnose tick fauna and investigate the prevalence of Rickettsia spp. in ticks collected from domestic animals and dogs in the rural regions of Kerman Province, Southeast Iran. In this study, tick species (fauna) were identified and 2100 ticks (350 pooled samples) from two genera and species including Rhipicephalus linnaei (1128) and Hyalomma deteritum (972) were tested to detect Rickettsia genus using Real-time PCR. The presence of the Rickettsia genus was observed in 24.9% (95%CI 20.28–29.52) of the pooled samples. Sequencing and phylogenetic analyses revealed the presence of Rickettsia aeschlimannii (48.98%), Rickettsia conorii israelensis (28.57%), Rickettsia sibirica (20.41%), and Rickettsia helvetica (2.04%) in the positive samples. The results showed a significant association between county variables and the following variables: tick spp. (p < 0.001), Rickettsia genus infection in ticks (p < 0.001) and Rickettsia spp. (p < 0.001). In addition, there was a significant association between tick species and host animals (dogs and domestic animals) (p < 0.001), Rickettsia spp infection in ticks (p < 0.001), and Rickettsia spp. (p < 0.001). This study indicates a high prevalence of Rickettsia spp. (SFG) in ticks of domestic animals and dogs in rural areas of Kerman Province. The health system should be informed of the possibility of rickettsiosis and the circulating species of Rickettsia in these areas.
Similar content being viewed by others
Introduction
Although vector-borne diseases (VBDs) are globally prevalence, they are mostly reported in tropical and subtropical countries. The prevalence of these diseases depends on human and natural factors, such as climatic conditions and the movement of humans and animals. This makes their control and treatment difficult, especially in poor countries and areas where access to the health care system is limited.
Rickettsiosis or diseases caused by Rickettsia species represents a very important group because of the emergent character of the illness [1]. The Rickettsiaceae family includes small Gram-negative obligate intracellular pleomorphic bacteria. Rickettsia can be transmitted to animals and humans by hematophagous arthropods, causing specific zoonotic diseases, termed rickettsioses. The main vectors are ticks, although the pathogen can also be transmitted by other arthropods such as fleas, lice, or mites [1]. Rickettsia bacteria were divided into four groups based on the new Rickettsia genus classification: the spotted fever group (including R. conorii, R. rickettsia, and several others), typhus group (i.e. R. typhi and R. prowazekii), and ancestral group (including R. Canadensis, R. bellii nonpathogenic are known), and transitional group (including R. felis, R. australis, and R. akari). Many novel Rickettsia clades have been discovered in a variety of new hosts, including amoebae, insects, and leeches, providing a broader view of the evolution of Rickettsia [2].
In recent years, rickettsial infection in humans, animals, and ticks have been reported in most of the various countries in the Middle East Countries [3, 4]. Limited information is available on Rickettsia spp in Iran. In a study to identify Rickettsia species in ticks collected from sheep in the Khuzestan province, Southwest Iran, the tick species were identified as Hyalomma marginatum, Hyalomma anatolicum, Hyalomma dromedarii, Hyalomma schulzei, Rhipicephalus bursa, and Rhipicephalus turanicus. Rickettsia spp. were observed in 50% of ticks collected (50%). Sequencing and phylogenetic analyses revealed the presence of Rickettsia aeschlimannii (60%), Rickettsia massiliae (30%), and Rickettsia conorii (10%) in infected ticks [5].
In 2017–2018, five cases of human Mediterranean spotted fever (MSF) infection (caused by R. conorii) were reported in southeast Iran [6]. Limited information is available on the prevalence of Rickettsia in humans, domestic animals, and vectors. Further investigation is required to understand the epidemiology of this disease in Iran. Screening ticks for disease-causing pathogens provides useful epidemiological information on their distribution and the prevalence of pathogens that pose veterinary and medical health risks. The present study aimed to investigate the possible circulation of Rickettsia species and identify the variables associated with ticks infesting ticks collected from rural areas of southeastern Iran.
