Abstract
Background
Health equity concerns the absence of avoidable and unfair differences in health. Randomized controlled trials (RCTs) can provide evidence about the impact of an intervention on health equity for specific disadvantaged populations or in general populations; this is important for equity-focused decision-making. Previous work has identified a lack of adequate reporting guidelines for assessing health equity in RCTs. The objective of this study is to develop guidelines to improve the reporting of health equity considerations in RCTs, as an extension of the Consolidated Standards of Reporting Trials (CONSORT).
Methods/design
A six-phase study using integrated knowledge translation governed by a study executive and advisory board will assemble empirical evidence to inform the CONSORT-equity extension. To create the guideline, the following steps are proposed: (1) develop a conceptual framework for identifying “equity-relevant trials,” (2) assess empirical evidence regarding reporting of equity-relevant trials, (3) consult with global methods and content experts on how to improve reporting of health equity in RCTs, (4) collect broad feedback and prioritize items needed to improve reporting of health equity in RCTs, (5) establish consensus on the CONSORT-equity extension: the guideline for equity-relevant trials, and (6) broadly disseminate and implement the CONSORT-equity extension.
Discussion
This work will be relevant to a broad range of RCTs addressing questions of effectiveness for strategies to improve practice and policy in the areas of social determinants of health, clinical care, health systems, public health, and international development, where health and/or access to health care is a primary outcome. The outcomes include a reporting guideline (CONSORT-equity extension) for equity-relevant RCTs and a knowledge translation strategy to broadly encourage its uptake and use by journal editors, authors, and funding agencies.
Similar content being viewed by others
Background
Health equity is defined as the absence of avoidable and unfair differences in health within and between populations [1] and is at the core of global health research priorities [2, 3]. The concept of health equity includes both access to health care as well as the broader concept of opportunities to achieve good health [4–6]. While there is a lack of consensus on the use of the terms health equity, health inequality, and health disparities, we have chosen the term “health equity” [7]. We consider differences in health outcomes across socially stratifying factors such as age, sex/gender, culture, and socioeconomic status to be health inequities when they are considered avoidable and unfair (Table 1). Absent and/or poor quality evidence about health equity is identified by policy makers as a key limitation of research [8, 9]. For example, evidence about poor or black or Hispanic populations is missing in reviews used for drug formulary development in the USA [10]. To address key public health objectives, decision-makers require the best evidence to guide appropriate consideration of the likely effects on health equity in their populations [3, 11, 12].
Randomized controlled trials (RCTs) are a powerful research design for ascertaining the impact of an intervention and for informing health decisions [13]. There is growing support for well-conducted RCTs to improve the evidence base for the growing field of personalized medicine [14] as well as population and public health [15] and international development [16]. We define RCTs that meet one (or more) of the following criteria as “equity-relevant trials”: (1) assessing effects in a disadvantaged population in relation to a less disadvantaged population (see Table 1), (2) assessing differences in effects between populations considered disadvantaged compared to a less disadvantaged group (see Table 1), or (3) assessing gradient of effects across levels of disadvantage.
There are a number of challenges to overcome if RCTs are to contribute to a robust evidence base for policy making that promotes health equity across populations [17, 18]. There is well-documented under-representation of populations in RCTs who may be disadvantaged due to their ethnicity within a population, or their age, or gender. Even when these groups are included in RCTs, the authors of the trials often fail to report basic sociodemographic details [19] and rarely are subgroup analyses conducted across PROGRESS-Plus characteristics [20, 21]. All too often, this makes it impossible to apply results to populations with particular characteristics or features. Cluster RCTs pose additional challenges when assessing effects on health equity because clusters may include individuals experiencing disadvantage and/or clusters may have different overall experience of disadvantage. Systematic reviews are completely dependent upon the constituent studies, so poor reporting of equity in RCTs is a major constraint on equity assessment in systematic reviews, as has been described frequently but specifically in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)-equity reporting guidelines [22].
Developing a CONSORT reporting guideline for equity informing trials
The Consolidated Standards for Reporting Trials (CONSORT) statement is an evidence-based guideline consisting of 25 items, intended to encourage completeness and transparency of reporting RCTs. CONSORT has been endorsed by over 50 % of the medical journals indexed in PubMed and has been shown by a systematic review to improve reporting [23]. CONSORT extension statements have been developed for specific issues, such as reporting of cluster RCTs, harms, pragmatic trials, non-pharmacologic therapy, and social and psychological interventions [24]. None of the existing or planned CONSORT extensions addresses reporting characteristics needed to assess the effects of an intervention on health equity (Appendix A).
