Abstract
Purpose
A substantial need for effective and safe treatment options is still unmet for patients with heavily pre-treated human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). Herein, we assessed the efficacy and safety of pyrotinib plus trastuzumab and chemotherapy in patients with heavily treated HER2-positive MBC.
Methods
In this single-arm exploratory phase II trial, patients with HER2-positive MBC previously treated with trastuzumab plus lapatinib or pertuzumab, received pyrotinib plus trastuzumab and chemotherapy. The primary end point was progression-free survival (PFS) in the total population (TP). Secondary end points included PFS in the subgroup with brain metastases (Sub-BrM), confirmed objective response rate (ORR), clinical benefit rate (CBR), disease control rate (DCR), exploration of predictive factors of PFS, and safety.
Results
Between November 1, 2018, and March 31, 2021, 40 patients were eligible for this study. The median PFS reached 7.5 months (95% confidence interval [CI] 4.7 to 9.9 months) and 9.4 months (95% CI 6.6 to 12.1 months) in the TP and Sub-BrM, respectively. ORR was 50.5% (20/40). CBR was 75.5% (30/40) and DCR reached 97.5% (39/40). Cox univariate and multivariate analyses demonstrated that liver or/and lung metastases was the significant adverse prognostic factor for PFS (p = 0.018; p = 0.026; respectively). The most frequent grade 3 or 4 treatment-related adverse events were diarrhea, neutropenia and leukopenia. No new safety signals were observed.
Conclusion
Pyrotinib plus trastuzumab and chemotherapy offered a promising option with manageable safety profile for heavily pre-treated HER2-positive MBC, especially for those without liver or/and lung metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) has surpassed lung cancer as the most commonly diagnosed malignancy in women according to Global Cancer statistics 2020 [1]. Approximately 15 to 20% of BC cases exhibit overexpression of the human epidermal growth factor receptor 2 (HER2) or/and amplification of the coding gene ERBB2, which is defined as HER2-positive subtype [2]. HER2-positive BC is more aggressive and prone to recurrence than HER2-negative tumors [3]. In the last decades, the clinical outcome of HER2-positive BC has been significantly improved since the introduction of HER2-targeted drugs mainly including monoclonal antibodies, tyrosine kinase inhibitors (TKIs) and antibody drug conjugates (ADCs). Currently, the standard of care for HER2-positive metastatic breast cancer (MBC) involves trastuzumab plus pertuzumab and a taxane as the first-line regimen, followed by second-line trastuzumab emtansine (T-DM1) or fam-trastuzumab deruxtecan-nxki (ds8201) for patients whose disease has progressed on prior dual HER2 blockade [4]. However, a large proportion of patients with HER2-positive MBC fail to be treated with standard second-line regimens due to economic and drug accessibility factors. Moreover, most patients inevitably experience disease progression on anti-HER2-targeted therapy due to de novo or acquired resistance. Therefore, it is necessary to explore novel approaches to overcome drug resistance. Key strategies include efficient suppression of the HER2 signaling pathway by dual blockade and development of more effective anti-HER2 therapies like antibody–drug conjugates, new anti-HER2 antibodies, bispecific antibodies and novel TKIs [5].
Brain metastases (BrM) is a common complication of advanced malignant disease and occurs in 1/3 of HER2-positive metastatic breast cancer [6]. Central nervous system-directed local therapies, including surgical resection and radiotherapy, are the foundations of BrM management [7]. Besides, TKIs and several chemotherapeutic drugs with blood–brain barrier penetrability have demonstrated efficacy in HER2-positive MBC patients with BrM. Despite sequential local and systemic treatment, resistance inevitably develops and options are limited for the control of BrM.
