Abstract
Background
Liquid biopsy has been widely researched for early diagnosis, prognostication and disease monitoring in lung cancer, but there is a need to investigate its clinical utility for early-stage non-small cell lung cancer (NSCLC).
Methods
We performed a meta-analysis and systematic review to evaluate diagnostic and prognostic values of liquid biopsy for early-stage NSCLC, regarding the common biomarkers, circulating tumor cells, circulating tumor DNA (ctDNA), methylation signatures, and microRNAs. Cochrane Library, PubMed, EMBASE databases, ClinicalTrials.gov, and reference lists were searched for eligible studies since inception to 17 May 2022. Sensitivity, specificity and area under the curve (AUC) were assessed for diagnostic values. Hazard ratio (HR) with a 95% confidence interval (CI) was extracted from the recurrence-free survival (RFS) and overall survival (OS) plots for prognostic analysis. Also, potential predictive values and treatment response evaluation were further investigated.
Results
In this meta-analysis, there were 34 studies eligible for diagnostic assessment and 21 for prognostic analysis. The estimated diagnostic values of biomarkers for early-stage NSCLC with AUCs ranged from 0.84 to 0.87. The factors TNM stage I, T1 stage, N0 stage, adenocarcinoma, young age, and nonsmoking contributed to a lower tumor burden, with a median cell-free DNA concentration of 8.64 ng/ml. For prognostic analysis, the presence of molecular residual disease (MRD) detection was a strong predictor of disease relapse (RFS, HR, 4.95; 95% CI, 3.06–8.02; p < 0.001) and inferior OS (HR, 3.93; 95% CI, 1.97–7.83; p < 0.001), with average lead time of 179 ± 74 days between molecular recurrence and radiographic progression. Predictive values analysis showed adjuvant therapy significantly benefited the RFS of MRD + patients (HR, 0.27; p < 0.001), while an opposite tendency was detected for MRD − patients (HR, 1.51; p = 0.19). For treatment response evaluation, a strong correlation between pathological response and ctDNA clearance was detected, and both were associated with longer survival after neoadjuvant therapy.
Conclusions
In conclusion, our study indicated liquid biopsy could reliably facilitate more precision and effective management of early-stage NSCLC. Improvement of liquid biopsy techniques and detection approaches and platforms is still needed, and higher-quality trials are required to provide more rigorous evidence prior to their routine clinical application.
Similar content being viewed by others
Background
Lung cancer was the second most commonly diagnosed cancer and the leading cause of cancer death in 2020, with an estimated 2.2 million new cancer cases and 1.8 million deaths worldwide [1]. Approximately 60% of lung cancer patients have distant metastasis at the initial diagnosis, and a substantial number of patients still progress to local recurrence or distant metastasis after curative-intent treatment [2, 3]. Low-dose computed tomography (LDCT) has been suggested for lung cancer screening, with a significant relative reduction in mortality for lung cancer patients [4]. However, distinguishing small malignant nodules in LDCT from benign lesions is particularly challenging due to the high false-positive rate [5,6,7]. In practice, a significant proportion of patients still suffer from ambiguous disease progression during radiological follow-up after radical surgery. Presently, the prognostic stratification of non-small cell lung cancer (NSCLC) using factors such as tumor, node, metastasis (TNM) classification, airway spread, and pathological subtype has shown limited effectiveness, with some low-risk patients experiencing postoperative relapse [8]. Therefore, more effective approaches and biomarkers will contribute to early detection, precision medicine, individualized treatment, and prognostication of lung cancer, and they are urgently needed.
Recently, with the advantages of noninvasiveness, ease of access, reproducibility, good reflection of the overall state of the tumor, and real-time surveillance, liquid biopsy has been widely researched and has shown promising efficacy in early diagnosis, prognostication, and disease monitoring in lung cancer. Circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), methylation signatures, and microRNAs are the most commonly detected biomarkers [9, 10]. The clinical applications of liquid biopsy have been gradually established for advanced-stage NSCLC and metastatic disease. With the development of molecular biological detection technologies and platforms, combined with multianalytical approaches and machine learning models, the clinical performance of liquid biopsy in detecting diagnostic, prognostic, and predictive biomarkers for early-stage NSCLC has been further investigated in the last few decades [11,12,13,14,15,16]. Here, we performed this systematic review and meta-analysis to discuss the advantages and current limitations of liquid biopsy in the management of localized NSCLC, regarding the commonly detected biomarkers, CTCs, ctDNA, methylation signatures, and microRNAs. The potential clinical utility of liquid biopsy in early-stage NSCLC diagnosis, prognosis, and clinical monitoring of treatment response or recurrence will also be explored.
Methods
Study selection
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statements checklist (Additional file 1: Table S1) [17]. Literature search was conducted in Cochrane Library, PubMed, EMBASE databases, ClinicalTrials.gov, and reference lists by 2 researchers (H.S. and Y.J.) independently. Studies published since inception to 17 May 2022 were included. To perform a comprehensive search, we used the following keywords and MeSH terms in different patterns: (“Lung Neoplasm”) AND (“Liquid Biopsy” OR “Circulating Tumor Cell” OR “Circulating Tumor DNA” OR “DNA Methylation” OR “Circulating Tumor RNA”) (Additional file 2: Table S1).
Eligibility criteria
The following criteria were used for study inclusion: studies that evaluated the diagnostic and prognostic values of liquid biopsy for early-stage NSCLC, regarding the common biomarkers, CTCs, ctDNA, methylation signatures, and microRNAs; adequate data to construct the diagnostic 2 × 2 table for diagnostic assessment; sufficient survival data to obtain the hazard ratios (HRs) for the prognostic analysis in the preoperative, postoperative, and postchemotherapy time point; and the most recent or completed study if based on overlapping patients. The exclusion criteria were as follows: studies without any relevant data for analysis; stage IV or advanced-stage NSCLC; the involved sample size was fewer than 10; papers that were not published in English; and commentaries, editorials, reports, reviews, letters, and experiments.
