Abstract
Background
Pyrotinib (an irreversible pan-ErbB inhibitor) plus capecitabine has survival benefits and acceptable tolerability in patients with HER2-positive metastatic breast cancer. We further assessed addition of pyrotinib to trastuzumab and docetaxel in the neoadjuvant setting.
Methods
In this multicenter, double-blind, phase 3 study (PHEDRA), treatment-naive women with HER2-positive early or locally advanced breast cancer were randomly assigned (1:1) to receive four neoadjuvant cycles of oral pyrotinib or placebo (400 mg) once daily, plus intravenous trastuzumab (8 mg/kg loading dose, followed by 6 mg/kg) and docetaxel (100 mg/m2) every 3 weeks. The primary endpoint was the total pathological complete response (tpCR; ypT0/is and ypN0) rate per independent central review.
Results
Between Jul 23, 2018, and Jan 8, 2021, 355 patients were randomly assigned, 178 to the pyrotinib group and 177 to the placebo group. The majority of patients completed four cycles of neoadjuvant treatment as planned (92.7% and 97.7% in the pyrotinib and placebo groups, respectively). The tpCR rate was 41.0% (95% CI 34.0 to 48.4) in the pyrotinib group compared with 22.0% (95% CI 16.6 to 28.7) in the placebo group (difference, 19.0% [95% CI 9.5 to 28.4]; one-sided P < 0.0001). The objective response rate per investigator was 91.6% (95% CI 86.6 to 94.8) in the pyrotinib group and 81.9% (95% CI 75.6 to 86.9) in the placebo group after the neoadjuvant treatment, resulting in an increase of 9.7% (95% CI 2.7 to 16.6). The most common grade 3 or worse adverse events were diarrhea (79 [44.4%] in the pyrotinib group and nine [5.1%] in the placebo group), neutropenia (33 [18.5%] and 36 [20.3%]), and decreased white blood cell count (29 [16.3%] and 24 [13.6%]). No deaths were reported during neoadjuvant treatment.
Conclusions
The primary endpoint of the study was met. Neoadjuvant pyrotinib, trastuzumab, and docetaxel significantly improved the tpCR rate compared with placebo, trastuzumab, and docetaxel, with manageable toxicity, providing a new option for HER2-positive early or locally advanced breast cancer.
Trial registration
ClinicalTrials.gov, NCT03588091
Similar content being viewed by others
Background
HER2 overexpression or gene amplification accounts for approximately 15 to 20% of all breast cancers [1]. For primary operable HER2-positive breast cancer, neoadjuvant anti-HER2 therapy has become a routine treatment strategy. The achievement of pathological complete response (pCR) after neoadjuvant therapy represents a well-established surrogate study endpoint for the long-term outcomes in terms of event-free survival (EFS) and overall survival, especially when defined as eradication of invasive tumor from both breast and lymph nodes [2, 3].
Humanized monoclonal antibody trastuzumab is the cornerstone of therapy for HER2-positive breast cancer. Despite the improvement in pCR rate and EFS, 15% of patients might relapse due to resistance to trastuzumab [4]. The proposed mechanisms might be involved with structural defects within the HER2 receptor, the constitutive activation of downstream elements, the activation of the downstream pathways by other members of the HER family, or intracellular alterations that affect the PI3K pathway [5, 6]. Therefore, adding a second anti-HER2 agent with trastuzumab-complementary activity represents a rationale strategy for neoadjuvant therapy [7].
Pertuzumab is a HER2-directed humanized monoclonal antibody with different binding sites to trastuzumab [8]. Until now, pertuzumab in combination with trastuzumab and chemotherapy remains the only approved dual anti-HER2 regimen in the neoadjuvant setting for patients with HER2-positive early breast cancer [9, 10]. Compared with monoclonal antibodies, tyrosine kinase inhibitors (TKIs) also show robust anti-tumor activities against breast cancer but have advantages of oral administration route, inhibition of both ligand-dependent and independent signaling, and low risk of cardiac toxicity.
