Abstract
Background
Given limited data regarding the involvement of disadvantaged groups in paediatric diabetes clinical trials, this study aimed to evaluate the socioeconomic representativeness of participants recruited into a multinational clinical trial in relation to regional and national type 1 diabetes reference populations.
Methods
Retrospective, cross-sectional evaluation of a subset of adolescent type 1 diabetes cardiorenal intervention trial (AdDIT) participants from Australia (n = 144), Canada (n = 312) and the UK (n = 173). Validated national measures of deprivation were used: the Index of Relative Socioeconomic Disadvantage (IRSD) 2016 (Australia), the Material Resources (MR) dimension of the Canadian Marginalisation index 2016 (Canada) and the Index of Multiple Deprivation (IMD) 2015 (UK). Representativeness was assessed by comparing the AdDIT cohort’s distribution of deprivation quintiles with that of the local paediatric type 1 diabetes population (regional), and the broader type 1 diabetes population for which the trial’s intervention was targeted (national).
Results
Recruited study cohorts from each country had higher proportions of participants with higher SES, and significant underrepresentation of lower SES, in relation to their national references. The socioeconomic make-up in Australia mirrored that of the regional population (p = 0.99). For Canada, the 2nd least deprived (p = 0.001) and the most deprived quintiles (p < 0.001) were over- and under-represented relative to the regional reference, while the UK featured higher regional and national SES bias with over-representation and under-representation from the least-deprived and most-deprived quintiles (p < 0.0001).
Conclusions
Significant national differences in trial participation of low SES participants were observed, highlighting limitations in access to clinical research and the importance of reporting sociodemographic representation in diabetes clinical trials.
Trial registration
NCT01581476. Registered on 20 April 2012.
Similar content being viewed by others
Background
Despite the increasing recognition that social determinants of health significantly impact clinical health outcomes, less than 15% of clinical trials published in leading medical journals report any data on participant socioeconomic status (SES) [1,2,3]. In the few studies reporting such data, participants of higher SES predominate with higher levels of reported household income and educational attainment [4,5,6]. The inclusion of participants into clinical trials from a wide variety of socioeconomic backgrounds is important to ensure that medical research innovations shown to be effective and safe have been evaluated and are thus generalizable to the broader clinic population [4]. This is particularly relevant in diabetes-related research, as many medication and medical device trials fail to report details relating to social or ethnic factors, or show low rates of participation of underrepresented groups [7].
Disadvantaged groups may experience barriers to high-quality care that include limited economic resources as well as limitations in accessing care centres due to obstacles in distance and time [8, 9]. In addition, many socially disadvantaged groups experience greater disease burden for whom clinical interventions may be more beneficial [10,11,12]. From a health equity perspective, individuals of lower SES should also be provided equal opportunity to participate in clinical research [13, 14].
There are few diabetes trials, particularly in paediatrics, reporting the participation of individuals with low SES [15]. The overall goal of this report was to examine the SES of participants recruited in the Adolescent type 1 Diabetes cardiorenal Intervention Trial (AdDIT). This study was conducted in three countries—Australia, Canada and the UK—with publicly funded healthcare systems as well as validated measures of SES to quantify social inequity within both the type 1 diabetes study participants and the general population. The specific study aims were to evaluate, within each individual country, how representative AdDIT study participants were in relation to (1) the local/regional population of persons with type 1 diabetes available for recruitment and (2) the national population of persons with type 1 diabetes.
Methods
Study population—AdDIT study cohort
AdDIT is an international (Canada, UK and Australia) study that evaluated adolescents for inclusion into an intervention (i.e. randomized control trial, RCT) or observational study arm between 2010 and 2016 [16]. Potential study participants for AdDIT were identified based on screening of albumin excretion (albumin-creatinine ratio, ACR) whereby high-risk subjects with increased albumin excretion (upper tertile of ACR) were eligible to participate in the intervention RCT, while those identified to be at low risk (lower and middle tertiles of ACR) were eligible to participate in the observational arm. ACR measurements were performed centrally at the WellChild Laboratory in London, England. Urine albumin was measured using nephelometric immunoassay according to the manufacturer’s instructions (Siemens BN Prospec). HbA1c was assessed locally using DCCT-aligned methods.