Materials and methods
Ethical code
The ethical code (IR. UK. VETMED. REC. 1399, 025) was obtained from the Ethics Committee of Shahid Bahonar University of Kerman. In addition, for the collection of ticks, verbal permission was obtained from the domestic animal owners.
Study area
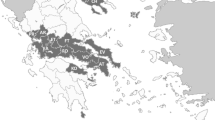
This study was carried out in Kerman Province in southeastern Iran in 2021 (January-September). The tick samples used in this study were collected from sheep, goats, cattle, and dogs from two counties in Kerman Province (Jiroft and Zarand). Kerman Province has a tropical climate, with an area of 182.301 km2 and a population of over 3 million people. Ticks of domestic animals and dogs were performed on farms in the villages of Zarand County (located in the northwestern part of Kerman Province with a population of 138,000, semi-arid climate, average annual precipitation of 140 mm, a height of 1664 m above sea level, and geographical location of 30.8 0N and 56.58 0E) and villages of Jiroft County (located in the southern part of Kerman Province with a population of 309,000, warm weather, average annual precipitation of 220 mm, height of 860 m above sea level, and geographical location of 28.91 0N and 57.66 0E) (Fig. 1).
Tick collection and identification
In this study, ticks were collected between January and September 2021. First, ten villages were randomly selected from each county. Twenty farms in each village were randomly selected and included in this study. A total of 400 farms were included in this study. Tick specimens were collected from sheep, goats, cattle, and dogs at the sampling sites (farms) and verbal consent was obtained from animal handlers before examining their domestic animals for ticks. Using blunt forceps, ticks were collected (from the abdomen, neck, internal sides of the rear legs, tail, and ear) and placed into labeled vials containing 70% ethanol. The ticks were then transported to the laboratory for identification under a light stereomicroscope (Olympus, Japan). All ticks were morphologically identified using taxonomic keys [7, 8]. The specimens were pooled according to the species, sex, study site, and host animal. The pooled samples consisted of six adult ticks (three males and three females), and were grouped into 350 pools: 180 pools from Zarand County and 170 pools from Jiroft. The tick specimens were then stored at -20 °C for further examination.
Extraction of tick nucleic acids
The DNA was extracted using the potassium acetate method [9]. Briefly, pooled ticks were homogenized in liquid nitrogen and sterile PBS, washed again in 70% ethanol, rinsed with sterile water, and dried. The ticks were frozen with liquid nitrogen and disrupted mechanically using 1.5 mL plastic microtubes with a pestle. Initially, 500 µL of lysis buffer [0.1 M Tris-HCl (pH 8.25), 0.05 M EDTA, 0.2 M sucrose, 0.5% SDS] and 20 µL proteinase K (10 mg/mL) were added to each tick lysate. The suspensions were incubated overnight at 56 °C. Next, 120 µL of 5 M potassium acetate was added to each sample and incubated on ice for 10 min. The samples were centrifuged at 12,000 xg for 10 min, and the supernatants were collected. For nucleic acid precipitation, 35 µL of 4 M sodium acetate, 0.25% acrylamide mix, and 1.0 mL of absolute ethanol were added to each supernatant, which was then incubated for 10 min at -20 °C, followed by centrifugation at 12,000 xg for 20 min. The 1.5 mL plastic microtubes were washed with 500 µL 70% ethanol and air-dried at room temperature. Finally, the extracts were resuspended in 75 µL of 1X TE buffer (1 mM Tris-HCl pH 8.0, 1 mM EDTA) and stored at -20 °C until use.
Detection of the Rickettsia genus
DNA extracted from ticks was analyzed to detect the gltA gene of the Rickettsia genus using Real-Time PCR. The 20 µLreactions contained, 10 µL commercial master mix (RealQ Plus 2x Master Mix Ampliqon, Denmark), 2.5 µL template DNA, 900 nmol (0.3 µL) of forward and reverse primers (Table 1) [10], and sterile distilled water to final volume (6.9 µL). R. conorii DNA (Amplirun, Vircell) and distilled water were included in all assays as positive and negative controls (2.5 µL), respectively. Amplification was performed in a Light Cycler 96 system (Germany) programmed for 10-min activation at 95 ˚C, followed by 45 cycles at 95 ˚C for 15 s, and 60 ˚C for 60 s. Quantitative analysis was performed using Rotor-Gene Q Series software, and readings were taken at the end of each cycle in green color at 60 ˚C. Samples with a cycle threshold (Ct) value lower than 37 and a suitable melting curve (73 ± 0.5 ̊C) were considered positive for Rickettsia spp. [10].