Pyrotinib is an irreversible pan-ErbB inhibitor targeting EGFR/HER1, HER2, and HER4 [11]. Compared with lapatinib plus capecitabine, pyrotinib plus capecitabine exhibited an over 20% increase in objective response rate (ORR), a significant benefit in progression-free survival (median, 12.5 versus 6.8 months; hazard ratio [HR], 0.39), and a clear trend towards improvement in overall survival (median, not reached versus 26.9 months; HR, 0.69) in patients with pretreated metastatic HER2-positive breast cancer [12,13,14]. Combined with the findings of other pivotal studies [15,16,17], pyrotinib in combination with capecitabine was approved as the second-line standard-of-care for HER2-positive metastatic breast cancer in China. In this context, we conducted a phase 3 study to further investigate the efficacy and safety of adding pyrotinib to trastuzumab and docetaxel in the neoadjuvant setting.
Methods
Study design and patients
The PHEDRA study was a multicenter, double-blind, randomized, placebo-controlled, phase 3 study done at 17 hospitals in China (NCT03588091). Eligible patients were treatment-naive women aged 18 to 75 years with pathologically confirmed HER2-positive, early (T2 to 3, N0 to 1, M0) or locally advanced (T2 to 3, N2 to 3, M0) breast cancer with primary tumor larger than 2 cm in diameter. HER2 positivity was determined locally and defined as 3+ staining intensity by immunohistochemistry or HER2 gene amplification by fluorescence in situ hybridization according to the 2013 American Society of Clinical Oncology/College of American Pathologists guidelines [18]. Other main inclusion criteria included Eastern Cooperative Oncology Group performance status of 0 or 1, known estrogen receptor (ER) and progesterone receptor (PR) status, and adequate hepatic, renal, bone marrow, and cardiac function based on laboratory assessments. For adequate cardiac function, baseline left ventricular ejection fraction (LVEF) of 55% or more as measured by echocardiography and Fridericia-corrected QT (QTcF) interval of less than 470 ms was required. Key exclusion criteria included metastatic disease (stage IV), inflammatory breast cancer, other malignancies, prior anti-cancer therapy or radiotherapy for any malignancy (except cured cervical carcinoma in situ, basal cell carcinoma, or squamous cell carcinoma), impaired cardiac function, uncontrolled hypertension, pregnancy, and refusal to use contraception.
The study protocol and all amendments were approved by the Ethics Committee of each study site. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Randomization and masking
Patients were randomly assigned (1:1) to receive either pyrotinib, trastuzumab, plus docetaxel (pyrotinib group) or placebo, trastuzumab, plus docetaxel (placebo group). A stratified, permuted block randomization with a block size of four was performed, with stratification by primary tumor size (>2 cm and ≤5 cm versus >5 cm) and hormone receptor status (ER and/or PR positive versus ER and PR negative, the positivity cutoff for both was ≥1%). All investigators, patients, and the funder of the study were masked to treatment allocation.
Interventions
Patients received oral pyrotinib 400 mg once daily or matched placebo in combination with intravenous trastuzumab (8 mg/kg loading dose, 6 mg/kg maintenance dose) and docetaxel (100 mg/m2) on day 1 of each 21-day cycle for four cycles, followed by surgery within 14 days (Additional file 1: Fig. S1). Thereafter, patients were given adjuvant therapy with three cycles of FEC (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2; all given intravenously every 3 weeks) and subsequent anti-cancer treatments at physicians’ discretion in accordance with clinical practice guidelines.
Primary prophylaxis for diarrhea was not prespecified. Based on the results of the interim analysis, the independent data monitoring committee (IDMC) recommended the implementation of proactive diarrhea management (PDM). Primary prophylaxis of neutropenia using a single, 6 mg fixed dose of mecapegfilgrastim on day 2 of each cycle was prespecified. Other granulocyte colony-stimulating factors were permitted if mecapegfilgrastim was intolerable or unavailable at the local study center.
Assessments
To assess the tumor response to neoadjuvant therapy, patients underwent mammography, ultrasounds, and MRI at baseline and after completion of the neoadjuvant therapy (before surgery). Objective responses were assessed by investigators according to the Response Evaluation Criteria in Solid Tumors, version 1.1. Pathological response was assessed by local pathology review and masked independent central review using tumor tissue resection specimens obtained at surgery.