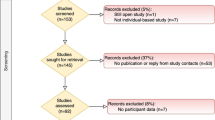
The current evaluation includes N = 631 of the 893 original AdDIT participants (70.4%) from both the intervention (n = 301/443; 67.9%) and observational (n = 330/450; 73.3%) study arms. Complete postal codes were available for participants from AdDIT Canada; however, local ethics restrictions pertaining to confidentiality limited access to postal code data for a subset of participants from AdDIT UK and AdDIT Australia, which did not allow the entire original AdDIT cohort to be evaluated as part of this study. Overall, the study included n = 144 of 201 from Australia, n = 312 of 323 from Canada and n = 175 of 369 from the UK (specifically England and Wales) (see Additional file 1: Fig. S1). Participant recruitment for AdDIT was completed at 6 sites across 4 Australian states for Australia, 5 sites in a single Canadian province (Ontario) for Canada and 23 sites across 8 of the 9 NHS health regions in England for the UK (see Fig. 1). This study was ethics approved at all study sites and the study protocol was in conformity with the Declaration of Helsinki.
Deprivation indices
The study used validated, aggregate-level, area-based measures of deprivation and marginalization developed by the governments of each country to approximate individual-level SES. The indices from each country were analysed as quintile scores to allow comparisons with local/regional and national reference populations. For each country, measures of deprivation/marginalization were linked to participant data using postal codes as described by the guidance documents of each index. Postcodes of all n = 144 of the Australian participants were directly linked to the Socio-Economic Indices For Areas (SEIFA) 2016 including the Index of Relative Socio-economic Disadvantage (IRSD) [16]. For Canada, the 6-digit postal codes of all n = 312 participants were linked to dissemination area (DA) codes, the smallest census-based geographic unit, using Statistics Canada’s Postal Code Conversion File (PCCF) before being linked to the Material Resources (MR) dimension of the Canadian Marginalisation Index (CAN-Marg) 2016 [17]. For the UK, the postcodes for n = 173 of 175 participants were successfully linked to the Lower Layer Super Output Area (LSOA) code in which they were located. The LSOA code was subsequently linked to the Index of Multiple Deprivation (IMD) 2015 quintile score for n = 172 participants with an England-based postcode and the Welsh Index of Multiple Deprivation (WIMD) 2014 quintile score for n = 1 participant with a Wales-based postcode [18]. The postcodes of n = 2 participants from the UK could not be linked to their corresponding LSOA and were thus excluded from the present analysis. Both the IRSD and IMD measures were scored from Q1 (most disadvantaged/deprived) to Q5 (least disadvantaged/deprived), while the CAN-Marg quintiles were scored inversely with Q1 representing the least deprived quintile and Q5, the most deprived quintile.
Local/regional and national paediatric type 1 diabetes reference populations
Aggregate data for both regional and national reference populations in England and Wales were extracted from the National Pediatric Diabetes Audit (NPDA) 2015–2016 and 2019–2020 Annual Report and Appendices [19]. The breakdown of paediatric T1D across IMD quintiles in the UK and in regions in which AdDIT sites were located were extracted directly from the NPDA reports.
Data from the paediatric type 1 diabetes population from the study’s main Canadian site, The Hospital for Sick Children (SickKids), and regional sites were used as the local/regional reference. Canadian reference data for paediatric type 1 diabetes were not readily available from government agencies at the national level as these data were consistently grouped alongside type 2 diabetes. In light of such a limitation, National references for paediatric type 1 diabetes in Canada were derived by combining data from the Pediatric Diabetes Network (PDN) and the Canadian Chronic Disease Surveillance System (CCDSS) [20, 21]. Due to the lack of linkage to CAN-Marg, the reference population for Canada was assumed to be evenly distributed across quintiles.
Regional reference data for 2015–2016 for Australian sites was obtained from the Australasian Diabetes Data Network (ADDN) in aggregate [22]. Postcodes from the ADDN dataset were linked to SEIFA 2016 indices using the method described above. Postcodes encompassing less than n = 4 participants were not included for confidentiality reasons. Nationally, aggregate prevalence data was extracted from the Australian Institute for Health and Wellness’s (AIHW) Diabetes 2020 Data Table 1.12, which broke down the prevalence of children and young adults aged 0 to 24 with type 1 diabetes in Australia (n = 20,664) across IRSD 2016 quintiles [22].