Determination of Rickettsia species
To select suitable samples and for final confirmation, the positive samples were sent to the Epidemiology Laboratory of the Pasteur Institute of Iran. Samples were tested using Taqman Real-time PCR assay (16 S rRNA) for confirmation of Rickettsia infection (Table 2) [12]. Samples with a cycle threshold (Ct) ≤ 30 in Taqman Real-time PCR assay were selected for the identification of Rickettsia species.
Using conventional PCR, Rickettsia species were determined by g1tA and ompA gene amplification. The primers used for g1tA and ompA gene amplification are shown in Table 3 [13].
The PCR products for each gene were sequenced (Genomin Co, Tehran, Iran). The sequences were analyzed using Chromas version 2.6.6. Finally, the g1tA and ompA gene sequences based on different Rickettsia spp. in the GenBank database were extracted, and phylogenetic analysis was performed using MEGA X (version 10.1).
Statistical analysis
Data analysis was performed using SPSS software (version 26). The prevalence of qualitative data was estimated using descriptive statistics (95% CIs). Moreover, to evaluate the effect and statistical correlation of the variables, the Chi-square test was used for data analysis. Statistical significance was set at P < 0.05.
Results
In this study, 2100 adult ticks (350 pools) were examined using molecular methods. After morphological examination, ticks were pooled according to species, sex, sampling location, and animal species in which they were collected. There were 1050 male ticks (50%) and 1050 female ticks (50%). A total of 1890 (315 pools = 90%) were collected from domestic livestock (cattle, sheep, and goats) and 210 (35 pools = 10%) were collected from dogs. In the present study, we identified two tick species using morphological keys. They were classified into two genera, Hyalomma deteritum, and Rhipicephalus linnaei, with the highest percentages of Rhipicephalus linnaei (1128 ticks = 53.71%) and Hyalomma deteritum (972 ticks = 46.29%) (Table 4).
Rickettsia detection by real-time PCR
In Kerman Province, of the 350 DNA pooled samples tested by Real-Time PCR, 87 pools (24.9%; 95%CI 20.28–29.52) were positive for Rickettsia. Among 180 DNA pooled samples from Zarand County, 70 pools (38.90%; 95%CI 33.70–44.10) were positive for Rickettsia, and in Jiroft County among 170 DNA pooled samples, 17 pools (10%; 95%CI 6.80–13.20) were positive for Rickettsia (Table 5). Hyalomma deteritum had a greater percentage of positive pools (37.66%), and Rhipicephalus linnaei had a lower percentage of positive pools (13.80%) (Table 6). According to the number of positive pools in each county (38.90% in Zarand County and 10% in Jiroft County), rickettsial infection was significantly higher in ticks from Zarand County than in those from Jiroft County (P < 0.001). There was no statistically significant difference in Rickettsia infection between the host animals variable (animal species) and the positive results of tick infection with the Rickettsia variable (P = 0.076).
Identification and phylogenetic analysis of Rickettsia species
A total of 49 pool samples positive for Rickettsia were selected for species identification in such a way that the selected samples included different tick species from all studied counties and hosts. In addition, the load of Rickettsia DNA (CT ≥ 30) was considered in sample selection for the phylogeny survey. Based on the results of sequence BLAST in GenBank and phylogenetic analysis, four distinct species were identified from 49 sequenced Rickettsia gltA and ompA samples, the majority of which were R. aeschlimannii (n = 24, 48.98%) and R. conorii israelensis (n = 14, 28.57%). Other Rickettsia species identified in the present study included R. sibirica (n = 10, 20.41%) and R. helvetica (n = 1, 2.04%) (Table 7).
