Abstract
Background
Sheep were among the first animals to be domesticated. They are raised all over the world and produce a major scale of animal-based protein for human consumption and play an important role in agricultural economy. Iran is one of the important locations for sheep genetic resources in the world. Here, we compared the Illumina Ovine SNP50 BeadChip data of three Iranian local breeds (Moghani, Afshari and Gezel), as a population that does not undergone artificial breeding programs as yet, and five other sheep breeds namely East Friesian white, East Friesian brown, Lacaune, DorsetHorn and Texel to detect genetic mechanisms underlying economical traits and daptation to harsh environments in sheep.
Results
To identify genomic regions that have been targeted by positive selection, we used fixation index (Fst) and nucleotide diversity (Pi) statistics. Further analysis indicated candidate genes involved in different important traits such as; wool production included crimp of wool (PTPN3, NBEA and KRTAP20–2 genes), fiber diameter (PIK3R4 gene), hair follicle development (LHX2 gene), the growth and development of fiber (COL17A1 gene)), adaptation to hot arid environments (CORIN gene), adaptive in deficit water status (CPQ gene), heat stress (PLCB4, FAM107B, NBEA, PIK3C2B and USP43 genes) in sheep.
Conclusions
We detected several candidate genes related to wool production traits and adaptation to hot arid environments in sheep that can be applicable for inbreeding goals. Our findings not only include the results of previous researches, but also identify a number of novel candidate genes related to studied traits. However, more works will be essential to acknowledge phenotype- genotype relationships of the identified genes in our study.
Similar content being viewed by others
Background
Small ruminants, especially domestic sheep (Ovis aries), have an vital role in the livelihood of a significant portion of human population in the developing and under developing countries [1, 2]. They probably domesticated about 11,000 years before present (BP) from Asian Mouflon (Ovis orientalis) in the Fertile cresecent, possibly southeast Anatolia and/or the Zagros [3]. After domestication, geographical isolations and artificial selection have led to substantial variation in the phenotypic traits of different sheep breeds. Today, they are raised all over the world and adaptable to different geographical climates due to their adaptability to low nutrition diets, endurance in intolerable climatic situations and manageable size [4, 5]. Around 44.9% of the world’s sheep populations are live in Asian continent [6], and Iran has the largest sheep population of 52 million in the Middle East. More than 27 sheep ecotypes have been recognized in Iran which are vary in different factors, including their genetic potential for milk, meat and wool production traits [7, 8]. These breeds are conventionally named in accordance with their geographical origin, also thay have categorized according to productive performance and morphological features [9,10,11,12]. Despite lower production efficiency of Iranian local sheep population than commercial dairy and meat breeds, they have adapted to various evolutionary trajectory based on genetic drift and regional adaptation, and have not been undergone artificial breeding programs as yet [13]. For example, Moghani, Afshari and Gezel are three well-known dual-purpose sheep breeds in Iran. The Ghezel is one of the native fat-tailed breeds in the north-western part of Iran, dual purpose (meal and milk). The Afshari is one the fat-tailed carpet wool sheep breeds, known for its large size, that has been originated from north-west of Iran (Zanjan province). The Moghani sheep is one of the medium-size Iranian fat-tailed sheep breeds, which can be found in the West Azerbaijan province. This breed is good for both the wool and meat production traits.
A considerable diversity in production [14] and daptation [15, 16] traits were detected across and within different sheep breeds [14]. Iran is placed in the Africa-Asia girdle with 90% of arid regions, which is considered as a hot and arid country in terms of climate in the world [17, 18]. Conserving these diversity is important for raising the efficiency of production and imporoving adaptation to environments. Until now, different studies have been done to identify genes related to production and daptation traits in sheep using methods such as methylated DNA immunoprecipitation sequencing [19,20,21], RNA-Seq analyses [22,23,24,25,26,27], whole genome sequencing nalyses [28, 29] and genome-wide association studies [30,31,32,33]. Although Iranian indigenous sheep breeds display high genetic potential for production, researches to increase their productivity is less carried out compared to other sheep breeds. There are only a few genome-wide association [34,35,36,37] and signatures selection mapping [38] studies related to some Iranian sheep breeds.
In this research, we used the fixation index (FST), as a measure of population differentiation due to genetic structur and nucleotide diversity (Pi), to compare the Illumina Ovine SNP50 BeadChip data of three Iranian local breeds (Moghani, Afshari and Gezel) with those of the dairy (East Friesian white, East Friesian brown and Lacaune) and meat (DorsetHorn and Texel) sheep breeds to explore novel candidate genes related to economic and adaptation traits in Iranian local sheep breeds.