Statistical analyses
All statistical analyses were completed using R Statistics v4.1.0 (www.r-project.org) Representativeness was first assessed overall by comparing the distribution of AdDIT participant cohorts across deprivation quintiles with that of their regional and national reference populations using chi-square tests. The distribution of the Australian cohort of AdDIT across IRSD quintiles was compared with the ADDN and AIHW references. The Canadian cohort of AdDIT was compared with the SickKids and inferred PDN/CCDSS references on the basis of their distribution across quintiles from the MR dimensions of the 2016 Can-Marg Index. The UK AdDIT cohort was compared with the regional and national NPDA references according to IMD 2015 quintiles. Confidence set at 95% (α = 0.05), such that P-values < 0.05 were deemed significant for this assessment.
Further testing assessing differences within quintiles was completed using Fisher’s Exact tests to evaluate over- and under-representation among quintile groups relative to their reference populations. To account for multiple testing within each deprivation index, a simple Bonferroni correction was used, whereby P-values < 0.005 (i.e. 0.05/10) were deemed significant. No comparisons were performed between the AdDIT cohorts from different countries; each cohort was analysed separately and compared with reference populations from their country using the country-specific indicators.
Results
AdDIT participant demographic data
The current study included N = 629 participants from the original AdDIT study for whom valid postal information was available. The assessed cohort was 46.9% female with a mean age of 14.1 ± 1.6 years, diabetes duration of 6.5 ± 3.2 years and mean HbA1c 8.5 ± 1.3% (69.2 ± 14.6 mmol/mol) (Table 1). Characteristics were clinically similar between countries aside from a higher proportion of insulin pump use (in relation to injection) in Canada, with 56.1% of Canadian participants reporting pump use as compared with Australia (36.8%) and the UK (35.1%); P < 0.001). Significant differences in self-identified ethnicity were seen, with a more diverse, non-White, ethnic composition seen in Canada (40.9%) in relation to the UK (6.2%) or Australia (4.7%) (P < 0.001). The proportion of participants from the interventional and observational arms of AdDIT did not differ across deprivation quintiles and were analysed as a single cohort as part of this study (see Additional file 1: Fig. S2).
Comparisons with regional paediatric type 1 diabetes references
The distribution of participants from the UK across IMD quintiles differed considerably when compared with the regional NPDA 2015–2016 reference population (P < 0.0001), as depicted in Fig. 2. Substantial variation in SES was observed among the AdDIT UK cohort, with a strong skew toward higher SES. Participants from both the least (37.6% vs. 17.9%; P < 0.0001) and 2nd least (28.9% vs. 18.0%; P < 0.001) deprived quintiles were greatly overrepresented when compared with the regional NPDA reference. Inversely, participants from the most (4.6% vs. 23.3%; P < 0.0001) and 2nd most (6.4% vs. 21.5%; P < 0.0001) deprived quintiles were underrepresented relative to the NPDA references.
Distribution of the UK, Canadian and Australian cohorts of AdDIT. A Distribution of the UK-based cohort of AdDIT, the NPDA regional reference and the NPDA national reference across quintiles of the Index of Multiple Deprivation (IMD) 2015. B Distribution of the Canadian cohort of AdDIT, the SickKids regional reference and the derived national reference across quintiles of the Material Resources (MR) 2016. C Index of Relative Socioeconomic Disadvantage (IRSD) 2016. Differences between the AdDIT cohort and both reference populations are presented. Error bars represent 95% confidence intervals
The Canadian AdDIT cohort was found to be significantly different than the local paediatric type 1 diabetes population reference with respect to their distribution across quintiles of the MR dimension (P < 0.0001). When examined further at the quintile level, the difference observed based on MR was an over-representation and under-representation of participants from the 2nd least deprived quintile (28.2% vs. 18.9%; P = 0.001) and under-representation of participants from the most deprived quintile (12.2% vs. 22.0%; P < 0.001) relative to their regional reference, respectively. While it did not reach significance, a slight overrepresentation of participants from the Q3 was observed relative to the regional reference (18.6% vs. 13.8%; P = 0.049).