In this study, R. conorii israelensis infection was detected in the ticks (Rh. linnaei) from different hosts (cattle, sheep, goats, and dogs) in Jiroft County. R. aeschlimannii and R. sibirica were identified in ticks (H. deteritum) collected from different hosts (cattle, sheep, goats, and dogs) in Zarand County. R. helvetica infection was detected in H. deteritum ticks isolated from cattle, sheep, and goats in Zarand County (Table 7).
The prevalence of R. conorii israelensis was 28.57% (28.57%; 95% CI 15.92–41.22) of 49 sequenced positive samples in Kerman Province (Jiroft County only). According to sequencing and BLAST analysis in GenBank, the gltA gene sequence in all positive samples for R. conorii israelensis, except for the J6GG sample, was identical (matched 100%) to each other, with 100% similarity with the sequence of human clinical cases reported for this bacteria from Kerman Province. The J6GG sample had only one nucleotide difference in sequence with the other samples of R. conorii israelensis obtained in this study (Fig. 2). In addition, the sequence obtained for the ompA gene for all positive samples of R. conorii israelensis was exactly similar to each other and had 100% similarity (matched 100%) with the sequence of human clinical cases reported for this bacterium from Kerman Province (Fig. 3).
The prevalence of R. sibirica was 20.41% (20.41%; 95% CI 9.12–31.70) of 49 sequenced positive samples in Kerman Province (Zarand County only). According to the sequencing and BLAST analysis in GenBank, the obtained sequences of the gltA gene of all samples related to this species (Z1G, Z3C, Z3E, Z3CC, Z5D, Z5H, Z6B, Z6G, and Z8D), except for the Z2I sample in this study, had the same sequence (matched 100%) (Fig. 2). In addition, according to sequencing and analysis of the ompA gene sequence, all R. sibirica samples in this study were similar to each other (matched 100%) (Fig. 3).
The prevalence of R. aeschlimannii was 48.98% (48.98%; 95%CI 34.99–62.97) of 49 sequenced positive samples in Kerman Province (Zarand County only). According to the sequencing and sequence analysis of the gltA gene, the sequences of all samples of R. aeschlimannii in this study were exactly similar (matched 100%) to each other (Fig. 2). Also, Z4G and Z2D samples had 100% identical sequences in ompA gene and sequences of these two samples had very little different from other identified R. aeschlimannii in this study (Fig. 3).
The prevalence of R. helvetica was 2.04% (2.04%; 95% CI 0–6) in the 49 sequenced positive samples from Kerman Province (Zarand County only). The sequence of a single positive sample of R. helvetica was the same as that recorded for the gltA gene in GenBank (100%match) (Fig. 2). In addition, due to the absence of the ompA gene in R. helvetica, the amplification result for single positive sample in our study was negative (Fig. 3).
The results showed a significant association between county variables and tick spp. (p < 0.001), Rickettsia genus infection in ticks (p < 0.001) and Rickettsia spp. infection (p < 0.001). In addition, there was a significant association between tick species. variable in association with host animals (p < 0.001), Rickettsia genus infection in ticks (p < 0.001), and Rickettsia spp. (p < 0.001) variables were observed. However, no significant association was observed between the host animal variable and Rickettsia genus infection in ticks (P = 0.076 > 0.05) or the host animal variable in association with Rickettsia spp. in ticks (P = 0.569 > 0.05).
The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with the sum of branch length = 1.15024671 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The rate variation among sites was modelled with a gamma distribution (shape parameter = 1). This analysis involved 40 nucleotide sequences. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option). There were a total of 462 positions in the final dataset. Evolutionary analyses were conducted in MEGA X.
The evolutionary history was inferred using the Neighbor-Joining method [1]. The optimal tree with the sum of branch length = 0.53847388 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [2]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method [3] and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter = 1). This analysis involved 45 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option). There were a total of 747 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [4].