Data extraction
The following data were extracted in a standardized form: the publication details, study design, patient characteristics, stages, biopsy method, type of biomarker, the true positive, false positive, false negative, true negative for the analysis of the sensitivity and specificity. Additionally, the concentrations of circulating cell-free DNA (cfDNA) in preoperative plasma of eligible cohorts were also extracted for the exploration of related clinical factors, including sex, age, TNM stage, smoking, and histopathology. And if the studies reported the association between circulating biomarkers and short- and long-term outcomes of the NSCLC patients, follow-up duration and the hazard ratio (HR) with a 95% confidence interval (CI) were extracted from the regression or survival plot of recurrence-free survival (RFS) and overall survival (OS). The prognostic analysis for molecular residual disease (MRD) detection focused on the survival data at postoperative time point. The reported lead time of biomarker (e.g., ctDNA) detection preceding radiographic progression was also listed and summarized. As for predictive value analysis, original survival data were extracted from the Kaplan–Meier curves comparing RFS between MRD/ctDNA-positive patients receiving adjuvant therapy and not receiving adjuvant therapy and comparing RFS between MRD/ctDNA-negative patients receiving adjuvant therapy and not receiving adjuvant therapy. In terms of treatment response evaluation for neoadjuvant therapy, relevant data in ctDNA clearance and assessment of pathological response were collected and analyzed. Any discrepancies were assessed by a third author (K.C.).
Risk of bias assessment
The quality assessment of the included studies was evaluated by the Quality Assessment for Studies of Diagnostic Accuracy Score-2 (QUADAS-2) tool [18], with 4 different domains: patient selection, index test, reference standard, and flow and timing. And for the cohort studies involved in prognostic analysis, the quality assessment was followed by the Newcastle–Ottawa Scale [19]. Publication bias was detected by Deeks’ funnel plot.
Statistical analysis
Diagnostic meta-analysis was based on the MIDAS module (Stata module for meta-analytical integration of diagnostic test accuracy studies) [20] and bivariate approach [21]. The pooled sensitivity and specificity were calculated by the accuracy data, and the summary receiver operative curve (SROC) was generated by “mada” package, and the area under the curve (AUC) was evaluated. Analysis of factors related to preoperative cfDNA concentration was conducted by Kruskal–Wallis test. In terms of prognostic analysis, the RFS and OS were measured by HRs and 95% CIs that were directly reported in the included studies. Otherwise, survival data that were not presented numerically in articles were extracted from the Kaplan–Meier curve using Engauge Digitizer version 12, and the HRs were calculated by the Parmar and Tierney methods [22, 23]. The random-effects model was pooled due to the high heterogeneity of the studies (p < 0.10 or I2 > 50%). Otherwise, the fixed-effects model was used. Subgroup analysis was performed by the preoperative, postoperative, and postchemotherapy time point, while the prognostic analysis for MRD detection was based on the survival data at postoperative time point. The lead time analysis was estimated and summarized by the Wan and Luo methods [24, 25] and then pooled and presented in an estimated average using the ggplot2 package. The forest plots of predictive value analysis were pooled in MRD/ctDNA-positive group and MRD/ctDNA-negative group. Correlation between pathological response and ctDNA clearance was conducted by the 2-sided Fisher’s exact test [26]. Survival analysis in pathological response and ctDNA clearance was also pooled in a forest plot. Statistical analyses were performed by Stata 15.0 software (Stata Corporation, College Station, TX) and RStudio 4.1.3. Statistical significance was set at p < 0.05.
Results
Literature selection and study characteristics
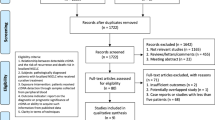
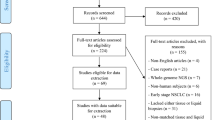
A flow diagram of the literature search is shown in Additional file 3: Fig. S1. After exclusion of studies, a total of 53 articles were included in the meta-analysis with 34 studies [14, 16, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58] eligible for diagnostic assessment and 21 [12,13,14,15, 33, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] for prognostic analysis. Summary characteristics of the included studies were demonstrated in Additional file 2: Table S2.
Assessment of bias
Assessment of the study quality was evaluated using the QUADAS-2 tool (Additional file 2: Table S3) and Newcastle–Ottawa Scale (Additional file 2: Table S4). There was no significant publication bias determined by the Deeks’ funnel plot (p = 0.39, Additional file 3: Fig. S2) in diagnostic study nor by formal statistical tests of Egger’s test in prognostic analysis (Additional file 3: Fig. S3).
Diagnostic performance
For the diagnostic analysis, 2917 healthy controls and 3015 patients with early-stage NSCLC were included, among whom 1537 patients were limited to stage I. The biomarkers included CTC in 6 eligible studies with 885 participants, ctDNA in 7 studies with 1001 participants, DNA methylation in 11 studies with 1888 participants, and microRNAs in 7 relevant articles with 1079 participants. The estimated diagnostic values of different common biomarkers for early-stage NSCLC with AUCs ranged from 0.84 to 0.87 (Fig. 1A). Additionally, the comparison of the diagnostic values between different biomarkers showed no significant differences, with a similar ROC-AUC and overlapping 95% confidence ellipses. In particular, a lower AUC was calculated when the analysis was limited to stage I disease (Additional file 2: Table S5).