The overall distribution of the Australian AdDIT cohort across IRSD (P = 0.98) quintiles was not significantly different when compared with the ADDN paediatric type 1 diabetes reference population. No additional within-quintile differences between the AdDIT Australia cohort and the ADDN reference were observed.
Comparisons with national paediatric type 1 diabetes references
The UK AdDIT cohort was also found to differ significantly relative to the national NPDA reference population with respect to their distribution across IMD 2015 quintiles (P < 0.0001). Similar to their regional findings, participants from both the least (37.6% vs. 19.7%; P < 0.0001) and 2nd least (28.9% vs. 19.4%; P = 0.0027) deprived quintile groups were considerably overrepresented, and the most (4.6% vs. 21.6%; P < 0.0001) and 2nd most (6.4% vs. 20.1%; P < 0.0001) deprived quintiles were underrepresented, relative to the national NPDA reference.
The overall distribution of the Canadian AdDIT cohort was also skewed relative to their derived national reference. For the MR dimension, a greater proportion of individuals from less marginalized areas, and a lower proportion of individuals from areas characterized by high marginalization, was observed. Individuals from the least (29.5%; P < 0.0001) and 2nd least (28.2%; P < 0.0001) marginalized quintile of the MR dimension were significantly overrepresented. Likewise, underrepresentation of individuals from the most (12.2%; P < 0.001) and 2nd most (11.5%; P < 0.001) marginalized quintiles was observed for the MR dimension.
Significant differences were observed between the Australian AdDIT cohort and AIHW reference population with respect to the IRSD 2016 index (P < 0.0001). Relative to their national reference population from the AIHW, on the basis of IRSD 2016 quintiles, the Australian AdDIT cohort was found to have a significantly greater proportion of individuals from the least deprived (36.1% vs. 18.3%; P < 0.0001) quintile group and a significantly lower proportion of individuals from the most (6.9% vs. 20.4%; P < 0.0001) deprived quintile. The 2nd most deprived quintile was also slightly underrepresented in the AdDIT Australia cohort (13.2%) compared the to national AIHW reference (21.1%), although this difference did not reach the threshold for significance (P = 0.018).
Discussion
In this international paediatric type 1 diabetes study, we report significant differences in SES between recruited study participants and reference regional and national populations with type 1 diabetes, within each of the AdDIT countries (see Table 2). Regional differences were seen between the three countries evaluated, such that research participation in the UK and Canada was overall under-represented for more deprived SES quintiles, while research participation in Australia matched their regional type 1 diabetes population. On a macro level, comparisons with all national reference populations showed that the recruited AdDIT study cohort was not representative of the overall population with type 1 diabetes.
Different recruitment approaches were used in each country. In the UK, a larger number of recruitment sites including towns and urban metropolitan areas were active from a wider geographic area. While this approach was intended to include a broader enrollment base, it meant that fewer participants per center were recruited (see Fig. 1). This may have contributed to resulted in a selection bias towards higher SES participants, who may have volunteered or have been preferentially selected based on greater interest, time or ability and perceived suitability for clinical research. Canada and Australia recruited from fewer sites, primarily represented by larger clinics in more urban areas. Canadian recruitment was centred in the Greater Toronto metropolitan area, and while recruitment was representative at higher SES quintiles, there was an underrepresentation of lower SES. Interestingly, the regional reference type 1 diabetes population in Canada shows a classic U-shaped curve of income polarization, whereby there is a loss of the middle SES quintiles and an over-representation of high and low marginalization. Income inequality is quantified using the Gini index, and Toronto has the highest national urban Gini index for Canada (https://www150.statcan.gc.ca/n1/daily-quotidien/220713/g-d007-eng.htm.). This trend of income polarization has intensified in Canadian and other cities globally alongside a progressive loss of middle-income earners in urban areas [23, 24]. Australian regional data were collected in moderate-sized clinics in many regional cities and describe a population weighted towards a higher SES overall, with much lower proportions of participants with greater deprivation/lower SES than Canada or the UK. Recruitment was highly representative of the regional population but reflects that, in Australia, the most advantaged SES quintiles are clustered around capital cities and selected coastal areas, while the most disadvantaged quintiles are in more remote and regional areas [25, 26].