Laboratory assessments and vital signs were done at baseline, at each cycle during neoadjuvant therapy, before surgery, and at each cycle during adjuvant therapy. Cardiac monitoring was done with echocardiography at baseline, at every two cycles during neoadjuvant therapy, and on the day before adjuvant therapy and with 12-lead electrocardiograms at baseline, at every two cycles during neoadjuvant therapy, and before surgery. Adverse events were monitored continuously until 28 days after the last dose of FEC treatment and graded according to the Common Terminology Criteria for Adverse Events, version 4.03.
Outcomes
The primary endpoint was the rate of total pathological complete response (tpCR), defined as the absence of any residual invasive cancer on hematoxylin and eosin staining of the resected breast specimen and all sampled ipsilateral lymph nodes (ypT0/is, ypN0) after neoadjuvant therapy and surgery, as assessed by independent central review. Protocol-defined secondary endpoints were tpCR rate per local pathology review, ORR (defined as the proportion of patients who had a best overall response of complete or partial response during neoadjuvant therapy), EFS (defined as the time from randomization to the first documentation of progressing disease while on study therapy, postoperative disease recurrence, or death from any cause), disease-free survival (DFS, time from surgery to the first documentation of postoperative disease recurrence or death from any cause), distant disease-free survival (DDFS, defined as the time from surgery to the first documentation of postoperative distant metastasis or death from any cause), and safety. Disease recurrence in EFS and DFS definitions referred to breast cancer recurrence, occurrence of second primary breast cancer, and occurrence of any other cancer. Exploratory endpoints included breast pathological complete response (bpCR) rate per local pathology review and independent central review. Results for EFS, DFS, and DDFS are not included in this report as the data are not mature.
Statistical analysis
With 294 patients, the study had 85% power to detect an increase of 18% in tpCR rate (from 30% in the placebo group to 48% in the pyrotinib group), at a one-sided α level of 0.025, considering that 10% of enrolled patients would be unevaluable for pathological response assessment. Comparison between groups was done using the Cochran-Mantel-Haenszel test stratified by the randomization strata. One prespecified interim analysis was planned when pathological responses were available for 158 patients. As of Aug 30, 2019, the pathological responses were assessable in 159 patients, and interim analysis was done by an independent statistics team from KNOWLANDS MedPharm Consulting (Shanghai, China). The IDMC reviewed the results on Nov 6, 2019, and recommended continuing the trial with an increased sample size of 354 patients for final analysis (the criteria for IDMC recommendation described in Additional file 1: Supplementary Methods). Considering the sample size was increased, the Cui, Hung, and Wang method was used in the primary analysis for the tpCR rate in order to control the type I error [19]. A one-sided P value was reported, and the value of less than 0.025 was considered significant.
Efficacy analyses were done in the full-analysis set, including all patients who underwent randomization and received at least one dose of study treatment, with patients analyzed according to their randomized assignment. Safety analyses were done in all patients who received at least one dose of study treatment, with patients included according to their actual treatment. The 95% CIs for the pCR rate were calculated using the Wilson method. The 95% CI for the between-group difference was calculated using the Wald method. Patients without a recorded assessment of pCR were regarded as non-responders. Prespecified subgroup analyses of the tpCR rate per independent central review were also done. Statistical analyses were conducted using SAS version 9.4 and sample size re-estimation was done using East version 6.5.
Results
Patients
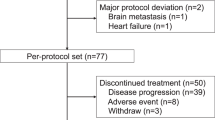
Between Jul 23, 2018, and Jan 8, 2021, 355 eligible patients were randomly assigned, 178 to receive pyrotinib, trastuzumab, and docetaxel and 177 to receive placebo, trastuzumab, and docetaxel (Fig. 1). The baseline characteristics were generally well balanced between the two groups (Table 1). The data cutoff date for the present analysis was Apr 30, 2021, which followed the last pathological assessment of enrolled patients.
All 355 patients received the study treatment. A total of 165 (92.7%) of 178 patients in the pyrotinib group and 173 (97.7%) of 177 patients in the placebo group completed four cycles of neoadjuvant treatment as planned (Fig. 1). The study treatment was discontinued in 13 (7.3%) patients in the pyrotinib group and four (2.3%) in the placebo group, mainly due to adverse events (6 [3.4%] and 1 [0.6%]), withdrawal of consent (5 [2.8%] and 1 [0.6%]), or start of new anti-cancer treatment (1 [0.6%] and 2 [1.1%]). No patients discontinued the neoadjuvant treatment because of disease progression. Five (2.8%) patients in the pyrotinib group and three (1.7%) in the placebo group who did not undergo surgery had no valid pathological assessment and were categorized as not achieving a pCR.