Results
Population genetic structure
A total of 324 individuals and 43,586 SNPs were selected for downstream analysis. After applying LD pruning, SNPs were used for ADMIXTURE analysis. The genetic distances tree showed that all three Iranian local sheep breeds shared a common clade and all other commercial breeds were placed into separated clades (Fig. 1a). To more study the relationships gained from phylogenetic analysis, we carry outed Admixture analysis based on a Bayesian model for different values of K (k = 2–6). We further found a clear differentiation between Iranian local sheep samples and other groups from K values in 3–6 (Fig. 1b).
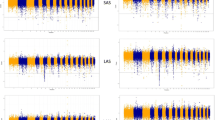
The results collectively showed that all three Iranian local sheep populations may have endured different evolutionary processes in accordance with their genetic breeding history and regional adaptation/selection following domestication. We then estimated the patterns of LD, which can be informative for different biological and historical factors including, recombination, selection, inbreeding, and population bottlenecks. The LD pattern showed that the mean of r2 in Iranian breeds was less than 0.5 in window different sizes (2.5, 5, 10, 20, 40, 80 and 160 Kb). The amount of LD was the highest in the East Friesian and DorsetHorn and the lowest in Moghani and Qezel respectively (Fig. 2A). In addition, we calculated runs of homozygosity (ROH) to assess recent inbreeding. The distribution of ROHs for all studied sheep breeds are shown in Fig. 2B. Here we found a markedly higher number of ROHs in DorsetHorn, Milk Lacaune and East Friesian whitegenomes than other sheep breeds, that could be a consequence of artificial selection for traits of interest, e.g. milk and meat production traits, in thire breeding programs. Also, whithin Iranian sheep breedsboth Moghani and Qezel breeds had the lowest number of ROHs (Fig. 2b). The results showing the less recent inbreeding in moghani and Qezel compared with that in the other breeds.
Genome-wide selective sweep analysis
In this study we used Ovine SNP50 BeadChip data for comparative genome analysis between Iranian sheep breeds (Moghani, Afshari and Gezel) and different commercial populations (dairy and meat types) to identify regions in the genome that are associated with different phenotypes. We applied FST nucleotide diversity (Pi) statistics to detect genomic footprints left by natural selection in local sheep breeds. The regions having lower levels of nucleotide diversity and extremely high FST values (top 1 and 5%) were studied to be regions potentially under selection. A number of genes that covers significant nucleotide diversity and FST values (Fig. 3 and supplementary file 1: Table S1) were identified in different comparations. To examine the functional complexity of each gene set, gene enrichment was performed on the gene lists produced by different comparations. Gene set enrichment analysis (GSEA) identified enriched categories related with “biological process”, “human phenotype” and “molecular function” (supplementary file 2: Table S2, supplementary file 3: Table S3, supplementary file 4: Table 4 and supplementary file 5: Table S5).
Discussion
We firstly applied different population genomic analyses to reveal genetic relationships among different sheep populations (Figs. 1 and 2). The phylogenetic distance based on the whole-genome information showed all Iranian native breeds have a obviously distinict genetic distance from the other commercial groups. Also Iranian sheep breeds were seen as a dependent group in admixture output (k values of 3–6). The ROHs and LD values represent the decrising of recent inbreeding in Iranian local sheep breeds due to no artificial selection compared with other commercial sheep breeds.
Our results collectively showed that all three Iranian local sheep breeds may have experience different evolutionary path based on genetic drift and various regional adaptation after domestication, which is in agreement with previous studies [8, 39, 40]. We then detected a number of candidate genes that previously have reported associated with production traits in sheep and other livestock species. The significant candidate genes related to wool trait and daptation to harsh and hot arid environments are summerized in supplementary file 1: Table S1.
In the following, only main candidate genes are mentioned below to discuss their potential involvements in controlling wool production traits and adaptation to hot arid environments.
Adaptation and genes associated with heat stress, hot arid and harsh environments
Heat stress has a negative effect on the biological functions of sheep and disrupts their production, fertility, and health characteristics [41]. Maintaining animal performance in hot climates can be achieved by identifying genetic exclusivity associated with heat tolerance [42]. Recent studies showed that functional genes are present in epigenetic thermoregulatory mechanisms that lead to adaptive behavioral and protective responses [43].
Phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 beta (PIK3C2B) gene encodes phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 beta and is located on sheep chromosome 12. In this research, PIK3C2B gene is considered as a region under selection through comparing Iranian native populations breeds with both commercial dairy and meat sheep breeds (top 5% for FST). A study has detected that genes enriched in the type II diabetes mellitus pathway including phosphoinositide 3-kinase genes are responsible for responding to adaptation mechanisms. These genes are responsible for many biological functions in the intracellular signal transduction pathways, such as immunity, metabolic control, and cardiovascular homeostasis. The PIK3C2B gene is grouped in a cluster of genes compatible with PI3K isoforms that cause energy homeostasis in response to relatively high energy demand under heat stress conditions and has an important relationship with adaptation mechanisms to heat stress in ducks [44].