Objectives
The main objective of this study is to develop guidelines to improve the reporting of health equity considerations in RCTs, as an extension of CONSORT. This program of research aims to meet the following specific objectives, each of which aligns with a study phase (Fig. 1):
-
1.
To develop a conceptual framework for identifying equity-relevant trials.
-
2.
To assess empirical evidence regarding reporting of equity-relevant trials.
-
3.
To consult with global methods and content experts on how to improve reporting of health equity in RCTs.
-
4.
To collect broad feedback and prioritize items needed to improve reporting of health equity in RCTs.
-
5.
To establish consensus on the CONSORT-equity extension: a reporting guideline for equity-relevant trials.
-
6.
To broadly disseminate and implement the CONSORT-equity extension.
Methods/design
The study consists of six phases, adapted from the guidance for developing reporting guidelines by Moher et al. [25]. These are identifying the need for the guideline, reviewing the literature, identifying participants, conducting a Delphi survey to gather opinions and set priorities, and holding a face-to-face consensus meeting. There will be two additions to these methods: (1) a review of guidance from research ethics boards and funding agencies about equity, inclusion, and diversity, and (2) consultation using key informant interviews across a broad range of disciplines to gather views on how to improve reporting of effects in subpopulations in RCTs (Fig. 1).
Ensuring the uptake of the CONSORT-equity extension is critical in order to influence reporting of future trials. Therefore, we are using an integrated knowledge translation approach that engages knowledge users as partners throughout the process and as a way to foster thinking about, inclusion, and respect for a multiplicity of perspectives [26]. The co-authors represent a range of disciplines including clinical epidemiology, social science, public health, and international development and are consulted regularly through quarterly meetings. We have developed an international advisory board from intended users across a range of perspectives: journal editors, trialists, bioethicists, patients and members of the public, clinicians, systematic review authors, policy makers, members of disadvantaged populations, and funders (Appendix B). Completion of all study objectives is anticipated by December 2017.
Phase 1: Development of a conceptual framework
We will use an iterative process to develop a conceptual framework for identifying equity-relevant RCTs. To date, we have consulted within the research team and advisory board and reviewed conceptual papers [27–31] to develop a draft conceptual framework (Fig. 2). We tested this framework on sample trials with subgroup analyses across one or more PROGRESS-Plus characteristic and trials conducted in populations who may be disadvantaged in some settings including children, older adults, and individuals with lower income identified by searching PubMed.
We acknowledge that although most health conditions exhibit a gradient of worse health for those experiencing more disadvantage and conventionally described or analyzed using socioeconomic variables such as ethnicity and educational attainment [32], many RCTs have not been powered or designed to provide evidence about health equity; often, feasibility and cost are used as defining reasons. Studies are to be considered for inclusion if they provide estimates of these effects, regardless of the sample or method used to do so. In addition, the appropriateness of sample and method will be assessed as part of the study. We will consider RCTs to be “equity-relevant” if they provide evidence about the following:
-
(1)
Effects in a population considered to be disadvantaged,
-
(2)
Difference or equivalence in effects across socially stratifying factors, or
-
(3)
Gradient of effects across socially stratifying factors.
The first criterion provides direct evidence about health equity by comparing effects across strata of disadvantage. The latter two criteria provide indirect evidence about health equity because there is no comparison to a more advantaged population (Table 2). This framework aligns closely with the framework by Hilary Graham that categorizes three approaches to tackling health inequalities and their implications for evaluation [27]. Furthermore, differences in effects may be assessed by different methods ranging from subgroup analyses to assessing external validity to populations not included in the trial (e.g., many disadvantaged groups are excluded from trials because of restrictive eligibility criteria such as presence of co-morbidities or age).
We will test the clarity, acceptability, and feasibility of using this conceptual framework by discussing sample trials with three types of stakeholders:
-
1.
Advisory board members (Appendix B)
-
2.
Investigators of effectiveness trials committed to informing equity
-
3.
Community representatives from disadvantaged populations
This may result in additional changes to the conceptual framework.
Phase 2: Assessing the evidence
We will address three research questions about the strengths and limitations of methods used to provide evidence about equity in RCTs as well as existing guidance regarding the use of these methods:
-
1.
What are the strengths and limitations of methods used in equity-relevant trials?