Studies have confirmed the activity of continued trastuzumab treatment beyond progression (TBP). The subgroup analysis of observational HERMINE study suggested that trastuzumab TBP offered a survival benefit to MBC patients treated with first-line trastuzumab [8]. Furthermore, a phase III study in HER2-positive BC showed that patients receiving continued trastuzumab TBP had a better post-progression survival than those not receiving (18.8 m vs 13.3 m, p = 0.02) [9]. Meanwhile, the potent anti-tumor efficacy of TKIs including lapatinib, neratinib, tucatinib and pyrotinib has been demonstrated in several pivotal studies [10,11,12,13,14]. Pyrotinib is an orally administered irreversible pan-ErbB TKI which shows anti-tumor activity and acceptable safety profile in HER2-positive advanced and metastatic breast cancer [14]. In a phase II study, pyrotinib plus capecitabine yielded statistically significant better overall response rate (ORR) and progression-free survival (PFS) than lapatinib plus capecitabine in patients with HER2-positive MBC previously treated with taxanes, anthracyclines, and/or trastuzumab [15]. Based on this phase II study, pyrotinib in combination with capecitabine received its first conditional approval in China for the treatment of HER2-positive, advanced or metastatic breast cancer previously exposed to anthracycline or taxane chemotherapy. Afterwards, a multi-center, open-label, randomized, controlled, phase III trial (PHOEBE) confirmed the efficacy and safety of pyrotinib plus capecitabine in patients with disease progression on previous trastuzumab [16]. Another phase III trial PHENIX further verified that pyrotinib plus capecitabine significantly improved PFS and ORR compared with capecitabine monotherapy in trastuzumab-treated patients with HER2-postive advanced or metastatic breast cancer, including those with brain metastases [17].
Dual HER2 blockade by trastuzumab plus TKI, simultaneously targeting the extra- and intra-cellular domains of HER2, showed encouraging anti-tumor activity in BC, including early breast cancer and MBC with intracranial metastases [18,19,20,21]. Our study aimed to explore the activity and safety of pyrotinib combined with trastuzumab and chemotherapy of physician’s choice in heavily pre-treated patients with HER2-positive MBC with or without BrM.
Methods
Patients and data collection
From November 1, 2018, to March 31, 2021, female patients aged ≥ 18 years old with histologically confirmed HER2-positive MBC at National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital (Shenzhen, Guangdong, China) were enrolled in this study. HER2 status was determined by central review based on immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) examination using primary or metastatic lesion samples. Patients had been previously treated with trastuzumab, pertuzumab and/or lapatinib in the (neo)adjuvant/metastatic setting. Additional requirements included an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 (on a 5-point scale by which greater scores reflect higher degree of disability); measurable lesions; and adequate organ function. Patients were excluded if they had previously received treatment with pyrotinib for metastatic disease; had symptomatic brain metastasis which necessitates immediate local intervention; or had leptomeningeal disease. All procedures performed involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was also approved by the institutional review board and ethics committee. All patients provided written informed consent for use of the medical information for research purpose.
Treatment
All eligible patients were treated with pyrotinib (240–400 mg orally once daily), trastuzumab (6 mg per kilogram of body weight intravenously per 21 days, with an initial loading dose of 8 mg per kilogram) and single chemotherapeutic agent (nab-paclitaxel, 260 mg per square meter of body-surface area intravenously on day 1 of each 21-day cycle; or capecitabine, 1000 mg per square meter of body-surface area orally twice daily on days 1 to 14 of each 21-day cycle; or gemcitabine, 1000 mg per square meter of body-surface area intravenously on days 1 and 8 of each 21-day cycle; or vinorelbine, 60 mg per square meter of body-surface area orally on days 1 and 8 of each 21-day cycle and dose elevation to 80 mg per square meter of body-surface area was allowed if well tolerated).