Phospholipase C beta 4 (PLCB4) gene encodes phospholipase C beta 4, was found in one of the selected regions on Chr. 13, between Iranian local sheep breeds with commercial dairy sheep breeds (top 5% for pi). Li et al. [45] have reported that PLCB4 gene is correlated with heat tolerance (oxidative stress response) in Dehong humped cattle. Also, Jin et al. [46], by studying of the catfish genome, showed that this gene is related to heat stress (energy metabolism) in aquatic species [46].
Another gene that responds to changes in heat stress levels is FAM107B (family with sequence similarity 107 member B) gene that was situated in one of the selected regions on Chr. 13, between Iranian native sheep breeds with commercial dairy sheep breeds (top 5% for pi). This gene plays a role in heat-shock induction [47]. A recent genome-wide association study in Holstein cattle has shown that the FAM107B gene is strongly associated with heat stress response in dairy cattle [48]. Also, USP43 (ubiquitin specific peptidase 43, Chr. 11 sheep, comparing between Iranian local sheep breeds and commercial dairy sheep breeds (top 5% for FST)) gene is involved in heat stress through the ubiquitination process, and its expression was increased in skeletal muscle lambs exposed to heat stress [49].
CORIN (corin, serine peptidase, Chr. 6 sheep, comparing between Iranian local sheep breeds commercial dairy sheep breeds (top 5% for FST)) gene encodes a serine proteinase that is involved in activating proteins related to volume and blood pressure [50, 51]. This gene is involved in the circulatory system and maintaining the proper volume of blood, so can play an important role in adapting to temperature regulation in hot and dry environments [52].
Carboxypeptidase Q (CPQ) gene encodes carboxypeptidase Q is located on the sheep chromosome 9. This gene is regarded as a region under selection through comparing Iranian local sheep breeds with both commercial dairy and meat sheep breeds (top 5% for FST) and Iranian native populations breeds with commercial dairy sheep breeds (top 1% for Pi). CPQ Gene is involved in proteolytic functions. Degradation of protein in plants, which depends on the levels of proteolytic enzymes, is an important part of the plant’s response to environmental stress. Drought affects on the function of these enzymes so that it leads to significant changes in proteolytic activity levels in plant leaves, which can be part of the drought-resistance mechanism [53]. accordingly, Almas et al., in a study of African indigenous chickens, introduced this gene as an important adaptive gene in deficit water status [54].
Neurobeachin (NBEA) gene encodes neurobeachin, was found in one of the selected regions on Chr. 10, between Iranian local sheep breeds with commercial meat sheep breeds (top 5% for FST and top 1% for Pi). NBEA gene has a relationship with regulating body temperature in cattle within heat stress [55] and also to contribute feed intake and body weight [56].
Genes associated with the wool trait
Wool is a valuable natural fiber that varies in crimp, elasticity, and diameter, and its quality affects the economic performance of wool sheep [57]. Carpet wool is developed by the thickening and elongation of the wool module in the primary wool follicles [57].
COL17A1 (collagen type XVII alpha 1 chain, Chr. 22) candidate gene related to coarse wool, was found between Iranian local sheep breeds with commercial meat sheep breeds (top 5% for FST). Nie et al. [58] Using transcriptome long non-coding RNAs (LncRNAs), and mRNAs involved in the excitation of the primary wool follicle in the skin of carpet embryo sheep, have reported that there is an intricate regulatory relation between LncRNAs and mRNAs in the formation of primary follicles. COL17A1 was one of the genes identified in their research in which mRNA of COL17A1 gene regulates the dermal-epidermal junction and basement membrane, and lncRNAs of COL17A1 gene are relevant to collagens [58].
Neurobeachin (NBEA) gene encodes neurobeachin, was found in one of the selected regions on Chr. 10, between Iranian local sheep breeds with commercial meat sheep breeds (top 5% for FST and top 1% for Pi). Studies show that the function of this gene is related to epithelial cells or skin development. Wang et al. [59] have reported that the NBEA gene is related to the crimp trait in the Chinese Merino sheep. In the current study, PIK3R4 (phosphoinositide-3-kinase, regulatory subunit 4) gene s located on Chr. 1 sheep, between Iranian local sheep breeds with commercial milk sheep breeds (top 5% for pi) and was identified as a gene related to wool diameter [59].