-
2.
What is the existing guidance on conducting equity-relevant trials?
-
3.
What is the quality of reporting of equity-relevant trials?
Phase 2, question 1: What are the strengths and limitations of methods used in equity-relevant trials?
We will conduct a Cochrane methodology systematic review to assess the strengths and limitations of methods used to address equity in equity-relevant trials. A methodology review is different from an intervention review since it aims to assess the strengths and limitations of methods used for research rather than the impact of interventions. For example, Welch and colleagues used a similar approach to assess the strengths and limitations of methods to assess equity in systematic reviews [33]. We will develop an a priori protocol, following Cochrane Handbook methods [34] and Cochrane Methodology Review Group guidance [35]. The eligible studies will be empirical studies of methods used in equity-relevant trials. We have identified examples of eligible studies in Table 3. We will design a search for evidence with a librarian scientist (JM) encompassing electronic databases (e.g., MEDLINE, The Cochrane Library, Cochrane Methodology register, Sociological abstracts) and gray literature, using a combination of text words and subject headings, and assess its ability to identify a reference set of known articles (Appendix C). We will also use Web of Science to search for studies that cite eligible studies [36]. The protocol will be submitted for publication in The Cochrane Library. The results of this review will contribute candidate items for reporting equity-relevant trials.
Phase 2, question 2: What is existing guidance on conducting equity-relevant trials?
We will conduct a review of existing guidance for equity-relevant trials such as inclusion of vulnerable populations and ethical concerns. This will include guidance developed by any type of organization, such as academic groups (e.g., CONSORT), funding agencies (e.g., NIH guidance on inclusion of women and minorities), governmental and non-governmental organizations (e.g., Institute of Medicine guidance on sex-specific reporting in research [37]), and the National Guidelines Clearinghouse (www.guideline.gov). We will design a search strategy according to the Peer-Reviewed Electronic Search Strategies (PRESS) guideline [36] that includes both electronic databases and targeted search of websites of relevant organizations with an experienced librarian scientist (JM). Websites of organizations that provide ethics guidance will be searched (e.g., Institute of Medicine, Council for International Organizations of Medical Sciences and World Association of Medical Editors), as well as conducting open web searches.
Data will be extracted using a pretested form on the methods of developing the guidance, values statements (if provided), items related to study design, inclusion and protection of different disadvantaged populations, and any evidence on validation, uptake, or use of the guidelines. We will assess the extent to which populations defined as disadvantaged across PROGRESS-Plus criteria are considered in the guidance. The items identified by this review will be considered for inclusion in CONSORT-equity.
Phase 2, question 3: What is the quality of reporting equity-relevant trials?
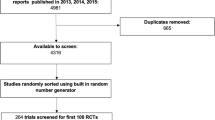
We will conduct an empirical review of a random sample of published equity-relevant trials to assess methodological quality and reporting of equity considerations. We will develop a search strategy for equity-relevant trials, in collaboration with a librarian scientist (JM), using both text words and MeSH headings, and test this search with a reference set of equity-relevant trials. We will search MEDLINE, Sociological abstracts, and Econlit to encompass medical, public health, and international development interventions. We will restrict the search to the last 3 years since reporting has improved over time, and we expect a sufficient number of RCTs in this period to meet our criteria. Two independent reviewers will screen titles and abstracts for eligibility using the conceptual framework to identify equity-relevant trials. We will select a random sample of 100 individually randomized trials and 100 cluster RCTs. We decided to balance the sample according to cluster versus individual randomization to ensure an adequate number of each design, because cluster RCTs have unique equity considerations and because they may require different analysis strategies [38]. We will extract data regarding the study design, populations, and analysis across PROGRESS-Plus characteristics using a pretested data extraction form, with two independent reviewers. We will assess reporting of methods using accepted criteria. For example, we will assess the extent to which subgroup analyses meet criteria for credibility [34]. We expect that the most commonly used methods will be subgroup analyses, and if these are used by 25 % of trials, each sample of 100 trials will provide a margin of error of ±8.5 % around this proportion. We will describe the range of methods used to provide evidence about equity (e.g., subgroup analysis, comparison of trial populations with target population to judge external validity [39]), any comparison between methods (if done), and quality of reporting or conduct for specific methods (e.g., quality of subgroup analyses). The results will be used to identify candidate items for the CONSORT-equity guideline.