Follow-up and assessment
All patients were followed up until January 20, 2022. Disease response was evaluated according to imaging reports from serial clinical assessments. Patient/disease response assessments were performed at baseline, every 6 weeks for 24 weeks, and every 9 weeks thereafter, including performance status, history, laboratory examinations, electrocardiogram, contrast-enhanced spiral computed tomography (CT) or positron emission tomography/CT (PET/CT), and contrast-enhanced magnetic resonance imaging (MRI). MRI of the head was obtained for all patients at baseline and head scans were repeated at the frequency described above if BrM had been detected at baseline. MRI of the breast was not required. Disease was evaluated in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1 [22]. Safety was assessed mainly via the incidence of treatment-related adverse events (TRAEs) using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
End points
The primary end point was PFS (calculated from the date of first treatment with double targeted therapy to the date of documented disease progression or death from any cause or the last follow-up visit) in TP. The secondary end points included PFS in the subgroup with BrM (Sub-BrM) at baseline; objective response rate (ORR, defined as the percentage of patients who had a confirmed complete response or partial response); clinical benefit rate (CBR, defined as the percentage of patients who had a confirmed complete response or partial response or stable disease for at least 24 weeks); disease control rate (DCR, defined as the percentage of patients who had a confirmed complete response or partial response or stable disease for at least 4 weeks); exploration of predictive factors of PFS; and safety.
Statistical analysis
All data were analyzed using SPSS 23.0 statistical software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Descriptive analysis was utilized to display clinicopathological features. The Kaplan–Meier method was used to estimate PFS and 95% confidence intervals (CIs) for the total population and the subgroups. Cox univariate and multivariate models were used to determine the predictive value of variables for PFS. In the analysis of progression-free survival, data from patients who did not have any documented event, were lost to follow-up or died from any cause were censored at the last date when the patient was known to be event-free. All reported p values were two-sided, with p < 0.05 being regarded as statistically significant.
Results
Patient clinicopathological characteristics
Between November 1, 2018, and March 31, 2021, a total of 40 patients were eligible for this study and included in the final analysis. In TP, the median age at diagnosis was 46 years (range 31–62 years). 33 patients (82.5%) were aged over 35 years old at diagnosis. ECOG performance status was 0 in 21 patients (52.5%) and 1 in 19 patients (47.5%). For HER2 status, HER2 3+ by IHC was recorded in 32 patients (80.0%), and HER2 2+ by IHC and amplification by FISH in 8 patients (20.0%). 21 patients (52.5%) were estrogen receptor (ER) and/or progesterone receptor (PR) positive and 19 patients (47.5%) were ER and PR negative. 39 patients (97.5%) had been treated with trastuzumab in the (neo)adjuvant or metastatic setting; 6 patients had received pertuzumab and trastuzumab as first-line therapeutic regimen; 21 patients (52.5%) received anti-HER2 therapy with TKI (lapatinib) for their metastatic disease; none of them was previously treated with TDM-1. 15 patients (37.5%) had brain metastases and 27 patients (67.5%) had lung or/and liver metastases at baseline. Of those patients with brain metastases, 7 patients had received central nervous system (CNS) local therapies including whole-brain radiotherapy (WBRT), stereotactic radiotherapy (SRT) and surgery plus SRT before study treatment; 3 patients whose intracranial disease progressed while remission of extra-cranial lesions lasted, received SRT with unchanged systemic therapy. 13 patients (32.5%) received at least 3 lines of therapy in the metastatic setting. Combined chemotherapeutic agents included vinorelbine (25, 62.5%), capecitabine (6, 15.0%), nab-paclitaxel (5, 12.5%) and gemcitabine (4, 10.0%). Patient clinicopathological characteristics at baseline were shown in Table 1.
Efficacy
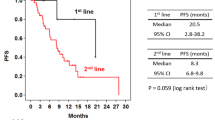
By the end of follow-up, 32 patients had experienced disease progression and 3 patients had died. The median duration of PFS was 7.5 months in TP (95% CI 4.7 to 9.9 months, Fig. 1) and was 9.4 months in Sub-BrM (95% CI 6.6 to 12.1 months, Fig. 2). Objective response was seen in 20 of 40 patients (including 1 patient with complete response and 19 patients with partial response, 50.0%, Table 2). All patients with objective response achieved disease remission within four cycles (12 weeks) after treatment administered. Clinical benefit was observed in 30 of 40 patients (75.5%) and disease control in 39 of 40 patients (97.5%, Table 2).