Analysis of the cfDNA concentration
Eight cohorts of 941 patients with early-stage NSCLC were reanalyzed [13,14,15, 35, 40, 42, 66, 69]. The relative factors included TNM stage (I, II, and III), T stage (T1, T2, T3, and T4), N stage (N0, N1, and N2), age (≥ 65 vs. < 65), sex, smoking status, and histopathology. The median cfDNA concentration of the preoperative plasma samples from all patients with stage I–III NSCLC was 8.64 ng/ml (Additional file 2: Table S6, original data in Additional file 4: Table S1). For TNM stage, the median cfDNA concentration in stage I was 7.58 ng/ml, significantly lower than the median concentrations in stage II (9.86 ng/ml, p < 0.001) and III (10.03 ng/ml, p < 0.0001). There was no significant difference between the median concentrations in stages II and III (p = 0.22) (Fig. 1B). The median cfDNA concentration in the T1 stage was 1.09 ng/ml, significantly lower than the median concentrations in the higher T stages. Similarly, the median cfDNA concentrations in N0 stage (1.70 ng/ml) were significantly lower than in N1 and N2 stages. Older patients had significantly higher cfDNA concentrations than younger patients (≥ 65 vs. < 65, 9.03 ng/ml vs. 6.99 ng/ml, p < 0.000001). Smoking was also associated with significantly higher cfDNA concentrations (smoking vs. nonsmoking, 10.64 ng/ml vs. 7.52 ng/ml, p < 0.000001). No significant difference was detected between the sexes (female vs. male, 7.83 ng/ml vs. 7.90 ng/ml, p = 0.98). For different histopathologies, the results showed that lung adenocarcinoma (LUAD) was associated with significantly lower cfDNA concentrations than lung squamous cell carcinoma (LUSC) (LUAD vs. LUSC, 9.03 ng/ml vs. 10.78 ng/ml, p = 0.014) (Fig. 1B).
Prognostic performance
To evaluate the prognostic values of liquid biopsy in early-stage NSCLC, 21 eligible studies with 2143 patients were included [12,13,14,15, 33, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74]. Most of the studies referred to ctDNA biomarker, with only one relevant study focusing on DNA methylation [33], 2 regarding CTCs [60, 61], and 1 referring to microRNAs [59]. Subgroup analysis was performed by the preoperative, postoperative, and postchemotherapy time point, while the prognostic analysis for MRD detection was based on the survival data at postoperative time point. The forest plots showed that MRD detection after curative intent treatment was a strong predictor of disease relapse (RFS, HR, 4.95; 95% CI, 3.06–8.02; p < 0.001, Fig. 2A) and a shorter OS (HR, 3.93; 95% CI, 1.97–7.83; p < 0.001, Fig. 2B). Likewise, biomarkers positive in the preoperative blood samples were also associated with significantly inferior RFS (HR, 3.00; 95% CI, 2.12–4.24; p < 0.001, Fig. 2A) and OS (HR, 3.65; 95% CI, 1.96–6.77; p < 0.001, Fig. 2B). Similar trends were detected at the postchemotherapy time point for both RFS (HR, 4.51; 95% CI, 2.27–8.94; p < 0.001, Fig. 2A) and OS (HR, 4.41; 95% CI, 0.52–37.29; p = 0.17, Fig. 2B).
Lead time analysis
A summary of the lead time by biomarker detection preceding radiographic progression was presented in Fig. 2C. All of the 9 eligible studies referred to ctDNA detection, with a total of 148 patients suffering disease progression [12, 13, 15, 68, 69, 71, 72, 74, 75]. Molecular recurrence was detected by next-generation sequencing (NGS) approaches and cancer personalized profiling by deep sequencing (CAPP-Seq), circulating single-molecule amplification and resequencing technology (cSMART), and multiplex polymerase chain reaction NGS (mPCR-NGS) platforms. The reported lead time ranged from 0 to 795 days. Taken together, our study found an average lead time of 179 ± 74 days.
Predictive value
The detailed data for predictive value analysis of MRD detection in adjuvant therapy guidance after lung cancer resection are presented in Fig. 3A [13, 66, 69, 72, 75, 76]. All of the 6 eligible cohorts explored the ctDNA-based MRD predictive value for adjuvant therapy (Additional file 4: Table S2). In this series, adjuvant therapy was found to confer a survival benefit for patients with detectable ctDNA-based MRD, while adjuvant therapy could not improve survival for undetectable MRD patients. Furthermore, the forest plot showed that the application of adjuvant therapy significantly benefited long-term survival in patients with ctDNA-based MRD + (RFS, HR, 0.27; 95% CI, 0.17–0.44; p < 0.001), while an opposite tendency was detected for MRD − patients (RFS, HR, 1.51; 95% CI, 0.81–2.79; p = 0.19) (Fig. 3B).
Treatment response evaluation
There were 3 studies eligible for the analysis of correlation between pathological response and ctDNA responder for neoadjuvant therapy (Additional file 2: Table S7) [71, 77, 78]. The percentage of patients with a major pathological response (MPR) or pathologic complete response (pCR) was higher among those with ctDNA responder (33% ~ 86%) than among those without ctDNA responder (0 ~ 17%) (Fig. 3C). Further Fisher’s exact test indicated a strong correlation between a pathological response and ctDNA responder (p < 0.00001). In addition, significantly improved long-term survivals were both observed for patients with pCR (HR, 0.41, 95% CI, 0.21–0.83, p < 0.05) and ctDNA clearance (HR, 0.16, 95% CI, 0.07–0.37, p < 0.001) after neoadjuvant therapy (Fig. 3D) [73, 77, 78].
Discussion
The study is a comprehensive systematic review and meta-analysis to evaluate the clinical utility of liquid biopsy focusing on the early-stage NSCLC, regarding the commonly detected biomarkers, CTCs, ctDNA, methylation signatures, and microRNAs. The present study indicated liquid biopsy could reliably facilitate more precision and effective management of early-stage NSCLC.