Another gene related to wool, KRTAP20–2 (keratin-associated protein 20–2) gene is located on Chr. 1 sheep, between Iranian local sheep breeds with commercial milk sheep breeds (top 5% for pi). Studies have shown that SNPs in the KRTAP20–2 gene are associated with cashmere weight and length in goats [60] and wool fiber curvature in sheep [61].
Another wool-related gene, protein tyrosine phosphatase non-receptor type 3 (PTPN3), was found in one of the selected regions on Chr. 2, between Iranian local sheep breeds with both commercial dairy and meat sheep breeds (top 1% for Pi). PTPN3 gene is a member of the FERM proteins family (4.1/ezrin/radixin/moesin). The PTPN3 gene product is a protein phosphatase and a structural component of the cytoskeleton that plays an important role in the maintenance of tight junction integrity between the cell membrane and the cytoskeleton. The PTPN3 gene product is associated with focal adhesions [62,63,64]. One previous study has reported that PTPN3 gene is associated with crimp traits in the wool of Chinese Merino sheep [59].
LIM homeobox 2 (LHX2) an encoding gene is located on Chr. 3, between Iranian local sheep breeds and commercial meat sheep breeds (top 1% for Pi). It is a flexible and hydrophobic protein and is involved in secondary hair follicle development [65] and studies have shown that LHX2 gene is not expressed in the resting phase of hair follicles but is active in their growth phase [66], this gene helps to maintain stem cell-related properties in hair follicles [67].
Conclusions
We detected several novel candidate genes related with wool production traits and adaptation to hot arid and harsh environments in sheep that can be applicable for inbreeding goals. However, more works will be essential to acknowledge phenotype- genotype relationships of the identified genes in our study.
Materials and methods
Population data and data preprocessing
The Illumina Ovine SNP50 BeadChip data of 324 sheep individuals, including 106 indigenous Iranian breeds (Moghani n = 34, Afshari n = 37 and Gezel n = 35) and four commercial breeds (DorsetHorn n = 21, Texel n = 46, Milk Lacaune n = 103, East Friesian white n = 9 and East Friesian brown n = 39) were obtained from the Sheep HapMap project database (Data Collection. https://doi.org/10.4225/08/51870B1E8EE56) [68]. The SNP quality control (QC) was performed by plink 1.07 program [69]. All SNPs were filtered based on minor allele frequency (maf > 0.01), call rate less than 90% and Hardy–Weinberg equilibrium (hwe > 10− 5).
Population structure, ROH and LD decay
To reduce the powerful influence of SNPs clusters in relatedness analysis, PLINK was used to prune the filtered SNPs (indep-pairwise 100 50 0.1). Admixture tool [70] was applied for visualize population structure in our sheep samples, with an ancestor population size ranging from 2 to 6 and 10,000 iterations for each run, based on the pruned data. Amounts ROHs (−-homozyg-kb and --homozyg-snp) lengths > 100 Kb and LD (with –r2 flag) for seven distance classes (2.5, 5, 10, 20, 40, 80 and 160 kb) were also computed using PLINK software. Genetic relationships among the individuals was investigated based on neighbor joining approach with pair-wide distances. Genetic distances tree was drawn using bootstrap method (No. of Bootstrap Replications, 1000) in MEGA7.
Signatures of positive selection and annotation
Recently different techniques have been used to reveal signatures of positive selection in genomes [71]. FST statistics is the most extensively applied and the strongest method that benefit from the variation across the genome [72, 73]. In this study in order to detect novel candidate genes included in genetic traits under selection, FST and nucleotide diversity (Pi) statistics were used to compare the SNP data of the Iranian breeds with those of dairy and meat breeds. Here, to identify the genomic regions under positive selection in eight sheep breeds, we detected the “outlier” loci with locus-specific FST estimates, as presented by Akey et al., (2002) as follows:
Here, MSP shows the observed mean square errors for Iranian sheep population and other sheep populations,
Where, MSG shows the observed mean square errors for loci within sheep populations.
In the above phrase, i shows the subpopulations (where i = 1, …, s), ni the sample size in subpopulation i, PAi the frequency of the SNP allele A in the ith subpopulation, nc is the mean sample size across samples [74], and
A sliding window method (100 kb with a step size of 50 kb) was applied to compute pairwise Fst using VCFtools 0.1.15 (http://vcftools.sourceforge.net/index.html) [75] and level of significance at cut off P ≤ 0.05 was used for FST values.