Phase 3: To consult with global experts on candidate items to improve reporting of equity-relevant trials
Health equity issues span a breadth of disciplinary fields that may have different approaches to reporting trials. Because we aim for broad relevance of this reporting guideline, we want to seek opinions regarding how to improve reporting of equity-relevant trials from different disciplinary perspectives. We will conduct key informant, semi-structured interviews since this is an efficient way to engage diverse stakeholders, and then collect and synthesize their views using thematic analysis. We will use results of the empirical studies (phase 2) to design a semi-structured interview guide. The interview guide will be designed to invite feedback on candidate items identified in the prior studies as well as seeking new items.
We will identify participants through our co-authors and advisory board as well as lead authors of empirical studies and other guidance (e.g., ethics, funding agencies). We will select participants to maximize variation of disciplines and stakeholder organizations (e.g., academic, non-governmental, research ethics boards, governmental). We will conduct interviews by phone or face to face and take notes during the interviews as well as tape record interviews. We will expand our sample by snowball sampling: an approach to recruit participants that builds on networks by asking each participant to suggest additional contacts [40]. Sample size will be determined by theoretical saturation, defined as when subsequent interviews contribute no new data, and is estimated to occur at 10–13 interviews [41]. Thematic analysis of transcribed interviews will be conducted by two coders, using NVivo qualitative software to facilitate analysis [42]. This portion of the study has been submitted to the Bruyère Research Institute Ethics Board for approval.
Phase 4: To prioritize candidate items for reporting guideline
Prior to seeking consensus (phase 5), it is important to seek external feedback on the importance of different candidate items identified in the preceding empirical studies [25]. In this phase, we aim to prioritize items and invite feedback on proposed items. We will use the Delphi process for this prioritization because it is a structured, iterative approach to obtaining information by posing a series of questions to a select group of experts in the area for which the information is sought [43]. To identify participants, we will use electronic mailing lists and social media to reach members of the intended users of the reporting guideline, such as trialists, methodologists, clinicians, and decision-makers. Patient engagement and citizen participation is critical from an equity perspective since patients and citizens are the intended beneficiaries of the results of trials. Patient and citizen views will be sought by engaging with networks of patients and citizens such as the Cochrane Consumer Network (led by AL, who is the consumer representative on the Cochrane Steering Group).
We will present a preliminary list of candidate items, with examples, and ask participants to rank their importance using an online survey tool. We will invite open-ended comments and suggestions for new items. We plan to conduct up to three rounds of an online Delphi survey, as for previous CONSORT guidelines [44–46]. If consensus is reached earlier, we will conduct fewer rounds of the survey. This study has been submitted to the Bruyère Research Institute Ethics Board for approval.
Phase 5: Consensus development
As recommended by Moher et al. [25], we will hold a 2-day face-to-face consensus meeting to engage and build consensus with users of the reporting guideline. We will design this meeting to facilitate engagement and equitable opportunities for contributions from all conference participants, by assigning roles as chairs, facilitators, discussants, and rapporteurs to each participant, as used previously by Welch et al. [22]. Participants will include members of the research team and advisory board and may include other external stakeholders.
During the meeting, we will present the results for each candidate item from the empirical studies and the Delphi process with examples and use a structured discussion to reach consensus on included items. The discussions will be audio-recorded and transcribed verbatim. As with other consensus meetings, word crafting will be left until post-meeting. We will use meeting transcripts and notes to finalize the CONSORT-equity reporting guideline.
Next, we will conduct an iterative process of usability testing of the guideline by intended users to assess clarity and acceptability of items. Written and oral feedback from these users will be incorporated. Consensus group members will participate in an iterative process of crafting the final guideline. We will develop an elaboration and explanation document with exemplars of good reporting and details of empiric evidence to support each item. This study has been submitted to the Bruyère Research Institute Ethics Board for approval.
Phase 6: Dissemination and implementation
We will develop a knowledge translation (KT) plan at the consensus conference, led by JMG, a known expert in the area of implementation science and KT. We will design strategies to promote implementation of the reporting guideline by thought leaders who publish, use, fund, or conduct RCTs across different disciplines. Possible strategies may include training workshops at events, such as at the Cochrane Colloquia and the Peer Review Congress, and webinars that will be made available on open-access websites such as the Campbell and Cochrane Equity Methods group website [47]. To reach journal editors and funders, we expect to use targeted approaches such as direct letters with specific messages (e.g., how to include this reporting guideline in instructions for authors and applicants). Throughout the study, we will use information technology to raise awareness, maintain a list of publications, and invite comments through an open-access website [48], writing blogs, and the use of social media, including twitter [49].