Discussion
Rickettsia occurs worldwide and rickettsiosis is recognized as an emerging infection in several parts of the world. Few studies have been conducted on the identification of tick fauna in Kerman Province. In our study, among the 2100 ticks collected, 1128 belonged to Rhipicephalus linnaei and 972 belonged to Hyalomma deteritum. In a study (2008–2009), was investigated the prevalence of hard ticks in cattle and sheep in southeastern Iran, Rhipicephalus and Hyalomma ticks have been identified as dominant ticks. A comparison of the results showed that Rhipicephalus and Hyalomma ticks were dominant in southeastern Iran. In addition, the difference in the species of these ticks may be due to, the large spread and vastness of rural areas in Kerman Province [14].
According to the results, the presence of the Rickettsia genus was observed in 24.9% (95%CI 20.28–29.52) of 350 samples. Sequencing and phylogenetic analyses revealed the presence of R. aeschlimannii (48.98%), R. conorii israelensis (28.57%), R. sibirica (20.41%), and R. helvetica (2.04%) in positive samples. In a similar study, Mostafavi et al. (2019–2020), reported a 20% prevalence of Rickettsia in ticks of stray dogs in Kerman city and the presence of Rickettsia spp. including R. massiliae, R. rhipicephali, and R. sibirica in Rhipicephalus sanguineus sensu lato ticks. Since both studies were conducted in Kerman Province, it can be concluded that the prevalence of Rickettsia in ticks was significant, therefore the prevalence of Rickettsia in our study was relatively higher, and the prevalence of R. sibirica in ticks in both studies was significant [15].
To date, few studies have been conducted on the prevalence and species of Rickettsia in domestic animals and dog ticks in Iran. In 2020, the prevalence of SFG Rickettsia in ticks collected from domestic animals and birds in nine provinces of Iran was 59%. The prevalence of rickettsia in this study was higher than that in our study, which could be due to the extent of the studied areas and the diversity of tick species [16]. In one study, a 50% prevalence of Rickettsia was observed in hard ticks collected from sheep in nine counties of the Khuzestan Province of Iran. According to sequencing and phylogenetic analyses, a significant presence of R. aeschlimannii (60%), R. massiliae (30%), and Rickettsia conorii (10%) was detected in infected ticks. The prevalence of Rickettsia in this study is higher than in our study, which could be due to extent of the studied areas and the diversity of tick species, compared to our study. However, the similarity between the two identified species, R. aeschlimannii and R. conorii, in both studies indicates the prevalence of these two species in southern Iran [5].
Based on a recent study in Iran’s northern provinces (Guilan, Mazandaran, and Golestan), 25.2% of collected ticks were positive for Rickettsia, and the 8 species of Rickettsia were identified including R. massiliae, R. sibirica, R. rhipicephali, R. aeschlimannii, R.helvetica, R. asiatica, R. monacensis, and R. raoultii. The similarity of the three species of Rickettsia (R.sibirica, R. aeschlimannii and R. helvetica) identified in this study with our study indicates the prevalence of these species in northern and southern Iran [17].
In other countries, the prevalence of Rickettsia in ticks in Pakistan 14% [18], in Italy 18.4% [19], in Ukraine 19.1% [20] and in Turkey 1.9% [21] has been reported. A comparison of the results of these studies with those of our study showed a significant prevalence of Rickettsia (24.9%). this could be due to differences in climatic conditions, the diversity of tick species, and an increase in the population of ticks in our study areas. However, in other studies, the prevalence of Rickettsia in ticks: in Italy at 52.25% [22], in Italy at 33% [23], Ghana at 45.6% [24], and France at 25.6% [25] has been reported. In these studies, a significant prevalence of Rickettsia compared to our study has been reported, which could be due to the favorable climatic conditions for the growth of ticks, diversity of tick species involved in the reproduction and transmission of Rickettsia, animal tick contamination, and increased tick populations.