The nucleotide diversity was calculated as the proportion of pairwise differences between two populations, (π(other sheep populations)- π(Iranian sheep population)), finally the top 1% was separated as positively selected regions. The nucleotide diversity (π) was calculated using a step size of 25 kb and window size of 50 kb. To assist following gene annotation, the UCSC liftOver tool [76] (https://genome.ucsc.edu/cgi-bin/hgLiftOver) was applied to upgrade the all genomic positions to accordance the sheep genome Oar_v4.0. Sheep gene IDs that involved selected genomic regions were obtained from Ensemble annotation. Gene ontology (GO) analysis was carried out in g:Profiler toolset [77] using Benjamini–Hochberg false discovery rate procedure and we only reported the significant terms (P-value < 0.05).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- COL17A1:
-
Collagen type XVII alpha 1
- CORIN:
-
Corin, serine peptidase
- CPQ:
-
Carboxypeptidase Q
- FAM107B:
-
Family with sequence similarity 107 member B
- FST:
-
Fixation index
- GO:
-
Gene ontology
- KRTAP20–2 :
-
Keratin-associated protein 20–2
- LD:
-
Linkage disequilibrium
- LHX2:
-
LIM homeobox 2
- LncRNAs:
-
Long non-coding RNAs
- MAF:
-
Minor allele frequency
- NBEA:
-
Neurobeachin
- PIK3C2B:
-
Phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 beta
- PIK3R4:
-
Phosphoinositide-3-kinase, regulatory subunit 4
- PLCB4:
-
Phospholipase C beta 4
- PTPN3:
-
Protein tyrosine phosphatase non-receptor type 3
- ROHs:
-
Runs of homozygosity.
- USP43:
-
Ubiquitin specific peptidase 43
References
Ahsani MR, Mohammadabadi MR, Shamsaddini MB. Clostridium perfringens isolate typing by multiplex PCR. J Venom Anim Toxins. 2010;16:573–8.
Vajed Ebrahimi MT, Mohammadabadi M, Esmailizadeh A. Using microsatellite markers to analyze genetic diversity in 14 sheep types in Iran. Arch Anim Breed. 2017;60:183–9.
Zeder MA. Animal domestication in the Zagros: an update and directions for future research. MOM Edition. 2008;49:243–77.
Kijas JW, Townley D, Dalrymple BP, Heaton MP, Maddox JF, McGrath A, et al. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds. PLoS One. 2009;4:e4668.
Soma P, Kotze A, Grobler JP, Van Wyk JB. South African sheep breeds: population genetic structure and conservation implications. Small Ruminant Res. 2012;103:112–9.
Skapetas B, Kalaitzidou M. Current status and perspectives of sheep sector in the world. Change. 2013;2000(2013):e2000.
Ghotbaldini H, Mohammadabadi MR, Nezamabadi-pour H, Babenko OI, Bushtruk M, Tkachenko SV. Predicting breeding value of body weight at 6-month age using Artificial Neural Networks in Kermani sheep breed. Acta Scientiarum Anim Sci. 2019;41:e45282.
Masoudzadeh SH, Mohammadabadi M, Khezri A, Stavetska RV, Oleshko VP, Babenko OI, et al. Effects of diets with different levels of fennel (Foeniculum vulgare) seed powder on DLK1 gene expression in brain, adipose tissue, femur muscle and rumen of Kermani lambs. Small Ruminant Res. 2020;193:e106276.
Zamani P, Akhondi M, Mohammadabadi MR, Saki AA, Ershadi A, Banabazi MH, et al. Genetic variation of Mehraban sheep using two intersimple sequence repeat (ISSR) markers. Afr J Biotechnol. 2011;10:1812–7.
Mohammadabadi MR, Jafari AHD, Bordbar F. Molecular analysis of CIB4 gene and protein in Kermani sheep. Braz J Med and Biol Res. 2017;50:e6177.
Ahsani MR, Bafti MS, Esmailizadeh AK, Mohammadabadi MR. Genotyping of isolates of Clostridium perfringens from vaccinated and unvaccinated sheep. Small Ruminant Res. 2011;95:65–9.
Mohammadabadi MR. Inter-simple sequence repeat loci associations with predicted breeding values of body weight in kermani sheep. Genet Third Millenn. 2016;14:4383–90.
Mohammadabadi M, Kord M, Nazari M. Studying expression of leptin gene in different tissues of Kermani Sheep using Real Time PCR. Agri Biotechnol J. 2018;10:111–23.
Xu SS, Li MH. Recent advances in understanding genetic variants associated with economically important traits in sheep (Ovis aries) revealed by high-throughput screening technologies. Fron Agri Sci Eng. 2017;4:279–88.
Wei C, Wang H, Liu G, Zhao F, Kijas JW, Ma Y, et al. Genome-wide analysis reveals adaptation to high altitudes in Tibetan sheep. Sci Rep. 2016;6:1–11.
Edea Z, Dadi H, Dessie T, Kim KS. Genomic signatures of high-altitude adaptation in Ethiopian sheep populations. Genes Genomics. 2019;41:973–81.