Discussion
Following the WHO Commission on the Social Determinants of Health (CSDH), there is increasing interest in health equity [17]. However, variable or inadequate reporting of data from trials which can inform health equity decisions could contribute to waste in research and may not serve the needs of people who are experiencing health inequities. This program of research is the first known attempt to improve the reporting of health equity in RCTs and has been designed to complement current and in-development CONSORT statement extensions. Improved reporting of equity considerations is relevant to health research funders, decision-makers, and practitioners who use evidence from RCTs to inform decisions, researchers conducting equity-oriented systematic reviews, and civil society who benefit from these decisions. We hope this reporting guideline will contribute to an improved evidence base for equity-oriented decisions and promote a broader agenda in relation to health equity.
References
Whitehead M. The concepts and principles of equity and health. Int J Health Serv. 1992;22(3):429–45.
World Health Organization. Changing mindsets—strategy on health policy and systems research. 2012. ISBN 9789241504409. Available from http://www.who.int/alliance-hpsr/alliancehpsr_changingmindsets_strategyhpsr.pdf. Accessed 13 July 2015.
World Health Assembly. WHO strategy on research for health WHA63.21. Accessed 19 Jun 2015 from http://www.who.int/phi/WHO_Strategy_on_research_for_health.pdf
Sen A. Why health equity? Health Econ. 2002;11(8):659–66. doi:10.1002/hec.762.
Levesque JF, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12:18. doi:10.1186/1475-9276-12-18.
Marmot M, Friel S. Global health equity: evidence for action on the social determinants of health. J Epidemiol Community Health. 2008;62(12):1095–7. doi:10.1136/jech.2008.081695.
Gwatkin DR. Health inequalities and the health of the poor: what do we know? What can we do? Bull World Health Organ. 2000;78(1):3–18.
Petticrew M, Whitehead M, Macintyre SJ, Graham H, Egan M. Evidence for public health policy on inequalities: 1: the reality according to policymakers. J Epidemiol Community Health. 2004;58(10):811–6. doi:10.1136/jech.2003.015289.
Tugwell P, Petticrew M, Kristjansson E, Welch V, Ueffing E, Waters E, et al. Assessing equity in systematic reviews: realising the recommendations of the Commission on Social Determinants of Health. BMJ. 2010;341:c4739. doi:10.1136/bmj.c4739.
Odierna DH, Bero LA. Systematic reviews reveal unrepresentative evidence for the development of drug formularies for poor and nonwhite populations. J Clin Epidemiol. 2009;62(12):1268–78. doi:10.1016/j.jclinepi.2009.01.009.
Pan American Health Organization, 49th Directing Council, 61st Session of the Regional Committee of WHO for the Americas. Policy on research for health: document CD49/10. Washington, DC: PAHO; 2009. http://www2.paho.org/hq/dmdocuments/2009/CD49-10-e.pdf.
Pan American Health Organization. Public health in the Americas. 2002. Washington, D.C.: PAHO; 2002. (SP-E-587). Accessed 19 Jun 2015 from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=4036:la-salud-publica-americas&Itemid=3617&lang=en
Sackett D, Rosenberg W, Gray J, Haynes R, Richardson W. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–2.
Dzau VJ, Ginsburg GS, Van Nuys K, Agus D, Goldman D. Aligning incentives to fulfil the promise of personalised medicine. Lancet. 2015;385(9982):2118–9. doi:10.1016/s0140-6736(15)60722-x.
Macintyre S. Good intentions and received wisdom are not good enough: the need for controlled trials in public health. (1470–2738 (Electronic)).
Banerjee A, Duflo E. Poor economics: a radical rethinking of the way to fight global poverty. New York: Public Affairs; 2011.
Marmot MG. Policy making with health equity at its heart. JAMA. 2012;307(19):2033–4. doi:10.1001/jama.2012.3534.
Kaufman JS, Harper S. Health equity: utopian and scientific. Prev Med. 2013;57(6):739–40. doi:10.1016/j.ypmed.2013.09.013.
Furler J, Magin P, Pirotta M, van Driel M. Participant demographics reported in “Table 1” of randomised controlled trials: a case of “inverse evidence”? Int J Equity Health. 2012;11:14. doi:10.1186/1475-9276-11-14.