Strengths of this report are that validated SES measures from publicly funded health care systems were used, which allowed for descriptive international comparisons that lessen issues related to participant health insurance status. In addition, this allows for a nuanced evaluation of SES and study enrolment, allowing for comparisons with both regional and national populations of individuals with type 1 diabetes. In addition, while these data report SES differences in study recruitment, they also show the engagement of participants across a wide range of socioeconomic backgrounds. Strategies to optimize convenience and the overall participant experience at sites included flexibility in data collection by combining study and diabetes clinic visits, the availability of early or late or weekend appointments, reimbursement for travel and the availability of shared health care for participants living away from some sites [6, 27]. In the AdDIT study, weekend and after-hours visits and shared care strategies were implemented at some sites based upon advice provided by participant and parent/caregiver advisory group who emphasized this need, in particular, for two-household families and parents with restrictive employment schedules. AdDIT was also conducted prior to the more recent wider adoption of virtual research visits, which may allow for greater engagement of the underserved [8, 28,29,30].
This study has some limitations. Only a subset of the original AdDIT cohort could be evaluated in the current study due to local ethics restrictions at certain sites/countries. This could have potentially impacted our results, particularly for AdDIT UK, as a large portion of the cohort could not be assessed. We used population-level measures developed by national agencies to quantify social inequity within populations that are created by agglomerating multiple socioeconomic and environmental census data sources. It is important to recognize that individual national indexes vary with regard to specific components used to assess marginalization and SES and this is the reason why data in this report are reported and described separately for each country [17]. While area-based measures of SES may not be an ideal proxy for individual-based SES measures, they are valuable in describing socioeconomic trends where participant-level data are not available [31, 32]. Our study was limited to aggregate-level data for most regional and national reference groups, limiting our ability to evaluate and compare clinical characteristics such as HbA1c, ACR and insulin therapy. This report also has limited data on the impact of ethnicity on recruitment, as this data was not universally available from regional and national sources. Lastly, our study applied an assumption of uniform SES distribution for paediatric type 1 diabetes in Canada due to limited reference data at the national level, which may not reflect the true distribution of SES for this population.
Conclusions
As a large, multicenter trial, this report is unique as it describes socioeconomic details of clinical trial participants and how they compare with the populations from which they were sampled. This report also highlights the realities and challenges of contemporary clinical research in the context of broader social and economic inequities. Around 40–60% of clinical trials have insufficient or delayed recruitment, and investigators are often challenged to counterpoise the pressures of recruitment using more available, higher SES participants with those who are more representative [33,34,35]. Elements to consider in trial design would include snowball or social network-based approaches and community partnerships in addition to flexible data collection strategies [6, 33]. Trial recruitment and conduct should include personalized, needs-based approaches that include appropriate financial compensation and address cultural and language barriers to participation. These strategies are important to not only increase the level of involvement and participation in health research for disadvantaged groups, but also ensure the outcomes and implementation of health research are relevant.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available but reasonable requests may be forwarded to the AdDIT Steering Committee for review.
Abbreviations
- ACR:
-
Albumin-creatinine ratio
- AdDIT:
-
Adolescent type 1 Diabetes cardiorenal Intervention Trial
- ADDN:
-
Australasian Diabetes Data Network
- AIHW:
-
Australian Institute for Health and Wellbeing
- CCDSS:
-
Canadian Chronic Disease Surveillance System
- CAN-Marg:
-
Canadian Marginalisation Index
- IMD:
-
Index of Multiple Deprivation
- IRSD:
-
Index of Relative Socioeconomic Disadvantage
- LSOA:
-
Lower Layer Super Output Area
- MR:
-
Material Resources
- NHS:
-
National Health Service
- NPDA:
-
National Pediatric Diabetes Audit
- PCCF:
-
Postal Code Conversion File
- PDN:
-
Pediatric Diabetes Network
- SEIFA:
-
Socio-Economic Index For Areas
- SES:
-
Socioeconomic status
- UK:
-
United Kingdom
- WIMD:
-
Welsh Index of Multiple Deprivation
References
Alegria M, Sud S, Steinberg BE, Gai N, Siddiqui A. Reporting of participant race, sex, and socioeconomic status in randomized clinical trials in general medical journals, 2015 vs 2019. JAMA Netw Open. 2021;4(5):e2111516.