Pourkhorsandi H, Gattacceca J, Rochette P, d'Orazio M, Kamali H, de Avillez R, et al. Meteorites from the Lut Desert (Iran). Meteorit Planet Sci. 2019;54(8):1737–63.
Nouri M, Homaee M. Drought trend, frequency and extremity across a wide range of climates over Iran. Meteorol Appl. 2020;27(2):e1899.
Cao J, Wei C, Liu D, Wang H, Wu M, Xie Z, et al. DNA methylation landscape of body size variation in sheep. Sci Rep. 2015;5:e13950.
Cao J, Wei C, Zhang S, Capellini TD, Zhang L, Zhao F, et al. Screening of reproduction-related single-nucleotide variations from MeDIP-seq data in sheep. Mol Reprod Dev. 2016;83:958–67.
Couldrey C, Brauning R, Bracegirdle J, Maclean P, Henderson HV, McEwan JC. Genome-wide DNA methylation patterns and transcription analysis in sheep muscle. PLoS One. 2014;9:e101853.
Giordani T, Vangelisti A, Conte G, Serra A, Natali L, Ranieri A, et al. Transcript profiling in the milk of dairy ewes fed extruded linseed. Genom Data. 2017;11:17–9.
Lv X, Sun W, Yin J, Ni R, Su R, Wang Q, et al. An integrated analysis of microRNA and mRNA expression profiles to identify RNA expression signatures in lambskin hair follicles in Hu sheep. PLoS One. 2016;11:e0157463.
Miao X, Luo Q, Qin X, Guo Y. Genome-wide analysis of microRNAs identifies the lipid metabolism pathway to be a defining factor in adipose tissue from different sheep. Sci Rep. 2015;5:1–9.
Sun L, Bai M, Xiang L, Zhang G, Ma W, Jiang H. Comparative transcriptome profiling of longissimus muscle tissues from Qianhua Mutton Merino and Small Tail Han sheep. Sci Rep. 2016;6:1–13.
Wang X, Zhou G, Xu X, Geng R, Zhou J, Yang Y, et al. Transcriptome profile analysis of adipose tissues from fat and short-tailed sheep. Gene. 2014;549(2):252–7.
Liu J, Yuan C, Lu Z, Zhuoga D, Guo T, Zhang J, et al. Comparative analysis of long non-coding RNA and mRNA expression provides insights into adaptation to hypoxia in Tibetan sheep. 2021. PREPRINT (Version 2) available at Research Square; https://doi.org/10.21203/rs.3.rs-77480/v2.
Yang JI, Li WR, Lv FH, He SG, Tian SL, Peng WF, et al. Whole-genome sequencing of native sheep provides insights into rapid adaptations to extreme environments. Mol Biol Evol. 2016;33(10):2576–92.
Wiener P, Robert C, Ahbara A, Salavati M, Abebe A, Kebede A, et al. Whole-genome sequence data suggest environmental adaptation of Ethiopian sheep populations. Genom Biol Evol. 2021;13(3):e014.
Gutierrez-Gil B, Arranz JJ, Pong-Wong R, García-Gámez E, Kijas J, Wiener P. Application of selection mapping to identify genomic regions associated with dairy production in sheep. PLoS One. 2014;9:e94623.
Johnson PL, Van Stijn TC, Henry H, McLean NJ, Lee M. Genome wide association study using the ovine SNP50 BeadChip and lambs selected for extremes for carcass leanmeat yield. Assoc Advmt Anim Breed Genet. 2013;20:495–8.
Rupp R, Senin P, Sarry J, Allain C, Tasca C, Ligat L, et al. A point mutation in suppressor of cytokine signalling 2 (Socs2) increases the susceptibility to inflammation of the mammary gland while associated with higher body weight and size and higher milk production in a sheep model. PLoS Genet. 2015;11:e1005629.
Wang H, Zhang L, Cao J, Wu M, Ma X, Liu Z, et al. Genome-wide specific selection in three domestic sheep breeds. PLoS One. 2015;10:e0128688.
Abdoli R, Mirhoseini S, Hossein-Zadeh NG, Zamani P, Ferdosi MH, Gondro C. Genome-wide association study of four composite reproductive traits in Iranian fat-tailed sheep. Reprod Fertil Dev. 2019;31:1127–33.
Almasi M, Zamani P, Mirhoseini SZ, Moradi MH. Genome-wide association study of weaning traits in Lori-Bakhtiari sheep. Ann Anim Sci. 2020;20:811–24.
Ghasemi M, Zamani P, Vatankhah M, Abdoli R. Genome-wide association study of birth weight in sheep. Animal. 2019;13:1797–803.