In the present study, two species of R. aeschlimannii and R. conorii israelensis had the highest prevalence, both of which are members of SFG Rickettsia. R. aeschlimannii is a tick-borne Rickettsia that is known as a pathogenic species in Europe and Africa [26]. R. aeschlimannii is associated with cases (diseases) similar to Mediterranean spotted fever (MSF) in Africa and is distributed in Mediterranean areas [27]. R. conorii is responsible for MSF, and Rhipicephalus sanguineus tick is considered the main vector [28]. Similar to our findings, in Italy, R. conorii israelensis from Rhipicephalus sanguineus ticks (17.6%; 95%CI 4.67–44.20) and R. aeschlimannii from Hyalomma marginatum marginatum ticks (8.3%; 95%CI 0.44–40.25) has been reported [29]. Another study in Italy reported a 33% prevalence of Rickettsia in ticks. In this study, R. aeschlimannii was identified in Hyalomma marginatum and Hyalomma lusitanicum ticks, and R. conorii was identified in Rhipicephalus sanguineus ticks [23]. In Turkey, 41% prevalence of Rickettsia in human ticks, R. aeschlimannii in Hyalomma marginatum, Hyalomma aegyptium ticks (12%), R. conorii conorii in Rhipicephalus bursa ticks (4%), and R. helvetica in Ixodes ricinus ticks (2.3%) has been reported [30]. Considering the prevalence of R. aeschlimannii and R. conorii in these areas and our study, it is recommended that the health system pay attention to the dangers of their spread.
The other Rickettsia spp. identified in our study were R. sibirica and R. helvetica, which were relatively less common. These two species were identified only in ticks from Zarand County. R. helvetica is classified as a pathogenic species in SFG Rickettsia [26]. R. helvetica is also involved as a human pathogen with fever, with or without rash, and in patients with meningitis and carditis [27]. Siberian tick-borne typhus (STT) is caused by R. sibirica, which was previously reported to be the only tick-borne rickettsiosis agent in the Asian part of Russia [31]. Lymphangitis-associated rickettsioses (LAR), caused by R. sibirica mongolotimonae, have been recognized in various European countries (France, Spain, Portugal, and Greece) [27].
Similar to our findings, in the Asian part of Russia, R. sibirica (12.1%) was detected in Dermacentor nuttalli ticks and R. helvetica (1.9%) in Ixodes persulcatus ticks [32]. In another study in Spain, a 17.6% prevalence of Rickettsia in ticks was reported. Additionally, R. sibirica (1.12) and R. helvetica (1.12) have been identified in Ixodes ricinus ticks [33]. In Sweden, the prevalence of Rickettsia in ticks was reported to be 9.54–9.6%. In addition, R. sibirica and R. helvetica (with the highest amounts) were detected in Ixodes ricinus ticks [34].
Our study and several published studies in Iran indicate the existence of different species of Rickettsia. Therefore, it is possible to identify these species by conducting extensive and comprehensive studies. The results of the present study showed a significant association between county variables and the following variables: tick spp. (p < 0.001), Rickettsia infection in ticks (p < 0.001) and Rickettsia spp. (p < 0.001). In addition, a significant association between tick species and host animals (dogs and domestic animals) (p < 0.001), Rickettsia infection in ticks (p < 0.001), and Rickettsia spp. (p < 0.001) was observed, because all ticks collected from Jiroft County belonged to Rh. linnaei, whereas in Zarand County Rh. linnaei was collected only from dogs, and H. deteritum was collected only from domestic animals. The prevalence of Rickettsia in ticks from Zarand County (38.9%) was higher than that in the ticks from Jiroft County (10%). Rickettsia spp. isolated from ticks in Zarand County (R. aeschlimannii, R. sibirica, and R. helvetica) differed from those isolated from ticks in Jiroft County (R. conorii israelensis). There were differences in the parasitization of animals by specific genera and species of ticks. For example, only Rh. linnaei was collected from dogs. Rickettsia infection in H. deteritum ticks (37.66%) was higher than in Rh. linnaei ticks (13.80%). Rickettsia spp isolated from Rh. linnaei and H. deteritum ticks were different. For example, R. conorii israelensis has been isolated only from Rh. linnaei ticks.
There was no statistical association between the host animal variables and the following variables: Rickettsia genus infection and Rickettsia spp. indicating a lack of a role for the host animal (dogs and domestic animals) in the prevalence of Rickettsia and its species in Kerman Province.