Univariate and multivariate analyses were performed to identify predictive factors of PFS for the 40 patients. By means of Cox univariate and multivariate analyses, we found that liver or/and lung metastases at baseline was the significant adverse prognostic factor for PFS (hazard ratio [HR] 0.322; 95% CI 0.132 to 0.831; p = 0.018; HR 0.281; 95% CI 0.093 to 0.876; p = 0.026; respectively; Table 3). The median PFS was 6.0 months (95% CI 2.8 to 9.2 months) in the subgroup with liver or/and lung metastases and was not reached in the subgroup without (Fig. 3).
Safety
All treatment-related adverse events (TRAEs) reported in the study were summarized in Table 4. The most common adverse events (frequency ≥ 10%) were diarrhoea (85.0%), leukopenia (42.5%), neutropenia (37.5%), fatigue (37.5%), vomiting (32.5%), and nausea (12.5%) in turn. Other recorded adverse events included hand-foot syndrome (10.0%), aspartate aminotransferase increase (10.0%), anorexia (7.5%), alanine aminotransferase increase (5.0%), anemia (5.0%), peripheral neurotoxicity (5.0%), rash (2.5%) and thrombocytopenia (2.5%). The most frequent grade 3 to 4 adverse events were diarrhoea (25.0%), neutropenia (15.0%), and leukopenia (12.5%). Grade 4 adverse events occurred in 1 patient (leukopenia and febrile neutropenia). No grade 4 diarrhea or cardiac-related events were reported. No one discontinued study treatment or died owing to TRAEs. However, 14 patients (35.0%) experienced pyrotinib dose reduction because of diarrhoea (10 patients reduced from 400 to 320 mg and 4 patients from 400 to 240 mg). 4 patients experienced chemotherapeutic drug dose reduction (nab-paclitaxel in 1 patient, capecitabine in 1 patient, and vinorelbine in 2 patients). Diarrhoea, the most common adverse event, mostly occurred within the first two weeks after treatment initiation and could be managed by the administration of loperamide or/and pyrotinib dose reduction.
Discussion
In this single-center study, pyrotinib combined with trastuzumab and chemotherapy demonstrated promising efficacy in heavily pre-treated HER2-positive MBC (92.5% of the patients with prior trastuzumab and 52.5% with lapatinib), with a median PFS of 7.5 months and an ORR of 50%, which implies that half of the patients could still respond to pyrotinib-based regimen even in later-line settings.