Gholizadeh M, Rahimi-Mianji G, Nejati-Javaremi A. Genome wide association study of body weight traits in Baluchi sheep. J Genet. 2015;94:143–6.
Manzari Z, Mehrabani-Yeganeh H, Nejati-Javaremi A, Moradi MH, Gholizadeh M. Detecting selection signatures in three Iranian sheep breeds. Anim Genet. 2019;50:298–302.
Ruiz-Larrañaga O, Nanaei HA, Montes I, Mehrgardi AA, Abdolmohammadi A, Kharrati-Koopaee H, et al. Genetic structure of Iranian indigenous sheep breeds: insights for conservation. Trop Anim Health Prod. 2020;52:2283–90.
Vahidi SMF, Faruque MO, Falahati Anbaran M, Afraz F, Mousavi SM, Boettcher P, et al. Multilocus genotypic data reveal high genetic diversity and low population genetic structure of Iranian indigenous sheep. Anim Genet. 2016;47:463–70.
Marai IF, El-Darawany AA, Fadiel A, Abdel-Hafez MA. Physiological traits as affected by heat stress in sheep—a review. Small Rumin Res. 2007;71(1–3):1–2.
West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci. 2003;86(6):2131–44.
Akerman AP, Tipton M, Minson CT, Cotter JD. Heat stress and dehydration in adapting for performance: good, bad, both, or neither? Temperature. 2016;3(3):412–36.
Kim JM, Lim KS, Byun M, Lee KT, Yang YR, Park M, et al. Identification of the acclimation genes in transcriptomic responses to heat stress of White Pekin duck. Cell Stress Chaperones. 2017;22(6):787–97.
Li R, Li C, Chen H, Li R, Chong Q, Xiao H, et al. Genome-wide scan of selection signatures in Dehong humped cattle for heat tolerance and disease resistance. Anim Genet. 2020;51(2):292–9.
Jin Y, Zhou T, Geng X, Liu S, Chen A, Yao J, et al. A genome-wide association study of heat stress-associated SNPs in catfish. Anim Genet. 2017;48(2):233–6.
Yonekura T, Nojima T, Tanaka H, Umehar N, Tomosugi T, Takata T, et al. Motoo induction of HITS, a newly identified family with sequence similarity 107 protein (FAM107B), in cancer cells by heat shock stimulation. Int J Oncol. 2010;37:e583.
Luo H, Li X, Lirong H, Wei X, Chu Q, Liu A, et al. Genomic analyses and biological validation of candidate genes for rectal temperature as an indicator of heat stress in Holstein cattle. J Dairy Sci. 2021;104(4):4441–51.
Kubik RM, Tietze SM, Schmidt TB, Yates DT, Petersen JL. Investigation of the skeletal muscle transcriptome in lambs fed β adrenergic agonists and subjected to heat stress for 21 d. translation. Anim Sci. 2018;2(suppl_1):S53–6.
Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97(15):8525–9.
Chen S, Cao P, Dong N, Peng J, Zhang C, Wang H, et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med. 2015;21(9):1048–53.
Gu J, Liang Q, Liu C, Li S. Genomic analyses reveal adaptation to hot arid and harsh environments in native chickens of China. Front Genet. 2020;11:582355.
Hieng B, Ugrinović K, Šuštar-Vozlič J, Kidrič M. Different classes of proteases are involved in the response to drought of Phaseolus vulgaris L. cultivars differing in sensitivity. J Plant Physiol. 2004;161(5):519–30.
Gheyas AA, Trujillo AV, Kebede A, Lozano-Jaramillo M, Dessie T, Smith J, et al. Integrated environmental and genomic analysis reveals the drivers of local adaptation in African indigenous chickens. Mol Biol Evol. 2021;38(10):4268-85.
Howard JT, Kachman SD, Snelling WM, Pollak EJ, Ciobanu DC, Kuehn LA, et al. Beef cattle body temperature during climatic stress: a genome-wide association study. Int J Biometeorol. 2014;58(7):1665–72.
Olszewski PK, Rozman J, Jacobsson JA, Rathkolb B, Strömberg S, Hans W, et al. A regulator of synaptic protein targeting, is associated with body fat mass and feeding behavior in mice and body-mass index in humans. PLoS Genet. 2012;8(3):e1002568.
Rogers GE. Biology of the wool follicle: an excursion into a unique tissue interaction system waiting to be re-discovered. Exp Dermatol. 2006;12:931–49.
Nie Y, Li S, Zheng X, Chen W, Li X, Liu Z, et al. Transcriptome reveals long non-coding RNAs and mRNAs involved in primary wool follicle induction in carpet sheep fetal skin. Front Physiol. 2018;15(9):446.