Our limitations in this study were the impossibility of collecting samples from more counties of Kerman Province. In addition, because of the possibility of the prevalence of Rickettsia in a wide range of ticks of birds and animals (domestic and wild) in Kerman Province, we could not solve these limitations due to the lack of facilities and time. Therefore, future studies should investigate the prevalence of Rickettsia spp. in wider areas and more animal ectoparasites.
Conclusion
According to the findings of this study, it is recommended that the health system be informed about Rickettsia species circulating in these areas. Therefore, to better understand the epidemiological situation of rickettsiosis in Iran, more studies should be conducted in the field of detection of Rickettsia species in animals and their external parasites (especially ticks and fleas), as well as a detailed investigation of suspected human cases in different regions of Iran.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Blanda V, et al. New real-time PCRs to differentiate Rickettsia spp. and Rickettsia conorii. Molecules. 2020;25(19):4431.
Mansueto P et al. New insight into immunity and immunopathology of Rickettsial diseases Clinical and Developmental Immunology, 2012. 2012.
Perveen N, Muzaffar SB, Al-Deeb MA. Ticks and tick-borne diseases of livestock in the Middle East and North Africa: a review. Insects. 2021;12(1):83.
Abdad MY, et al. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. 2018;56(8):e01728–17.
Afzalkhani A et al. Molecular detection and diversity of spotted fever group Rickettsia isolated from ticks in Iran 2022.
Farrokhnia M, et al. Cases of Mediterranean spotted fever in southeast of Iran. Iran J Microbiol. 2020;12(3):256.
Walker AR. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports Edinburgh; 2003.
Šlapeta J, et al. Rhipicephalus linnaei (Audouin, 1826) recognised as the tropical lineage of the brown dog tick Rhipicephalus sanguineus Sensu Lato: neotype designation, redescription, and establishment of morphological and molecular reference. Volume 13. Ticks and Tick-borne Diseases; 2022. p. 102024. 6.
Rodríguez I, et al. An alternative and rapid method for the extraction of nucleic acids from ixodid ticks by potassium acetate procedure. Brazilian Archives Biology Technol. 2014;57:542–7.
Giulieri S, et al. Development of a duplex real time PCR for the detection of Rickettsia spp. and typhus group rickettsia in clinical samples. FEMS Immunol Med Microbiol. 2012;64(1):92–7.
Portillo A, et al. Guidelines for the detection of Rickettsia spp. Vector-Borne Zoonotic Dis. 2017;17(1):23–32.
Baseri N, et al. Investigation of Rickettsia conorii in patients suspected of having Crimean-Congo Hemorrhagic Fever. Pathogens. 2022;11(9):973.
Labruna MB, et al. Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J Med Entomol. 2004;41(3):533–7.
Dehaghi MM, et al. Prevalence of ixodid ticks on cattle and sheep southeast of Iran. Trop Anim Health Prod. 2011;43:459–61.
Mostafavi SM, et al. Rickettsia spp. in Rhipicephalus sanguineus Sensu lato ticks collected from stray dogs in Kerman city, Iran. Volume 13. Ticks and Tick-borne Diseases; 2022. p. 101985. 5.
Hosseini-Chegeni A, et al. Molecular detection of spotted fever group Rickettsia (Rickettsiales: Rickettsiaceae) in ticks of Iran. Volume 75. Archives of Razi Institute; 2020. p. 317. 3.
Ghasemi A, et al. Molecular surveillance for Rickettsia spp. and Bartonella spp. in ticks from Northern Iran. PLoS ONE. 2022;17(12):e0278579.
Ali A, et al. Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Volume 14. Parasites & Vectors; 2021. pp. 1–12. 1.
Morganti G, et al. Molecular survey on Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi Sensu Lato, and Babesia spp. in Ixodes ricinus ticks infesting dogs in central Italy. Vector-Borne Zoonotic Dis. 2017;17(11):743–8.
Alieva E, et al. The role of Rhipicephalus sanguineus ticks parasitizing dogs in the spread of tick-borne rickettsial pathogens in the city of Sevastopol. New Microbes new Infections. 2020;36:p100704.