Efficacy
According to the independent central review, the tpCR (ypT0/is, ypN0) rate was significantly higher in the pyrotinib group than that in the placebo group (41.0% [95% CI 34.0 to 48.4] versus 22.0% [95% CI 16.6 to 28.7]; absolute difference, 19.0% [95% CI 9.5 to 28.4], one-sided P < 0.0001, Fig. 2A). Local pathologists assessed tpCR rate also indicated similar improvement (44.4% [95% CI 37.3 to 51.7] in the pyrotinib group versus 24.3% [95% CI 18.6 to 31.1] in the placebo group; absolute difference, 20.1% [95% CI 10.4 to 29.7], Fig. 2B). The rate of bpCR (ypT0/is) was higher in the pyrotinib group either according to independent central review (43.8% [95% CI 36.5 to 51.1] versus 23.7% [95% CI 17.5 to 30.0]; absolute difference, 20.1% [95% CI, 9.9 to 29.7]) or local pathology review (46.6% [95% CI 39.4 to 54.0] versus 26.6% [95% CI 20.6 to 33.5]; absolute difference, 20.1% [95% CI, 10.3 to 29.9], Fig. 2A, B).
Total pathological complete response (tpCR) rate and breast pathological complete response (bpCR) rate. A Per independent central review; B per local pathology review. Patients with missing or unevaluable pCR status were considered non-responders. Error bars show 95% CIs for the pCR rate in each group, which were calculated using the Wilson method. Comparison between groups was done using the Cochran-Mantel-Haenszel test stratified by the randomization strata, and the 95% CI for the between-group difference was calculated using the Wald method
Consistent with the overall result, improvements in tpCR with pyrotinib, trastuzumab, and docetaxel were clearly observed across all predefined subgroups (Fig. 3).
The ORR per investigator was 91.6% (95% CI 86.6 to 94.8) in the pyrotinib group and 81.9% (95% CI 75.6 to 86.9) in the placebo group after the neoadjuvant treatment, resulting in an increase of 9.7% (95% CI 2.7 to 16.6, Additional file 1: Table S1). Complete responses were achieved by 28 (15.7%) of 178 patients in the pyrotinib group and 11 (6.2%) of 177 patients in the placebo group.
Safety
Exposure of the individual components of neoadjuvant treatment is shown in Additional file 1: Table S2. A total of 127 (71.3%) patients in the pyrotinib group and 66 (37.3%) in the placebo group experienced adverse events of grade 3 or 4 (Table 2). The grade 3 or 4 adverse events occurring in at least 15% of patients in either group were diarrhea (79 [44.4%] in the pyrotinib group and nine [5.1%] in the placebo group), neutropenia (33 [18.5%] and 36 [20.3%]), and decreased white blood cell count (29 [16.3%] and 24 [13.6%]). Serious adverse events were reported in 26 (14.6%) patients in the pyrotinib group and 12 (6.8%) patients in the placebo group (Additional file 1: Table S3). No deaths occurred during the neoadjuvant treatment.
The worst diarrhea in severity was grade 3. It mainly occurred in the first cycle and gradually decreased during treatment (Additional file 1: Fig. S2). In the pyrotinib group, the median time from the first dose to the onset of grade 3 diarrhea was 9 days, with a median duration of 2 days per event and a median cumulative duration of 5 days (Additional file 1: Table S4). Discontinuation of pyrotinib due to diarrhea was required in only one (0.6%) patient. Of the 178 patients in the pyrotinib group, 106 were recruited before the implementation of the PDM strategy, including the use of loperamide as the first choice of anti-diarrheal agents and strict application of loperamide recommended dose (4 mg initially and an additional 2 mg following each diarrhea stool, not exceeding 16 mg/day), and 72 were recruited after the implementation of PDM strategy (Additional file 1: Table S5). The incidence of grade 3 diarrhea after PDM implementation decreased to 36.1% (26/72), compared with 50.0% (53/106) before PDM implementation (cycle 1, 29.2% versus 44.3%; cycle 2, 10.1% versus 21.8%; cycle 3, 7.2% versus 14.1%; cycle 4, 4.5% versus 11.1%). The median cumulative duration of grade 3 diarrhea was shortened in patients enrolled after the PDM implementation (2 days versus 6 days).