The early detection of lung cancer is critical for reducing its mortality and morbidity. In recent decades, many studies have investigated the screening and diagnostic values of different biomarkers, including ctDNA, CTCs, DNA methylation, and microRNAs. Our analysis indicated that these common biomarkers could provide similar diagnostic accuracy for early-stage NSCLC, with acceptable effectiveness in AUCs ranging from 0.84 to 0.87. With the development of platforms and technology, ctDNA analysis has provided a practical approach to the noninvasive detection of early-stage tumors [14, 35, 39]. In addition, the analysis of cfDNA fragmentomics profiles was newly highlighted as the novel approach and pattern for the accurate screening, early detection, and monitoring of human cancer [79, 80]. With the advantages of an early appearance in the disease course, cancer specificity, biological stability, and ready accessibility in bodily fluids [81], aberrant DNA methylation analysis combined with machine learning provided the opportunity to overcome the challenge of the low abundance of ctDNA in plasma samples [28, 29, 34, 58, 82, 83]. CTCs in the blood could serve as effective screening and diagnostic markers to discriminate small malignant pulmonary nodules from benign lesions [54, 56]. The profiling of circulating microRNAs has promising accuracy for discriminating lung cancer patients from healthy controls and could be developed as a supplement in future screening [51, 84]. Recently, integrative multi-analytical models combining clinical features and multiple biomarkers have achieved a better balance of predictive sensitivity and specificity [16, 40, 53]. Multiomics analysis combining ctDNA detection with methylation, exosomes, circulating microRNAs, circulating tumor cells, metabonomics, and molecular imaging methods should be considered to improve the efficacy and provide more practical value for clinical applications.
In this study, there was a lower diagnostic accuracy for stage I NSCLC. The low concentration of cfDNA molecules in plasma introduces a biological limitation for detecting early-stage tumors [85, 86]. Similar to the previous studies [85, 87], our analysis pooled a median cfDNA concentration of 8.64 ng/ml in early-stage NSCLC and suggested that TNM stage I, T1 stage, N0 stage, adenocarcinoma, young age, and nonsmoking were associated with significantly lower cfDNA concentrations. The summarized data on the limited tumor burden highlighted the physical limitation of ctDNA analyses, particularly for the early detection of NSCLC patients with T1a-c stage. Additionally, clonal hematopoiesis of undetermined potential (CHIP) during aging is a common confounding factor influencing ctDNA detection. Deep sequencing of both white blood cell DNA and cfDNA might be required to identify and filter out CHIP-related mutations to reduce false-positive ctDNA detection rates [85].
With the development of new technologies and platforms, the feasibility and efficacy of MRD assessment and postoperative disease monitoring for early-stage NSCLC have gradually been investigated [85, 88, 89]. It is now widely recognized that patients with positive ctDNA and MRD detection after curative intent treatment have a worse prognosis than those with undetectable MRD [12, 15, 66, 72, 75]. In addition, the promising ctDNA MRD analysis for the early detection of disease recurrence preceding radiographic progression by an average lead time of 179 ± 74 days could reliably facilitate more effective interventions, offering the chance for earlier treatment strategy decision-making and to treat patients at their lowest tumor burden [12, 13, 15, 71, 74]. Although the other biomarkers including CTC, DNA methylation and microRNAs also showed the promising prognostic values, there is still lack of clinical cohort regarding their MRD detection during post-treatment monitoring [59, 60, 84].
A summary of the four different MRD detection patterns is presented in Table 1. For assay design, a tumor-naive panel involves sequencing several genes commonly known to be mutated in lung cancer, and thus there is no need for individualized tumor information [90]. The novel CAPP-Seq strategy has developed and achieved the enhancements in ctDNA analysis, with the ultrasensitive detection efficiency under the ultralow detection limit [14, 91]. Correspondingly, tumor-informed assays were designed based on the whole exome/genome sequencing of tumor tissues and tumor-adjacent normal tissues. The personalized panels take advantage of accurately tracking a larger number of mutations for each patient and improving the sensitivity of ctDNA detection [92]. However, the method is more expensive and has limitations in detecting de novo resistance/oncogenic alterations. The novel MRDetect model demonstrated that increased breadth or an expanded number of targeted mutations via genome wide mutational integration could effectively overcome the limitation of cfDNA abundance with a modest sequencing depth [11]. Honestly, DNA methylation analysis was proved to provide the promising efficacy in cancer detection and screening [82, 83], but there is still a lack of large-scale validations and clinical implementations regarding their MRD detection during post-treatment monitoring in early-stage lung cancer [93]. The currently ongoing prospective cohort, which was designed to investigate the feasibility of tumor-informed methylation-based MRD detection and postoperative cancer surveillance, is fully expected [94].
Increasing evidence indicates that ctDNA-based MRD detection could provide good prognostic value for early-stage NSCLC after surgical resection and adjuvant therapy. However, evidence of the predictive value of MRD detection for adjuvant therapy guidance is still lacking. Our results indicated that the application of adjuvant therapy significantly benefited long-term survival in patients with ctDNA-based MRD + , while adjuvant therapy could not improve survival for MRD − patients [13, 66, 69, 72, 75, 76]. These results clearly revealed that longitudinal MRD monitoring could provide clinical utility for individualized patient care with adjuvant therapies and could efficiently avoid overtreatment of low-risk patients. However, the evidence should be interpreted cautiously because of the small sample sizes and lack of prospective interventional study designs. Thus, randomized controlled trials are necessary to confirm the predictive value of MRD. The MERMAID-1 trial [95] is ongoing to assess the efficacy of adjuvant durvalumab combined with chemotherapy in postsurgical MRD + patients with stage II–III NSCLC. Meanwhile, the ongoing MERMAID-2 trial [96] is designed to evaluate the efficacy and safety of durvalumab adjuvant therapy in stage II–III NSCLC patients who become MRD + during the surveillance period after curative intent therapy. The results of these relevant trials are highly anticipated, and high-level evidence is urgently awaited (Table 2) [97,98,99].