Buttery SC, Philip KEJ, Alghamdi SM, Williams PJ, Quint JK, Hopkinson NS. Reporting of data on participant ethnicity and socioeconomic status in high-impact medical journals: a targeted literature review. BMJ Open. 2022;12(8):e064276.
Magin P, Victoire A, Zhen XM, Furler J, Pirotta M, Lasserson DS, et al. Under-reporting of socioeconomic status of patients in stroke trials: adherence to CONSORT principles. Stroke. 2013;44(10):2920–2.
Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess. 2005;9(38):iii-iv, ix-x, 1–152.
Lee SJ, Kavanaugh A. A need for greater reporting of socioeconomic status and race in clinical trials. Ann Rheum Dis. 2004;63(12):1700–1.
Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: Critical Need for New Standards. Diabetes Care. 2021;44(6):e121–3.
Leach CR, Schoenberg NE, Hatcher J. Factors associated with participation in cancer prevention and control studies among rural Appalachian women. Fam Community Health. 2011;34(2):119–25.
Blumenthal DS, Sung J, Coates R, Williams J, Liff J. Recruitment and retention of subjects for a longitudinal cancer prevention study in an inner-city black community. Health Serv Res. 1995;30(1 Pt 2):197–205.
Zuijdwijk CS, Cuerden M, Mahmud FH. Social determinants of health on glycemic control in pediatric type 1 diabetes. J Pediatr. 2013;162(4):730–5.
Hershey JA, Morone J, Lipman TH, Hawkes CP. Social determinants of health, goals and outcomes in high-risk children with type 1 diabetes. Can J Diabetes. 2021;45(5):444-50.e1.
Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258–79.
Welch V, Jull J, Petkovic J, Armstrong R, Boyer Y, Cuervo LG, et al. Protocol for the development of a CONSORT-equity guideline to improve reporting of health equity in randomized trials. Implement Sci. 2015;10:146.
Gagné T, Ghenadenik AE. Rethinking the relationship between socioeconomic status and health: challenging how socioeconomic status is currently used in health inequality research. Scand J Public Health. 2018;46(1):53–6.
Brahan D, Bauchner H. Changes in reporting of race/ethnicity, socioeconomic status, gender, and age over 10 years. Pediatrics. 2005;115(2):e163–6.
Marcovecchio ML, Chiesa ST, Bond S, Daneman D, Dawson S, Donaghue KC, et al. ACE inhibitors and statins in adolescents with type 1 diabetes. N Engl J Med. 2017;377(18):1733–45.
Matheson FI, Dunn JR, Smith KL, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health. 2012;103(8 Suppl 2):S12-6.
Kontopantelis E, Mamas MA, van Marwijk H, Ryan AM, Buchan IE, Ashcroft DM, et al. Geographical epidemiology of health and overall deprivation in England, its changes and persistence from 2004 to 2015: a longitudinal spatial population study. J Epidemiol Community Health. 2018;72(2):140–7.
2019 NPDA. https://www.rcpch.ac.uk/work-we-do/clinical-audits/npda. Accessed 5 Sep 2023.
Lix LM, Ayles J, Bartholomew S, Cooke CA, Ellison J, Emond V, et al. The Canadian Chronic Disease Surveillance System: a model for collaborative surveillance. Int J Popul Data Sci. 2018;3(3):433.
Nakhla M, Rahme E, Simard M, Guttmann A. Outcomes associated with a pediatric clinical diabetes network in Ontario: a population-based time-trend analysis. CMAJ Open. 2017;5(3):E586–93.
Welfare AIoHa. https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/diabetes/overview.
Zwiers M, Kleinhans R, van Ham M. Divided cities: increasing socio-spatial polarization within large cities in the Netherlands. St. Louis: Federal Reserve Bank of St Louis; 2015.
Breau S, Shin M, Burkhart N. Pulling apart: new perspectives on the spatial dimensions of neighbourhood income disparities in Canadian cities. J Geogr Syst. 2018;20(1):1–25.