Welch V, Petticrew M, Ueffing E, Benkhalti Jandu M, Brand K, Dhaliwal B, et al. Does consideration and assessment of effects on health equity affect the conclusions of systematic reviews? A methodology study. PLoS ONE. 2012;7(3):e31360. doi:10.1371/journal.pone.0031360.
Tugwell P, Maxwell L, Welch V, Kristjansson E, Petticrew M, Wells G, et al. Is health equity considered in systematic reviews of the Cochrane Musculoskeletal Group? Arthritis Rheum. 2008;59(11):1603–10. doi:10.1002/art.24206.
Welch V, Petticrew M, Tugwell P, Moher D, O’Neill J, Waters E, et al. PRISMA-equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333. doi:10.1371/journal.pmed.1001333.
Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Syst Reviews. 2012;1:60. doi:10.1186/2046-4053-1-60.
CONSORT extension statements. http://www.consort-statement.org/extensions. Accessed 04 September, 2015
Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2):e1000217. doi:10.1371/journal.pmed.1000217.
Canadian Institute of Health Research. Guide to knowledge translation planning at CIHR: integrated and end-of-grant approaches. 2015. available at: http://www.cihr-irsc.gc.ca/e/45321.html
Graham H. Tackling inequalities in health in England: remedying health disadvantages, narrowing health gaps or reducing health gradients? J Soc Policy. 2004;33(01):115–31. doi:10.1017/S0047279403007220.
Marmot M, Friel S, Bell R, Houweling TA, Taylor S, Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–9. doi:10.1016/S0140-6736(08)61690-6.
Whitehead M. A typology of actions to tackle social inequalities in health. J Epidemiol Community Health. 2007;61(6):473–8. doi:10.1136/jech.2005.037242.
Ogilvie D, Fayter D, Petticrew M, Sowden A, Thomas S, Whitehead M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8:8. doi:10.1186/1471-2288-8-8.
Benach J, Malmusi D, Yasui Y, Martinez JM. A new typology of policies to tackle health inequalities and scenarios of impact based on Rose’s population approach. J Epidemiol Community Health. 2013;67(3):286–91. doi:10.1136/jech-2011-200363.
Marmot M, Commission on Social Determinants of Health. Achieving health equity: from root causes to fair outcomes. Lancet. 2007;370(9593):1153–63. doi:10.1016/S0140-6736(07)61385-3.
Welch V, Tugwell P, Petticrew M, de Montigny J, Ueffing E, Kristjansson B, et al. How effects on health equity are assessed in systematic reviews of interventions. Cochrane Database Syst Reviews. 2010;12:MR000028. doi:10.1002/14651858.MR000028.pub2.
Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. The influence of study characteristics on reporting of subgroup analyses in randomised controlled trials: systematic review. BMJ. 2011;342:d1569. doi:10.1136/bmj.d1569.
The Editorial Team. Cochrane Methodology Review Group. 2012.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–52. doi:10.1016/j.jclinepi.2008.10.012.
Institute of Medicine. Sex-specific reporting of scientific research, Available at: http://www.iom.edu/Reports/2012/Sex-Specific-Reporting-of-Scientific-Research.aspx 2012.
Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi:10.1136/bmj.e5661.
Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4(2):112–21.
Marshall MN. Sampling for qualitative research. Fam Pract. 1996;13(6):522–5.
Francis J, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles M, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229–45.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1):e1000393. doi:10.1371/journal.pmed.1000393.
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi:10.1371/journal.pmed.0050020.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Montgomery P, Grant S, Hopewell S, Macdonald G, Moher D, Michie S, et al. Protocol for CONSORT-SPI: an extension for social and psychological interventions. Implementation Sci IS. 2013;8:99. doi:10.1186/1748-5908-8-99.
The Campbell and Cochrane Equity Methods Group. http://equity.cochrane.org/. Accessed 04 September 2015.
CONSORT equity. http://equity.cochrane.org/consort-equity
Petkovic J. #cochraneequity.
Last JM. A dictionary of epidemiology. J Epidemiol Community Health. 1993;47(5):430.
Tan-Torres T. Health, poverty and equity at the close of the century scholar. Ottawa: Forging Links for Health Research: Perspectives from the Council on Health Research for Development; 2001.
Peter F, Evans T. Ethical dimensions of health equity. Challenging inequities in health. From ethics to action. New York, NY, USA: Oxford University Press; 2001.