Recently, the clinical utility of ctDNA in predicting the response to neoadjuvant treatments and assessing the prognosis of NSCLC has gradually been explored [71, 77, 100]. The NADIM trial [73] reported for the first time a significant association between ctDNA levels after neoadjuvant chemoimmunotherapy and survival outcomes in operable NSCLC. The data were supported by 3-year OS and revealed that ctDNA outperformed radiologic assessments in the prediction of survival, which highlighted the usefulness of ctDNA as an early surrogate end point for neoadjuvant treatment. Likewise, CheckMate 816 [78] showed that ctDNA clearance was associated with a pathologic response, and event-free survival appeared longer in patients with ctDNA clearance than in those without, suggesting that clearance during neoadjuvant therapy may be an early predictor of favorable outcomes. Correspondingly, our study detected a strong correlation between pathological response and ctDNA clearance, and significantly improved long-term survivals were both observed for patients with pCR and ctDNA clearance after neoadjuvant therapy. These results revealed the effective utility of ctDNA status and dynamics analysis and provided a landmark approach for evaluating the response of therapeutic outcomes for resectable NSCLC patients treated with neoadjuvant therapy. We require more evidence and data regarding the treatment response evaluation of ctDNA analysis in lung cancer disease monitoring.
Limitations
Several limitations of this study should be considered. First, the types of liquid biomarkers, detection technologies, and platforms and the lack of a standardized ctDNA detection manual all contribute to the great heterogeneity among the included studies. Second, there were 10 extensive stage patients enrolled in our analysis [16, 63, 69], but their impact may be negligible. In addition, even though we have completed a comprehensive and systematic search of the literature, publication bias is still inevitable. Finally, some studies did not report detailed information on histology and radiology, and further analysis was therefore limited.
Conclusions
In conclusion, our study indicated liquid biopsy could reliably facilitate more precision and effective management of early-stage NSCLC, regarding the commonly detected biomarkers, CTCs, ctDNA, methylation signatures, and microRNAs. The lead time analysis suggested ctDNA detection monitoring could provide the opportunity for earlier interventions during disease surveillance. Improvement of liquid biopsy techniques and detection approaches and platforms are still needed, and higher-quality trails are required to provide more rigorous evidence prior to their routine clinical application.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- AUC:
-
Area under the curve
- CAPP-seq:
-
Cancer personalized profiling by deep sequencing
- cfDNA :
-
Circulating cell-free DNA
- CHIP :
-
Clonal hematopoiesis of undetermined potential
- CI:
-
Confidence interval
- cSMART:
-
Circulating single-molecule amplification and resequencing technology
- CTC:
-
Circulating tumor cell
- ctDNA :
-
Circulating tumor DNA
- HR:
-
Hazard ratio
- LDCT:
-
Low-dose computed tomography
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MEDAL:
-
Methylation based dynamic analysis for lung cancer
- MIDAS:
-
Stata module for meta-analytical integration of diagnostic test accuracy studies
- mPCR-NGS:
-
Multiplex PCR NGS platforms
- MPR:
-
Major pathological response
- MRD :
-
Molecular residual disease
- NGS:
-
Next-generation sequencing
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PCR:
-
Polymerase chain reaction
- pCR:
-
Pathologic complete response
- RFS:
-
Recurrence-free survival
- SROC:
-
Summary receiver operative curve
- TNM:
-
Tumor, node, metastasis classification
- TRACERx:
-
Tracking non-small cell lung cancer evolution through therapy (Rx) study
- WGS:
-
Whole-genome sequencing
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, Kelsey CR. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7(4):723–30.
Watanabe K, Tsuboi M, Sakamaki K, Nishii T, Yamamoto T, Nagashima T, Ando K, Ishikawa Y, Woo T, Adachi H, et al. Postoperative follow-up strategy based on recurrence dynamics for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2016;49(6):1624–31.
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920–31.
Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, Nackaerts K, Vliegenthart R, ten Haaf K, Yousaf-Khan UA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15(12):1342–50.
Liu Y, Wang H, Li Q, McGettigan MJ, Balagurunathan Y, Garcia AL, Thompson ZJ, Heine JJ, Ye Z, Gillies RJ, et al. Radiologic features of small pulmonary nodules and lung cancer risk in the national lung screening trial: a nested case-control study. Radiology. 2018;286(1):298–306.
Wang C, Wu Y, Shao J, Liu D, Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020;20(1):150.
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, Tang XM, Sun F, Lu HM, Deng J, et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21(1):25.
Di Capua D, Bracken-Clarke D, Ronan K, Baird AM, Finn S. The liquid biopsy for lung cancer: state of the art, limitations and future developments. Cancers (Basel). 2021;13(16):3923.
Zviran A, Schulman RC, Shah M, Hill STK, Deochand S, Khamnei CC, Maloney D, Patel K, Liao W, Widman AJ, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med. 2020;26(7):1114–24.
Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA, Veeriah S, Rosenthal R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–51.
Chen K, Zhao H, Shi Y, Yang F, Wang LT, Kang G, Nie Y, Wang J. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC). Clin Cancer Res. 2019;25(23):7058–67.
Chabon JJ, Hamilton EG, Kurtz DM, Esfahani MS, Moding EJ, Stehr H, Schroers-Martin J, Nabet BY, Chen B, Chaudhuri AA, et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580(7802):245–51.
Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, Khodadoust MS, Esfahani MS, Liu CL, Zhou L, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7(12):1394–403.