Daley J, Wood D and Chivers C 2017, Regional patterns of Australia’s economy and population. Grattan Instutute Regional Patterns of Australia’s economy and population. Grattan Instutute; 2017. https://grattan.edu.au/wp-content/uploads/2017/08/890-Regional-patterns.pdf.
Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:MR000013. https://doi.org/10.1002/14651858.MR000013.pub6. Accessed 11 Nov 2023.
Peyser ND, Marcus GM, Beatty AL, Olgin JE, Pletcher MJ. Digital platforms for clinical trials: The Eureka experience. Contemp Clin Trials. 2022;115: 106710.
Agarwal S, Simmonds I, Myers AK. The use of diabetes technology to address inequity in health outcomes: limitations and opportunities. Curr Diab Rep. 2022;22(7):275–81.
Susukida R, Crum RM, Stuart EA, Ebnesajjad C, Mojtabai R. Assessing sample representativeness in randomized controlled trials: application to the National Institute of Drug Abuse Clinical Trials Network. Addiction. 2016;111(7):1226–34.
Pichora E, Polsky JY, Catley C, Perumal N, Jin J, Allin S. Comparing individual and area-based income measures: impact on analysis of inequality in smoking, obesity, and diabetes rates in Canadians 2003–2013. Can J Public Health. 2018;109(3):410–8.
Buajitti E, Chiodo S, Rosella LC. Agreement between area- and individual-level income measures in a population-based cohort: implications for population health research. SSM Popul Health. 2020;10: 100553.
Watson SE, Smith P, Snowden J, Vaughn V, Cottrell L, Madden CA, et al. Facilitators and barriers to pediatric clinical trial recruitment and retention in rural and community settings: A scoping review of the literature. Clin Transl Sci. 2022;15(4):838–53.
Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110(11):2385–93.
Tanner A, Kim SH, Friedman DB, Foster C, Bergeron CD. Promoting clinical research to medically underserved communities: current practices and perceptions about clinical trial recruiting strategies. Contemp Clin Trials. 2015;41:39–44.
Acknowledgements
The authors want to acknowledge persons with type 1 diabetes for their support and participation in the AdDIT study. We would also like to recognize and acknowledge the invaluable contributions and leadership of Prof. David Dunger as part of the AdDIT study.
Funding
This work was supported by the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) Network and funded by the Canadian Institute of Health Research – Strategies for Patient Oriented Research and the Juvenile Diabetes Research Foundation-Canadian Clinical Trials Network. The AdDIT study was also supported by The British Heart Foundation, Diabetes UK, Juvenile Diabetes Research Foundation, Canadian Diabetes Association and Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Contributions
F.H.M., A.B.M.C. and Y.E. were involved in the conception, design and conduct of the study in addition to the analysis and interpretation of the results. F.H.M., A.B.M.C. and Y.E. wrote the first draft of the manuscript. F.H.M., A.B.M.C, Y.E., J.C., P.B.A., F.J.C, S.T.C, C.C., J.J.C., M.E.C., R.N.D., D.D., E.A.D., J.E.D., K.C.D., T.W.J., S.M.M., A.N. and M.L.M. were involved in the conduct of the AdDIT clinical trial and edited, reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained for all participants involved in this study (SickKids REB 1000012240).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Country-specific measures of deprivation, and sources of regional and national reference type 1 diabetes population data. Table S2. Raw counts and proportions of the UK, Canadian and Australian cohorts of AdDIT across deprivation quintiles. Intra-quintile differences are presented. Figure S1. Flowchart of study participants from the original AdDIT UK, AdDIT Canada and AdDIT Australia cohorts included in the current study. Figure S2. Distribution of the Experimental and Observational study arms among the A) AdDIT UK and B) AdDIT Canada cohorts across country-specific deprivation quintiles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmud, F.H., Clarke, A.B.M., Elia, Y. et al. Socioeconomic representativeness of Australian, Canadian and British cohorts from the paediatric diabetes AdDIT study: comparisons to regional and national data. BMC Med 21, 506 (2023). https://doi.org/10.1186/s12916-023-03222-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-03222-w