Grade 3 neutropenia was reported in 15 (8.4%) versus 20 (11.3%) patients in the pyrotinib versus placebo group, and grade 4 neutropenia occurred in 18 (10.1%) versus 16 (9.0%) patients, respectively (Additional file 1: Table S6). Five (2.8%) patients in the pyrotinib group and two (1.1%) patients in the placebo group developed febrile neutropenia of any grade. Generally, grade 3 or 4 neutropenia, febrile neutropenia, and decreased white blood cell count mainly occurred during the first cycle of treatment in both pyrotinib and placebo groups and reduced in the second cycle and thereafter.
No major cardiac toxicities were observed in terms of changes in LVEF and QTcF values during treatment. No patients in the pyrotinib group and one (0.6%) in the placebo group had a LVEF of less than 50% and a decrease of 10% or more from baseline. Four (2.2%) patients in the pyrotinib and five (2.8%) in the placebo group had a QTcF of 480 ms or more and a change of at least 60 ms from baseline.
Discussion
The PHEDRA trial met its primary endpoint, demonstrating that neoadjuvant pyrotinib, trastuzumab, and docetaxel achieved a significantly higher rate of tpCR than placebo, trastuzumab, and docetaxel (41.0% versus 22.0%; absolute difference, 19.0%, one-sided P < 0.0001) in patients with HER2-positive early or locally advanced breast cancer.
Similar to the studies of pertuzumab, we adopted 12 weeks of neoadjuvant therapy followed by surgery and adjuvant FEC therapy. In the NeoSphere international phase 2 study, pertuzumab plus trastuzumab and docetaxel showed a significantly improved bpCR compared with trastuzumab and docetaxel (45.8% versus 29.0%) [9]. The tpCR rate was also higher with pertuzumab plus trastuzumab and docetaxel (39.3% versus 21.5%) [9]. The PEONY study reported a consistent result in the tpCR rate in the Asian population (39.3% with pertuzumab plus trastuzumab and docetaxel versus 21.8% with placebo plus trastuzumab and docetaxel; difference, 17.5% [95% CI 6.9 to 28.0]) [10]. Our study indicated comparable results with the PEONY study, both in the dual HER2 blockade group and single blockade control group, suggesting that pyrotinib plus trastuzumab and docetaxel might be an alternative option for HER2-positive breast cancer in the neoadjuvant setting.
Several clinical trials compared neoadjuvant therapy with a TKI plus trastuzumab-based regimen versus trastuzumab-based regimen [20,21,22,23,24,25,26]. Although all studies reported a numeric increase in bpCR or tpCR rate, only the CHER-LOB and NeoALTTO studies reached statistical significance. Differences in chemotherapy regimen, administration sequence of chemotherapy and anti-HER2 drugs, and duration of neoadjuvant therapy (16 to 26 weeks) might contribute to the inconsistent findings. In the CHER-LOB study, lapatinib and trastuzumab were administered for 26 weeks (throughout a sequential regimen of 12-week paclitaxel followed by 12-week FEC after 2 weeks of interval) [20]. The tpCR was 46.7% with lapatinib plus trastuzumab and chemotherapy versus 25% with trastuzumab and chemotherapy. In the NeoALTTO study, lapatinib and trastuzumab were administered for 18 weeks with paclitaxel being started at week 6 [25]. The tpCR rate was 46.8% with lapatinib and trastuzumab plus chemotherapy versus 27.6% with trastuzumab plus chemotherapy. These data, both in the dual HER2 blockade group and single blockade control group, were relatively higher in contrast to those reported in studies of pertuzumab and this study of pyrotinib, which might be caused by longer duration of neoadjuvant therapy with trastuzumab with or without lapatinib. Of note, only 60.5% of patients in the lapatinib plus trastuzumab and chemotherapy group completed planned neoadjuvant treatment, compared with 91.9% of patients in the trastuzumab and chemotherapy group [25]. In our study, 92.7% of patients completed the 12-week neoadjuvant pyrotinib plus trastuzumab and docetaxel as planned, suggesting that this neoadjuvant regimen represented a new effective option with short duration of neoadjuvant therapy and high compliance.