Chen K, Sun J, Zhao H, Jiang R, Zheng J, Li Z, Peng J, Shen H, Zhang K, Zhao J, et al. Non-invasive lung cancer diagnosis and prognosis based on multi-analyte liquid biopsy. Mol Cancer. 2021;20(1):23.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: The Ottawa Hospital Research Institute, 2021. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 17 May 2022.
Dwamena B: MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical Software Components S456880, Boston College Department of Economics, revised 05 Feb 2009. https://ideas.repec.org/c/boc/bocode/s456880.html. Accessed 17 May 2022.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Jung SH. Stratified Fisher’s exact test and its sample size calculation. Biom J. 2014;56(1):129–40.
Sozzi G, Musso K, Ratcliffe C, Goldstraw P, Pierotti MA, Pastorino U. Detection of microsatellite alterations in plasma DNA of non-small cell lung cancer patients: a prospect for early diagnosis. Clin Cancer Res. 1999;5(10):2689–92.
Ostrow KL, Hoque MO, Loyo M, Brait M, Greenberg A, Siegfried JM, Grandis JR, Gaither Davis A, Bigbee WL, Rom W, et al. Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative methylation-specific PCR. Clin Cancer Res. 2010;16(13):3463–72.
Begum S, Brait M, Dasgupta S, Ostrow KL, Zahurak M, Carvalho AL, Califano JA, Goodman SN, Westra WH, Hoque MO, et al. An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin Cancer Res. 2011;17(13):4494–503.
Kneip C, Schmidt B, Seegebarth A, Weickmann S, Fleischhacker M, Liebenberg V, Field JK, Dietrich D. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer in plasma. J Thorac Oncol. 2011;6(10):1632–8.
Wozniak MB, Scelo G, Muller DC, Mukeria A, Zaridze D, Brennan P. Circulating MicroRNAs as non-invasive biomarkers for early detection of non-small-cell lung cancer. PLoS ONE. 2015;10(5):e0125026.
Halvorsen AR, Bjaanæs M, LeBlanc M, Holm AM, Bolstad N, Rubio L, Peñalver JC, Cervera J, Mojarrieta JC, López-Guerrero JA, et al. A unique set of 6 circulating microRNAs for early detection of non-small cell lung cancer. Oncotarget. 2016;7(24):37250–9.
Balgkouranidou I, Chimonidou M, Milaki G, Tsaroucha E, Kakolyris S, Georgoulias V, Lianidou E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin Chem Lab Med. 2016;54(8):1385–93.
Ooki A, Maleki Z, Tsay JJ, Goparaju C, Brait M, Turaga N, Nam HS, Rom WN, Pass HI, Sidransky D, et al. A panel of novel detection and prognostic methylated DNA markers in primary non-small cell lung cancer and serum DNA. Clin Cancer Res. 2017;23(22):7141–52.
Phallen J, Sausen M, Adleff V, Leal A, Hruban C, White J, Anagnostou V, Fiksel J, Cristiano S, Papp E, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9(403):eaan2415.
Powrózek T, Kuźnar-Kamińska B, Dziedzic M, Mlak R, Batura-Gabryel H, Sagan D, Krawczyk P, Milanowski J, Małecka-Massalska T. The diagnostic role of plasma circulating precursors of miRNA-944 and miRNA-3662 for non-small cell lung cancer detection. Pathol Res Pract. 2017;213(11):1384–7.
Sun Y, Mei H, Xu C, Tang H, Wei W. Circulating microRNA-339-5p and -21 in plasma as an early detection predictors of lung adenocarcinoma. Pathol Res Pract. 2018;214(1):119–25.
Wei F, Strom CM, Cheng J, Lin CC, Hsu CY, Soo Hoo GW, Chia D, Kim Y, Li F, Elashoff D, et al. Electric field-induced release and measurement liquid biopsy for noninvasive early lung cancer assessment. J Mol Diagn. 2018;20(6):738–42.
Wan Y, Liu B, Lei H, Zhang B, Wang Y, Huang H, Chen S, Feng Y, Zhu L, Gu Y, et al. Nanoscale extracellular vesicle-derived DNA is superior to circulating cell-free DNA for mutation detection in early-stage non-small-cell lung cancer. Ann Oncol. 2018;29(12):2379–83.
Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926–30.
Liang W, Zhao Y, Huang W, Gao Y, Xu W, Tao J, Yang M, Li L, Ping W, Shen H, et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics. 2019;9(7):2056–70.
Peng M, Xie Y, Li X, Qian Y, Tu X, Yao X, Cheng F, Xu F, Kong D, He B, et al. Resectable lung lesions malignancy assessment and cancer detection by ultra-deep sequencing of targeted gene mutations in plasma cell-free DNA. J Med Genet. 2019;56(10):647–53.
Villalba M, Exposito F, Pajares MJ, Sainz C, Redrado M, Remirez A, Wistuba I, Behrens C, Jantus-Lewintre E, Camps C, et al. TMPRSS4: a novel tumor prognostic indicator for the stratification of stage IA tumors and a liquid biopsy biomarker for NSCLC patients. J Clin Med. 2019;8(12):2134.
Liu J, Han M, Huang H. Validation of the diagnostic efficiency of folate receptor-positive circulating tumor cells in lung cancers: a prospective observational study. Transl Cancer Res. 2019;8(4):1242–8.
Yang Z, Qi W, Sun L, Zhou H, Zhou B, Hu Y. DNA methylation analysis of selected genes for the detection of early-stage lung cancer using circulating cell-free DNA. Adv Clin Exp Med. 2019;28(3):355–60.
Yang Y, Zheng D, Wu C, Lizaso A, Ye J, Chuai S, Ni J, Xu J, Jiang G. Detecting ultralow frequency mutation in circulating cell-free DNA of early-stage nonsmall cell lung cancer patients with unique molecular identifiers. Small Methods. 2019;3:1900206.