As is well known, efficacy and safety of pyrotinib in patients with HER2-positive MBC have been verified in several pivotal studies. In the phase I study pyrotinib monotherapy demonstrated an ORR of 83.3% in trastuzumab-naive patients and 33.3% in trastuzumab-pre-treated patients [14]. The benefit of continued use of trastuzumab beyond disease progression was also confirmed in earlier studies [8, 9]. The anti-tumor activity and favorable tolerability of dual HER2 blockade with TKI plus trastuzumab has been reported previously and mechanisms of synergistic interaction may involve enhanced apoptosis of cancer cells, increased stabilization and degradation of HER2 receptors, and reversion of resistance to trastuzumab by accumulation of HER2 receptors on the surface of breast cancer cells [20, 21]. Impressively, Murthy and colleagues reported that tucatinib, which was an investigational, oral, highly selective inhibitor of the HER2 tyrosine kinase, was an alternative regimen in heavily pre-treated metastatic breast cancer when combined with trastuzumab and capecitabine, with a median PFS of 7.8 months in the study population and 7.6 months in the brain metastases subgroup [19]. Another single-arm exploratory phase II trial has demonstrated that pyrotinib plus trastuzumab and albumin-bound paclitaxel generated an encouraging pCR rate and an ORR of 100% in the neoadjuvant setting [18]. To our knowledge, there exists no published data investigating the activity and safety of pyrotinib plus trastuzumab and chemotherapy in MBC patients previously treated. Our findings are fairly consistent with and further consolidate the above-mentioned data. The PFS duration reported here is similar with that in the HER2CLIMB trial. Noteworthy, the median PFS of BrM subset that have presumably worse prognosis, was numerically superior to that of the total population. The previous therapeutic lines may predominantly account for this result, since 32.5% of the total population versus 20.0% of BrM subgroup received ≥ 2 lines of therapy previously. Besides, concerning the patients with intracranial metastasis, the PFS duration estimated here (9.4 months) was also longer than that in the HER2CLIMB trial (7.6 months). The potent activity of pyrotinib per se for intracranial metastases may partly account for this finding. Another possible explanation is that local therapy for intracranial metastases (46.7% of the patients with brain metastases received local therapy) improved blood brain barrier permeability and thus the intracranial concentration of anti-tumor drugs increased.
Up to now, evidences on head-to-head comparison of TKI plus trastuzumab versus TKI alone are lacking. A randomized phase II trial, which was designed to assess the efficacy and tolerability of pyrotinib plus capecitabine versus lapatinib plus capecitabine in women with HER2-positive MBC previously treated with taxanes, anthracyclines, and/or trastuzumab, reported that the ORR was 78.5% and the median PFS was 18.1 months in the pyrotinib arm [15]. Similarly, PHENIX study showed that the ORR and median PFS of pyrotinib plus capecitabine were 68.6% and 11.1 months, respectively [17]. Numerically, these findings were superior to the data reported in our study, which might be partly attributed to different distribution of patient characteristics. The phase II study and PHENIX study enrolled patients with no more than 2 lines of prior treatment in the metastatic setting, and nearly 70% of the patients were trastuzumab-naïve. However, 67.5% of the patients in our study received ≥ 2 lines of therapy previously, and almost all patients had received prior trastuzumab for their metastatic disease [15]. Furthermore, our data demonstrated that 52.5% of patients had been exposed to prior lapatinib, which shows a phenomenon that more than half of lapatinib-pre-treated patients still respond to pyrotinib combined with trastuzumab and chemotherapy. Pyrotinib is an irreversible pan-ErbB TKI blocking HER1, HER2, and HER4 while lapatinib is a reversible small dual TKI of HER1 and HER2. Therefore, the response in previous lapatinib-treated patients may partly due to the chemotherapeutic agents and partly due to continuous inhibition of HER2 signal pathway through the reversion of resistance to lapatinib by combination of pyrotinib and trastuzumab. The result further implies the potential activity of pyrotinib plus trastuzumab beyond progression on lapatinib treatment, which merits further evaluation in future studies.
In addition, we further analyzed the predictive factors of PFS and found that liver or/and lung metastases was independent adverse factor for PFS, which suggested that PFS of patients without liver or/and lung metastases were much longer than those with liver or/and lung metastases. It has been reported that live or/and lung is a poor predictor of OS of MBC and a challenge to overcome in clinical practice [23, 24]. In PHENIX trial, subgroup analyses of PFS indicated that pyrotinib plus capecitabine exhibited PFS benefit and significantly decrease recurrence risk compared with placebo plus capecitabine in patients with non-visceral metastases [17]. Wang et al. reported the significant therapeutic effect of pyrotinib on cutaneous metastases of HER2-positive BC for the first time [25]. However, they have not further explored the efficacy and safety of pyrotinib on patients with visceral-metastases. Similarly, data on patients with visceral-metastases were not available in HER2CLIMB trial [19]. Our results demonstrated that patients without liver or/and lung metastases might better benefit from this combination therapy and this finding may provide guidance for patient selection and optimize clinical management.