Demir S, et al. Molecular investigation of Rickettsia spp. and Francisella tularensis in ticks from three provinces of Turkey. Exp Appl Acarol. 2020;81:239–53.
Pascucci I, et al. Diversity of Rickettsia in ticks collected in Abruzzi and Molise regions (central Italy). Microorganisms. 2019;7(12):696.
Blanda V, et al. A retrospective study of the characterization of Rickettsia species in ticks collected from humans. Ticks tick-borne Dis. 2017;8(4):610–4.
Nimo-Paintsil SC, et al. Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Volume 15. Parasites & Vectors; 2022. p. 86. 1.
Cicculli V, et al. Molecular detection of spotted-fever group rickettsiae in ticks collected from domestic and wild animals in Corsica, France. Pathogens. 2019;8(3):138.
Chisu V et al. Detection of Rickettsia hoogstraalii, Rickettsia helvetica, Rickettsia massiliae, Rickettsia slovaca and Rickettsia aeschlimannii in ticks from Sardinia, Italy Ticks and tick-borne diseases, 2017. 8(3): p. 347–52.
Oteo JA, Portillo A. Tick-borne rickettsioses in Europe. Ticks tick-borne Dis. 2012;3(5–6):271–8.
Psaroulaki A, et al. First isolation and genotypic identification of Rickettsia conorii Malish 7 from a patient in Greece. Eur J Clin Microbiol Infect Dis. 2005;24(4):297–8.
Chisu V, et al. Rickettsia conorii israelensis in Rhipicephalus sanguineus ticks, Sardinia, Italy. Ticks Tick-borne Dis. 2014;5(4):446–8.
Gargili A, et al. Rickettsia species in ticks removed from humans in Istanbul, Turkey. Vector-Borne Zoonotic Dis. 2012;12(11):938–41.
Igolkina Y, et al. Detection of causative agents of tick-borne rickettsioses in Western Siberia, Russia: identification of Rickettsia raoultii and Rickettsia sibirica DNA in clinical samples. Clin Microbiol Infect. 2018;24(2):e1999–19912.
Shpynov S, et al. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Volume 1078. Annals of the New York Academy of Sciences; 2006. pp. 378–83. 1.
Palomar AM et al. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerging infectious diseases, 2012. 18(7): p. 1188.
Wallménius K, et al. Prevalence of Rickettsia spp., Anaplasma phagocytophilum, and Coxiella burnetii in adult Ixodes ricinus ticks from 29 study areas in central and southern Sweden. Volume 3. Ticks and tick-borne diseases; 2012. pp. 100–6. 2.
Acknowledgements
The authors would like to thank Miss Mina Latifian (Pasteur Institute of Iran) for assistance with Rickettsia typing.
Funding
This research was partially funded by Shahid Bahonar University of Kerman (Grant number 94154).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.K, and SN; data analysis and curation: E.M, and A.Q; visualization: A.Q; investigation: A.Q, M.K, S.N and S.E; methodology: E.S, M.D, M.F, and E.M; project administration and supervision: M.K and S.N; figurePrepration: A.Q; funding acquisition: M.K; writing original draft: A.Q; writing-review and editing: M.K, E.S, E.M, S.N and M.F All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was received from the Institutional Animal Care and Use Committee (ARC-IACUC) of the Ethics Committee of Shahid Bahonar University of Kerman (IR. UK. VETMED. REC. 1399, 025) and all methods were performed under relevant guidance and regulations. The oral informed consent from the domestic animal owners was approved by the Ethics Committee of Shahid Bahonar University of Kerman. All methods were carried out according to the relevant guidelines and regulations. This study was approved by. The verbal permission of Informed consent was taken from the domestic animal owners for the collection of ticks.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qorbani, A., Khalili, M., Nourollahifard, S. et al. Diversity of Rickettsia species in collected ticks from Southeast Iran. BMC Vet Res 20, 279 (2024). https://doi.org/10.1186/s12917-024-04142-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04142-4