Evans T, Brown H. Road traffic crashes: operationalizing equity in the context of health sector reform. Inj Control Saf Promot. 2003;10(1–2):11–2. doi:10.1076/icsp.10.1.11.14117.
O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M et al. Applying an equity lens to interventions: using PROGRESS to ensure consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology. 2013;in press.
Oliver S KJ, Kavanagh J, Caird J, Lorenc T, Oliver K, Harden A, et al. Health promotion, inequalities and young people’s health. A systematic review of research. EPPI-Centre. 2008.
Oliver S, Dickson K, Newman M. Getting started with a review. In: Gough SO D, Thomas J, editors. An introduction to systematic reviews. London, UK: SAGE Publications; 2012.
Campbell and Cochrane Equity Methods Group. Cochrane Equity. @CochraneEquity; 2015. Available from: https://twitter.com/cochraneequity
Rousseau C, Beauregard C, Daignault K, Petrakos H, Thombs BD, Steele R, et al. A cluster randomized-controlled trial of a classroom-based drama workshop program to improve mental health outcomes among immigrant and refugee youth in special classes. PLoS One. 2014;9(8):e104704. doi:10.1371/journal.pone.0104704.
Andersson N, Shea B, Amaratunga C, McGuire P, Sioui G. Rebuilding from resilience: research framework for a randomized controlled trial of community-led interventions to prevent domestic violence in aboriginal communities. Pimatisiwin. 2010;8(2):61–88.
Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64(16):1669–78. doi:10.1016/j.jacc.2014.04.087.
Marteau TM, Mann E, Prevost AT, Vasconcelos JC, Kellar I, Sanderson S, et al. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial. BMJ. 2010;340:c2138. doi:10.1136/bmj.c2138.
Kendzor DE, Reitzel LR, Mazas CA, Cofta-Woerpel LM, Cao Y, Ji L, et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med. 2012;74(9):1394–401. doi:10.1016/j.socscimed.2012.01.013.
Yamashita S, Takahashi S, Osaka Y, Fujikura K, Tabata K, Tanaka M. Efficacy of the transillumination method for appropriate tracheal tube placement in small children: a randomized controlled trial. J Clin Anesth. 2015;27(1):12–6. doi:10.1016/j.jclinane.2014.09.003.
Hui C, Joughin E, Nettel-Aguirre A, Goldstein S, Harder J, Kiefer G, et al. Comparison of cast materials for the treatment of congenital idiopathic clubfoot using the Ponseti method: a prospective randomized controlled trial. Can J Surg. 2014;57(4):247–53.
Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants? Trials. 2015;16:80. doi:10.1186/s13063-015-0582-8.
Aulakh AK, Anand SS. Sex and gender subgroup analyses of randomized trials. Women’s Health Issues. 2007;17(6):342–50. doi:10.1016/j.whi.2007.04.002.
Acknowledgements
We thank Dr. Susan Norris for her thorough and thoughtful review and comments on this work. We thank Manosila Yoganathan who has assisted with formatting references for this document.
YB holds a Canada Research Chair in Aboriginal Health and Wellness. DM holds a University Research Chair in Systematic Reviews. MW holds a W.H. Duncan Chair of Public Health. JMG holds a Canada Research Chair in Health Knowledge Transfer and Uptake. PT holds a Canada Research Chair in Health Equity. JP is supported by a CIHR Doctoral Research Award. Vivian Welch holds an Ontario Early Researcher Award.
We are greatly saddened by the loss of our colleague, Liz Waters, whose contributions to this work were invaluable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
PT is a co-convenor of the Campbell and Cochrane Equity Methods group.
VW is a co-convenor of the Campbell and Cochrane Equity Methods group.
JJ is a member of the Campbell and Cochrane Equity Methods group.
JP is the coordinator of the Campbell and Cochrane Equity Methods group.
JM is a member of the Campbell and Cochrane Equity Methods group and co-convenor of the Cochrane Information Retrieval Methods Group.
TP and JMG are editors with the Cochrane Effective Practice and Organisation of Care (EPOC) group.
Authors’ contributions
VW had the idea for the project and is responsible for the first and final draft of this manuscript. JJ, JP, PT, VW, MP, BS, CW, and MT have held monthly meetings to develop the conceptual framework. JJ is leading the advisory board formation and conduct. JP is leading the systematic review of equity. TR and JM contributed to the search methods. All authors have made contributions to previous drafts of this manuscript and approved the final version.