Wang Z, Zhang H, Yang H, Wang S, Rong E, Pei W, et al. Genome-wide association study for wool production traits in a Chinese Merino sheep population. PLoS One. 2014;9(9):e107101.
Wang J, Zhou H, Zhu J, Hu J, Liu X, Li S, et al. Identification of the ovine keratin-associated protein 15-1 gene (KRTAP15-1) and genetic variation in its coding sequence. Small Rumin Res. 2017;153:131–6.
Bai L, Gong H, Zhou H, Tao J, Hickford JG. A nucleotide substitution in the ovine KAP20-2 gene leads to a premature stop codon that affects wool fibre curvature. Anim Genet. 2018;49:357–8.
Zhang SH, Kobayashi R, Graves PR, Piwnica-Worms H, Tonks NK. Serine phosphorylation-dependent association of the Band 4.1-related protein-tyrosine phosphatase PTPH1 with 14-3-3ॆ protein. J Biol Chem. 1997;272(43):27281–7.
Itoh F, Ikuta S, Hinoda Y, Arimura Y, Ohe M, Adachi M, et al. Expression and chromosomal assignment of PTPH1 gene encoding a cytosolic protein tyrosine phosphatase homologous to cytoskeletal-associated proteins. Int J Cancer. 1993;55(6):947–51.
Brown EL, Below JE, Fischer RS, Essigmann HT, Hu H, Huff C, et al. Genome-wide association study of Staphylococcus aureus carriage in a community-based sample of Mexican-Americans in Starr county, Texas. PLoS One. 2015;10(11):e0142130.
Wang S, Jin H, Cao Y, Lu C, Zhang X, Sun F, et al. Cloning and sequence analysis of LHx2 gene cDAN from China Xinji fine wool sheep. Chinese J Vet Sci. 2015;35(12):1979–83.
Törnqvist G, Sandberg A, Hägglund AC, Carlsson L. Cyclic expression of lhx2 regulates hair formation. PLoS Genet. 2010;6(4):e1000904.
Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Sci. 2006;312(5782):1946–9.
Kijas J. ISGC SNP50 HapMap and sheep breed diversity genotypes v1. Canberra: CSIRO; 2013. Data collection. https://doi.org/10.4225/08/51870B1E8EE56
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Alexander DH, Lange K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatic. 2011;12:1–6.
Nielsen R, Williamson S, Kim Y, Hubisz MJ, Clark AG, Bustamante C. Genomic scans for selective sweeps using SNP data. Genome Res. 2005;15:1566–75.
Akey JM, Zhang G, Zhang K, Jin L, Shriver MD. Interrogating a high-density SNP map for signatures of natural selection. Genome Res. 2002;12:1805–14.
Garrigan D, Lewontin R, Wakeley J. Measuring the sensitivity of single-locus “neutrality tests” using a direct perturbation approach. Mol Biol Evol. 2010;27:73–89.
Weir BS. Population substructure. Genetic data analysis II. Sunderland: Sinauer Associates; 1996. p. 161–73.
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–8.
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006.
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. g: Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191–8.
Acknowledgements
The authors thank all staff of Shahid Bahonar University of Kerman, Bila Tserkva National Agrarian University, Sumy National Agrarian University and Polissia National University that have took part in the collection of data and analysis of samples for the study.
Funding
This work was supported by the Vice Chancellor for Research and Technology of Shahid Bahonar University of Kerman (Grant number: G-311/8720). The funding bodies provided all the help in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
MRM and OB planed the research and revised the manuscript. LM, HAN, SR and OK analysed the data. ZAG, DK and OK-Y drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In this research, we analysed the datasets freely accessible in the public area, so we did not need ethical permission.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Candidate genes related to production traits identified through signature selection analysis in sheep.
Additional file 2: Table S2.
Gene enrichment among regions under selection through compare the SNP data of the Iranian breeds with those of meat breeds using Fst method.
Additional file 3: Table S3.
Gene enrichment among regions under selection through compare the SNP data of the Iranian breeds with those of dairy breeds using Fst method.
Additional file 4: Table S4.
Gene enrichment among regions under selection through compare the SNP data of the Iranian breeds with those of meat breeds using nucleotide diversity (Pi).
Additional file 5: Table S5.
Gene enrichment among regions under selection through compare the SNP data of the Iranian breeds with those of dairy breeds using nucleotide diversity (Pi).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohamadipoor Saadatabadi, L., Mohammadabadi, M., Amiri Ghanatsaman, Z. et al. Signature selection analysis reveals candidate genes associated with production traits in Iranian sheep breeds. BMC Vet Res 17, 369 (2021). https://doi.org/10.1186/s12917-021-03077-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-021-03077-4