Referring to safety, the overall incidences of TRAEs were similar to that of previous report and no new TRAEs were reported. The majority of TRAEs were Grade 1 or 2 in severity and most of TRAEs could be managed by dose reduction and supportive treatment. No one discontinued study treatment or died due to TRAEs. Diarrhea, usually occurring on days 4–14 after the first dose, is the most common TRAE which is mainly induced by pyrotinib and could be managed by dose reduction and loperamide, turning mostly tolerable after treatment administered for 1 month. The majority of diarrhea events were Grade 1 or 2, and 25% of them were Grade 3 or 4. Prophylaxis use of loperamide could effectively reduce incidence of diarrhoea caused by neratinib. However, evidence is scarce supporting the prophylaxis use of loperamide in pyrotinib treatment. Of note, no cardiac-related events were recorded in our study. The low incidence of severe adverse events demonstrated the safety of pyrotinib plus trastuzumab in heavily pre-treated MBC patients.
The limitation of this study includes the fact that the study is an initial single-center investigation based on a small Chinese cohort. Another limitation is the relatively low rate of standard care administration in previous lines of treatment among the enrolled patients. As mentioned above, only 6 patients received pertuzumab and trastuzumab as first-line therapeutic regimen and none of them had received TDM-1, ds8201 and tucatinib pre-treatment owing to financial and drug accessibility factors. Therefore, due to the small overall number of patients and the relatively low rate of standard care, this study will always lack sufficient power to observe the efficacy of this combination regimen on patients pre-treated with multiple anti-HER2-target agents as well as the discrepancies between this combination regimen and current standard regimens. However, pyrotinib in combination with trastuzumab and chemotherapy still be a potentially effective alternative regimen in heavily pre-treated patients with HER2-positive MBC. Multi-center randomized controlled trials in larger cohorts are needed to further validate the efficacy and safety of this combination regimen.
In conclusion, pyrotinib in combination with trastuzumab and chemotherapy offer an active option with a favorable safety profile in heavily pre-treated patients with HER2-positive MBC, including those with brain metastases. Multi-center randomized controlled trials are warranted to validate the results.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Untch M, Smith I, Gianni L, Baselga J, Al-Sakaff N, Lauer S, McFadden E, Leyland-Jones B, Bell R, Dowsett M, Jackisch C (2017) 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. The Lancet 389:1195–1205. https://doi.org/10.1016/s0140-6736(16)32616-2
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
National Comprehensive Cancer Network (NCCN) (2021) Clinical practice guidelines in oncology: breast cancer. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Pernas S, Tolaney SM (2019) HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Therap Adv Med Oncol 11:175883591983351. https://doi.org/10.1177/1758835919833519
Brosnan EM, Anders CK (2018) Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med 6:163. https://doi.org/10.21037/atm.2018.04.35
Sammons S, Van Swearingen AED, Chung C, Anders CK (2021) Advances in the management of breast cancer brain metastases. Neuro-oncol Adv 3:v63–v74. https://doi.org/10.1093/noajnl/vdab119
Extra JM, Antoine EC, Vincent-Salomon A, Delozier T, Kerbrat P, Bethune-Volters A, Guastalla JP, Spielmann M, Mauriac L, Misset JL, Serin D, Campone M, Hebert C, Remblier C, Bergougnoux L, Campana F, Namer M (2010) Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 15:799–809. https://doi.org/10.1634/theoncologist.2009-0029
von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J, Harbeck N et al (2011) Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in H ER2-positive breast cancer. Eur J Cancer 47:2273–2281
Mukherjee A, Dhadda AS, Shehata M, Chan S (2007) Lapatinib: a tyrosine kinase inhibitor with a clinical role in breast cancer. Expert Opin Pharmacother 8:2189–2204
Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, Casey MA, Berger MS, Stein SH, Sledge GW (2008) Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26:2999–3005
Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A (2004) Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64:3958–3965