The most common severe toxicity with neoadjuvant pyrotinib plus trastuzumab and docetaxel was consistent with the known safety profile of individual components. Diarrhea in the pyrotinib group was characterized by mild or moderate severity, early onset, and short duration. Generally, diarrhea was manageable with anti-diarrheal agents, and only one patient discontinued pyrotinib due to diarrhea. Of note, implementation of the PDM strategy obviously reduced the incidence of grade 3 diarrhea in each cycle of the neoadjuvant therapy and shortened the cumulative duration of grade 3 diarrhea. Thus, when diarrhea occurs in clinical practice, loperamide at recommended dose should be given followed by close follow-up or observation.
When docetaxel at a dose of 75 mg/m2 was used, in either dual or single HER2 blockade groups, 45 to 57% of patients in the NeoSphere study and 32.7 to 38.1% of patients in the PEONY study suffered grade 3 or worse neutropenia, and 7 to 8% of patients in the NeoSphere study suffered febrile neutropenia. Mecapegfilgrastim is a long-acting recombinant human G-CSF with the advantage of once-per-cycle dosing and convenient dose management [27]. In our study, 100 mg/m2 docetaxel was used, and the majority of patients (82.0%) received 6 mg fixed dose of mecapegfilgrastim as primary prophylaxis for neoadjuvant chemotherapy-induced neutropenia. Grade 3 or 4 neutropenia occurred in 18.5% of patients in the pyrotinib group and 20.3% of patients in the placebo group, and febrile neutropenia occurred in 2.8% and 1.1% of patients, respectively. These results indicated that the addition of pyrotinib did not increase the incidence of neutropenia, and primary prophylaxis for neutropenia using mecapegfilgrastim was effective.
This study had some limitations. First, placebo, trastuzumab, and docetaxel was chosen as the control, because pertuzumab was not approved as a component of neoadjuvant therapy in China at the study design. Second, this report was for the final analysis of the primary endpoint tpCR; survival data were immature. The study is still ongoing and the data would be reported in the future. In addition, a phase 3 study of pyrotinib versus placebo after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (NCT03980054) is being conducted to investigate whether the positive results in the neoadjuvant setting would be substantiated in the adjuvant setting in terms of invasive DFS.
Conclusions
Overall, pyrotinib, trastuzumab, and docetaxel as neoadjuvant treatment achieved a statistically significant and clinically meaningful improvement in the tpCR rate for patients with HER2-positive early or locally advanced breast cancer compared with placebo, trastuzumab, and docetaxel, with an acceptable safety profile, supporting the approval of this combination as a new neoadjuvant treatment option in China. To our knowledge, this is the first phase 3 study supporting 12-week neoadjuvant treatment with a HER2-directed small-molecule TKI plus trastuzumab and chemotherapy in breast cancer patients.
Availability of data and materials
Clinical data may be requested 24 months after study completion. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requiring the data. The leading clinical site and sponsor will check whether the request is subject to any intellectual property restriction. The use of data must also comply with the requirements of the Human Genetics Resources Administration of China and other country- or region-specific regulations. A signed data access agreement with the sponsor is required before accessing shared data.
Abbreviations
- bpCR:
-
Breast pathological complete response
- DDFS:
-
Distant disease-free survival
- DFS:
-
Disease-free survival
- ECOG:
-
Eastern Cooperative Oncology Group
- EFS:
-
Event-free survival
- ER:
-
Estrogen receptor
- IDMC:
-
Independent data monitoring committee
- LVEF:
-
Left ventricular ejection fraction
- ORR:
-
Objective response rate
- pCR:
-
Pathological complete response
- PDM:
-
Proactive diarrhea management
- PR:
-
Progesterone receptor
- QTcF:
-
Fridericia-corrected QT
- TKI:
-
Tyrosine kinase inhibitor
- tpCR:
-
Total pathological complete response
References
Sareyeldin RM, Gupta I, Al-Hashimi I, Al-Thawadi HA, Al Farsi HF, Vranic S, et al. Gene expression and miRNAs profiling: function and regulation in human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Cancers (Basel). 2019;11(5):646.
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26:2838–48.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Patel A, Unni N, Peng Y. The changing paradigm for the treatment of HER2-positive breast cancer. Cancers (Basel). 2020;12(8):2081.
Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–38.
Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62.
Jackisch C, Cortazar P, Geyer CE Jr, Gianni L, Gligorov J, Machackova Z, et al. Risk-based decision-making in the treatment of HER2-positive early breast cancer: recommendations based on the current state of knowledge. Cancer Treat Rev. 2021;99:102229.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2020;6:e193692.
Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61.
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351–60.
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37:2610–9.
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Updated overall survival results from the phase 3 PHOEBE trial of pyrotinib versus lapatinib in combination with capecitabine in patients with HER2-positive metastatic breast cancer. In: San Antonio Breast Cancer Symposium (SABCS): 2021; 2021.
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35:3105–12.
Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019;25:5212–20.
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q, Feng J, et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res. 2020;1:1–13.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Cui L, Hung HM, Wang SJ. Modification of sample size in group sequential clinical trials. Biometrics. 1999;55:853–7.
Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–95.
Holmes FA, Espina V, Liotta LA, Nagarwala YM, Danso M, McIntyre KJ, et al. Pathologic complete response after preoperative anti-HER2 therapy correlates with alterations in PTEN, FOXO, phosphorylated Stat5, and autophagy protein signaling. BMC Res Notes. 2013;6:507.
Jacobs SA, Robidoux A, Abraham J, Perez-Garcia JM, La Verde N, Orcutt JM, et al. NSABP FB-7: a phase II randomized neoadjuvant trial with paclitaxel + trastuzumab and/or neratinib followed by chemotherapy and postoperative trastuzumab in HER2(+) breast cancer. Breast Cancer Res. 2019;21:133.
Bonnefoi H, Jacot W, Saghatchian M, Moldovan C, Venat-Bouvet L, Zaman K, et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline-based chemotherapy in HER2-positive breast cancer: results of the randomised phase II EORTC 10054 study. Ann Oncol. 2015;26:325–32.
Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34:542–9.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40.
Robidoux A, Tang G, Rastogi P, Geyer CE Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1183–92.
Xu F, Zhang Y, Miao Z, Zeng X, Wu B, Cai L, et al. Efficacy and safety of mecapegfilgrastim for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer: a randomized, multicenter, active-controlled phase III trial. Ann Transl Med. 2019;7:482.
Acknowledgements
We are grateful to all patients, their families, the investigators, the site staff, and the trial teams. Medical writing support was provided by Hui Dong (PhD, medical writer at Hengrui) according to Good Publication Practice Guidelines.
Funding
This study was funded by Jiangsu Hengrui Pharmaceuticals Co, Ltd.
Author information
Authors and Affiliations
Contributions
Concept and design: JW, ZJ, and JZ. Acquisition, analysis, or interpretation of data: JW, ZJ, ZL, BY, HY, JT, KW, YL, HW, PF, SZ, QL, SW1, JH, CW, SW2, YW, LZ, XZ, FW, XL, and JZ. Drafting of the manuscript: JW, ZJ, ZL, BY, XZ, FW, and XL. Critical revision of the manuscript for important intellectual content: HY, JT, KW, YL, HW, PF, SZ, QL, SW1, JH, CW, SW2, YW, LZ, and JZ. Statistical analysis: XL. Administrative, technical, or material support: JW, ZJ, ZL, BY, XZ, FW, XL, and JZ. Supervision: JW, ZJ, and JZ. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol and all amendments were approved by the Ethics Committee of each study site. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
XZ, FW, XL, and JZ reported being employed by Hengrui when conducting this study. No other disclosures were reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Study diagram. Figure S2. Incidence of grade 3 diarrhea during neoadjuvant treatment. Table S1. Clinical responses following neoadjuvant treatment assessed by local investigator per RECIST v1.1. Table S2. Exposure of the individual components of study treatment. Table S3. Serious adverse events during neoadjuvant therapy. Table S4. Summary of data on diarrhea during neoadjuvant treatment. Table S5. Characteristics of diarrhea before and after proactive management. Table S6. Overview of neutropenia, febrile neutropenia, and decreased white blood cell count during neoadjuvant treatment period.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, J., Jiang, Z., Liu, Z. et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): a double-blind, randomized phase 3 trial. BMC Med 20, 498 (2022). https://doi.org/10.1186/s12916-022-02708-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02708-3