Appendix A: CONSORT standard items, selected extensions, and possible CONSORT-equity items

Appendix B: CONSORT-equity advisory board members
CONSORT-equity advisory board
-
1.
Yvonne Boyer
Canada Research Chair in Aboriginal Health and Wellness
Brandon University
-
2.
Luis Gabriel Cuervo
Senior Advisor for Research Promotion and Development, PAHO
-
3.
Sarah Edwards
University College London
-
4.
Caroline Kisia
Executive Director, Action Africa Health-International
-
5.
Anne Lydiatt
Patient partner, Canadian Institutes of Health Research Strategy for Patient Oriented Research
-
6.
Lawrence Mbuagbaw
Assistant Professor, Epidemiology and Biostatistics, McMaster University
Centre for Development of Best Practices in Health, Yaoundé Central Hospital, Cameroon
-
7.
Janet Smylie
CIHR Applied Public Health Chair and Director, Well Living House Action Research Centre for Indigenous Infant, Child and Family Health and Wellbeing, St. Michael’s Hospital and DLSPH, University of Toronto
-
8.
Jimmy Volmink
Dean, Centre for Evidence-Based Health Care, Faculty of Medicine and Health Sciences, Stellenbosch University and Cochrane South Africa, South African Medical Research Council, Cape Town, South Africa
-
9.
Margaret Whitehead
WH Duncan Professor of Public Health, Division of Public Health, School of Population, Community and Behavioural Sciences, The University of Liverpool
Appendix C: Search strategy to identify equity-relevant trials
-
1.
exp Gender Identity/
-
2.
(gender-based or gender-related or gender factors).tw.
-
3.
((sex or gender) adj3 (analysis or factor$ or inequit$ or disparit$ or inequalit$ or difference$ or interact$)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
4.
exp sex factors/
-
5.
exp geriatrics/
-
6.
((ethnic$ or race or racial or religio$ or cultur$ or minorit$ or refugee or indigenous or aboriginal or African american) adj3 (analysis or disparit$ or inequalit$ or inequit$ or difference$ or predict$ or interact$)).tw.
-
7.
exp homosexuality/
-
8.
exp disabled persons/
-
9.
((poverty or low-income or “lower income” or socioeconomic$ or socio-economic or social) adj3 (analysis or disadvantage$ or factor$ or inequalit$ or depriv$ or inequit$ or disparit$ or difference$ or predict$ or interact$)).tw.
-
10.
exp Educational Status/
-
11.
exp Socioeconomic Factors/
-
12.
((discriminat$ or social exclu$ or social inclu$) adj3 (religion or culture or race or racial or aboriginal or indigenous or ethnic$)).tw.
-
13.
((urban or rural or inner-city or remote or slum) adj3 (analysis or inequit$ or disparit$ or inequalit$ or difference$ or predict$ or interact$)).tw.
-
14.
((resource-poor or (“low income” adj countr$) or (“middle income” adj countr$) or africa or developing countr$ or “south america” or china or asia or “latin america”) adj3 (relevance or analysis or applicab$ or inequit$ or disparit$ or inequalit$ or difference$ or predict$ or interact$)).tw.
-
15.
(inequalit$ or in-equalit or equit$ or inequit$ or in-equit or disparit$ or underserved or marginali$ed).tw.
-
16.
exp indigenous populations/
-
17.
((native* or Indian or aborigin*) adj3 (American* or Canadian* or Alaska*)).tw.
-
18.
(first adj2 nation*).tw.
-
19.
(aborigin$ or metis or inuit$ or eskimo$ or native or esquimaux or aleut or yuit or inughuit or unanga* or alutiiq or inup#ia* or kalaallit or Inuktitut or Nunavut or nunavik or cree or dene or haida or salish or Mohawk or ojibway or yupik or tribal or arctic).tw.
-
20.
exp american native continental ancestry group/ or oceanic ancestry group/
-
21.
exp rural health/
-
22.
or/1–21
-
23.
randomized controlled trial.pt.
-
24.
(randomized or placebo).mp.
-
25.
(cluster$ adj2 randomi$).tw.
-
26.
or/23–25
-
27.
22 and 26
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Welch, V., Jull, J., Petkovic, J. et al. Protocol for the development of a CONSORT-equity guideline to improve reporting of health equity in randomized trials. Implementation Sci 10, 146 (2015). https://doi.org/10.1186/s13012-015-0332-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13012-015-0332-z