Ghany SMA, Ali EMA, Ahmed AE, Hozayen WG, Mohamed-Hussein AAR, Elnaggar MS, Hetta HF. Circulating mirna-30a and mirna-221 as novel biomarkers for the early detection of non-small-cell lung cancer. Middle East J Cancer. 2020;11(1):50–8.
Zhang ZJ, Song XG, Xie L, Wang KY, Tang YY, Yu M, Feng XD, Song XR. Circulating serum exosomal miR-20b-5p and miR-3187-5p as efficient diagnostic biomarkers for early-stage non-small cell lung cancer. Exp Biol Med (Maywood). 2020;245(16):1428–36.
He Y, Shi J, Schmidt B, Liu Q, Shi G, Xu X, Liu C, Gao Z, Guo T, Shan B. Circulating tumor cells as a biomarker to assist molecular diagnosis for early stage non-small cell lung cancer. Cancer Manag Res. 2020;12:841–54.
Liu WR, Zhang B, Chen C, Li Y, Ye X, Tang DJ, Zhang JC, Ma J, Zhou YL, Fan XJ, et al. Detection of circulating genetically abnormal cells in peripheral blood for early diagnosis of non-small cell lung cancer. Thorac Cancer. 2020;11(11):3234–42.
Wang W, Chen D, Chen W, Xin Z, Huang Z, Zhang X, Xi K, Wang G, Zhang R, Zhao D, et al. Early detection of non-small cell lung cancer by using a 12-microRNA panel and a nomogram for assistant diagnosis. Front Oncol. 2020;10:855.
Chen C, Huang X, Yin W, Peng M, Wu F, Wu X, Tang J, Chen M, Wang X, Hulbert A, et al. Ultrasensitive DNA hypermethylation detection using plasma for early detection of NSCLC: a study in Chinese patients with very small nodules. Clin Epigenetics. 2020;12(1):39.
Liu QX, Zhou D, Han TC, Lu X, Hou B, Li MY, Yang GX, Li QY, Pei ZH, Hong YY, et al. A noninvasive multianalytical approach for lung cancer diagnosis of patients with pulmonary nodules. Adv Sci (Weinh). 2021;8(13):2100104.
Ye M, Zheng X, Ye X, Zhang J, Huang C, Liu Z, Huang M, Fan X, Chen Y, Xiao B, et al. Circulating genetically abnormal cells add non-invasive diagnosis value to discriminate lung cancer in patients with pulmonary nodules ≤10 mm. Front Oncol. 2021;11:638223.
Feng M, Ye X, Chen B, Zhang J, Lin M, Zhou H, Huang M, Chen Y, Zhu Y, Xiao B, et al. Detection of circulating genetically abnormal cells using 4-color fluorescence in situ hybridization for the early detection of lung cancer. J Cancer Res Clin Oncol. 2021;147(8):2397–405.
Liu C, Chen H, Sun T, Wang H, Chen B, Wang X. The value of circulating tumor cells with positive centromere probe 8 in the diagnosis of small pulmonary nodules. Transl Oncol. 2021;14(5):101052.
Liang W, Chen Z, Li C, Liu J, Tao J, Liu X, Zhao D, Yin W, Chen H, Cheng C, et al. Accurate diagnosis of pulmonary nodules using a noninvasive DNA methylation test. J Clin Invest. 2021;131(10):e145973.
Liang N, Li B, Jia Z, Wang C, Wu P, Zheng T, Wang Y, Qiu F, Wu Y, Su J, et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat Biomed Eng. 2021;5(6):586–99.
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28(10):1721–6.
Dandachi N, Tiran V, Lindenmann J, Brcic L, Fink-Neuboeck N, Kashofer K, Absenger G, Bezan A, Cote RJ, Datar R, et al. Frequency and clinical impact of preoperative circulating tumor cells in resectable non-metastatic lung adenocarcinomas. Lung Cancer. 2017;113:152–7.
de Miguel-Pérez D, Bayarri-Lara CI, Ortega FG, Russo A, Moyano Rodriguez MJ, Alvarez-Cubero MJ, Maza Serrano E, Lorente JA, Rolfo C, Serrano MJ. Post-surgery circulating tumor cells and AXL overexpression as new poor prognostic biomarkers in resected lung adenocarcinoma. Cancers (Basel). 2019;11(11):1750.
Isaksson S, George AM, Jönsson M, Cirenajwis H, Jönsson P, Bendahl PO, Brunnström H, Staaf J, Saal LH, Planck M. Pre-operative plasma cell-free circulating tumor DNA and serum protein tumor markers as predictors of lung adenocarcinoma recurrence. Acta Oncol. 2019;58(8):1079–86.
Peng M, Huang Q, Yin W, Tan S, Chen C, Liu W, Tang J, Wang X, Zhang B, Zou M, et al. Circulating tumor DNA as a prognostic biomarker in localized non-small cell lung cancer. Front Oncol. 2020;10:561598.
Yang W, You N, Jia M, Yeung SJ, Ou W, Yu M, Wang Y, Fu X, Zhang Z, Yang J, et al. Undetectable circulating tumor DNA levels correlate with low risk of recurrence/metastasis in postoperative pathologic stage I lung adenocarcinoma patients. Lung Cancer. 2020;146:327–34.
Kuang PP, Li N, Liu Z, Sun TY, Wang SQ, Hu J, Ou W, Wang SY. Circulating tumor DNA analyses as a potential marker of recurrence and effectiveness of adjuvant chemotherapy for resected non-small-cell lung cancer. Front Oncol. 2020;10:595650.
Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, Feng G, Deng Y, Gan F, Lin Y, et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res. 2022;28(15):3308-17.