Moulder SL, Borges VF, Baetz T, McSpadden T, Fernetich G, Murthy RK, Chavira R, Guthrie K, Barrett E, Chia SK (2017) Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC). Clin Cancer Res 23:3529–3536
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, Luo Y, Xing P, Lan B, Li M, Yi Z, Cai R, Yuan P, Zhang P, Li Q, Xu B (2017) Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 35:3105–3112
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, Hu X, Zou J, Zhu X, Xu B (2019) Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prio R taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol 37:2610–2619
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, Tong Z, Li H, Zhang Q, Sun T, Wang X, Yin Y, Cheng Y, Li W, Gu Y, Chen Q, Liu J, Cheng J, Geng C, Qin S, Wang S, Lu J, Shen K, Liu Q, Wang X, Wang H, Luo T, Yang J, Wu Y, Yu Z, Zhu X, Chen C, Zou J, Investigators P (2021) Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive met astatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22:351–360
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q, Feng J, Yin Y, Sun T, Tong Z, Wang X, Yao H, Zou J, Zhu X, Jiang Z (2020) Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res. https://doi.org/10.21037/tbcr-20-25
Zhong X, He P, Chen J, Yan X, Wei B, Zhang Z, Bu H, Li J, Tian T, Lv Q, Wang X, Li H, Wang J, Huang J, Suo J, Liu X, Zheng H, Luo T (2022) Neoadjuvant pyrotinib plus trastuzumab and nab-paclitaxel for HER2-positive early or locally advanced breast cancer: an exploratory phase II trial. Gland Surg 11:216–225
Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Muller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP (2020) Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597–609. https://doi.org/10.1056/NEJMoa1914609
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124–1130
Blackwell KL, Zaman K, Qin S, Tkaczuk KHR, Campone M, Hunt D, Bryce R, Goldstein LJ (2019) Neratinib in combination with trastuzumab for the treatment of patients with advanced HER2-positive breast cancer: a phase I/II study. Clin Breast Cancer 19:97–104
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F (2015) Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metast 32:125–133
Eng LG, Dawood S, Sopik V, Haaland B, Tan PS, Bhoo-Pathy N, Warner E, Iqbal J, Narod SA, Dent R (2016) Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res Tr 160:145–152
Wang N, Li L, Xiong Y, Chi J, Liu X, Zhong C, Wang F, Gu Y (2021) Case report: significant efficacy of pyrotinib in the treatment of extensive human epidermal growth factor receptor 2-positive breast cancer cutaneous metastases: a report of five cases. Front Oncol 11:729212
Acknowledgements
The authors are grateful to the patients and all the researchers, including the physicians, pathologists, and technicians, who participated in this study.
Funding
This work was supported by the Chinese Society of Clinical Oncology (CSCO) Research Foundation in Beijing and fund from ShenZhen Science and Technology Program (2018, JCYJ20180306171227129). Cai-Wen Du has received researches supported from Beijing CSCO clinical Oncology Research Foundation and Shenzhen Science and Technology Innovation Commission.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by X-FX, Q-YZ, J-YH, L-PC, S-JG, S-LX, and X-FL. Writing-reviewing and editing were performed by X-XF, XB, LS. The first draft of the manuscript was written by X-FX and C-WD. Supervision and project administration was performed by C-WD. All authors are responsible for the contents and have read and approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
All procedures performed involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was also approved by institutional review boards and ethics committees (ethical code: 2019-35) of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Informed consent
Informed consent was obtained from all individual participants included in the study. The authors affirmed that human research participants provided informed consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, XF., Zhang, QY., Huang, JY. et al. Pyrotinib combined with trastuzumab and chemotherapy for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: a single-arm exploratory phase II trial. Breast Cancer Res Treat 197, 93–101 (2023). https://doi.org/10.1007/s10549-022-06770-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06770-6