Ito M, Miyata Y, Hirano S, Irisuna F, Kushitani K, Kai Y, Kishi N, Tsutani Y, Takeshima Y, Okada M. Sensitivity and optimal clinicopathological features for mutation-targeted liquid biopsy in pN0M0 EGFR-mutant lung adenocarcinoma. J Cancer Res Clin Oncol. 2022;148(6):1419-28.
Li N, Wang BX, Li J, Shao Y, Li MT, Li JJ, Kuang PP, Liu Z, Sun TY, Wu HQ, et al. Perioperative circulating tumor DNA as a potential prognostic marker for operable stage I to IIIA non-small cell lung cancer. Cancer. 2022;128(4):708-18.
Qiu B, Guo W, Zhang F, Lv F, Ji Y, Peng Y, Chen X, Bao H, Xu Y, Shao Y, et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat Commun. 2021;12(1):6770.
Guo K, Shao C, Han L, Liu H, Ma Z, Yang Y, Feng Y, Pan M, Santarpia M, Carmo-Fonseca M, et al. Detection of epidermal growth factor receptor (EGFR) mutations from preoperative circulating tumor DNA (ctDNA) as a prognostic predictor for stage I-III non-small cell lung cancer (NSCLC) patients with baseline tissue EGFR mutations. Transl Lung Cancer Res. 2021;10(7):3213–25.
Yue D, Liu W, Chen C, Zhang T, Ma Y, Cui L, Gu Y, Bei T, Zhao X, Zhang B, et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl Lung Cancer Res. 2022;11(2):263–76.
Zhang JT, Liu SY, Gao W, Liu SM, Yan HH, Ji L, Chen Y, Gong Y, Lu HL, Lin JT, et al. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non-small cell lung cancer. Cancer Discov. 2022;12(7):1690-701.
Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, Dómine M, Majem M, Rodríguez-Abreu D, Martínez-Martí A, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial). J Clin Oncol. 2022;40(32):3785.
Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, Rundell V, Wulff J, Sharma G, Knock H, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. 2022;33(5):500–10.
Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, Bonilla RF, Ko RB, Yoo CH, Gojenola L, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer. 2020;1(2):176–83.
Zhou C, Das Thakur M, Srivastava MK, Zou W, Xu H, Ballinger M, Felip E, Wakelee H, Altorki NK, Reck M, et al. 2O IMpower010: biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann Oncol. 2021;32:S1374.
Kris MG, Grindheim JM, Chaft JE, Lee JM, Johnson BE, Rusch VW, Bunn PA, Pass H, Schum E, Carlisle J, et al. 1O dynamic circulating tumour DNA (ctDNA) response to neoadjuvant (NA) atezolizumab (atezo) and surgery (surg) and association with outcomes in patients (pts) with NSCLC. Ann Oncol. 2021;32:S1373.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022.
Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen S, Medina JE, Hruban C, White JR, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385–9.
Mathios D, Johansen JS, Cristiano S, Medina JE, Phallen J, Larsen KR, Bruhm DC, Niknafs N, Ferreira L, Adleff V, et al. Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat Commun. 2021;12(1):5060.
Andersen RF. Tumor-specific methylations in circulating cell-free DNA as clinically applicable markers with potential to substitute mutational analyses. Expert Rev Mol Diagn. 2018;18(12):1011–9.
Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, Zuzarte PC, Borgida A, Wang TT, Li T, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563(7732):579–83.
Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745–59.
Nagasaka M, Uddin MH, Al-Hallak MN, Rahman S, Balasubramanian S, Sukari A, Azmi AS. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol Cancer. 2021;20(1):82.
Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018;15(9):577–86.
Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20(2):71–88.
Avanzini S, Kurtz DM, Chabon JJ, Moding EJ, Hori SS, Gambhir SS, Alizadeh AA, Diehn M, Reiter JG. A mathematical model of ctDNA shedding predicts tumor detection size. Sci Adv. 2020;6(50):eabc4308.
Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting liquid remnants of solid tumors: circulating tumor DNA minimal residual disease. Cancer Discov. 2021;11(12):2968–86.
Pellini B, Chaudhuri AA. Circulating tumor DNA minimal residual disease detection of non-small-cell lung cancer treated with curative intent. J Clin Oncol. 2022;40(6):567–75.
Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–24.
Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–54.
Abbosh C, Frankell A, Garnett A, Harrison T, Weichert M, Licon A, Veeriah S, Daber B, Moreau M, Chesh A, et al. Abstract CT023: phylogenetic tracking and minimal residual disease detection using ctDNA in early-stage NSCLC: a lung TRACERx study. Cancer Res. 2020;80(16_Supplement):CT023–CT023.
Luo H, Wei W, Ye Z, Zheng J, Xu R-H. Liquid biopsy of methylation biomarkers in cell-free DNA. Trends Mol Med. 2021;27(5):482–500.
Kang G, Chen K, Yang F, Chuai S, Zhao H, Zhang K, Li B, Zhang Z, Wang J. Monitoring of circulating tumor DNA and its aberrant methylation in the surveillance of surgical lung Cancer patients: protocol for a prospective observational study. BMC Cancer. 2019;19(1):579.
Phase III study to determine the efficacy of durvalumab in combination with chemotherapy in completely resected stage II-III non-small cell lung cancer (NSCLC) (the MERMAID-1 Trial). https://www.clinicaltrials.gov/ct2/show/NCT04385368. Accessed on 17 May 2022.
Phase III study to determine efficacy of durvalumab in stage II-III non-small cell lung cancer (NSCLC) after curative intent therapy (the MERMAID-2 Trial). https://www.clinicaltrials.gov/ct2/show/NCT04642469. Accessed on 17 May 2022.
Adjuvant treatment with cisplatin-based chemotherapy plus concomitant atezolizumab in patients with stage i (tumors ≥ 4cm), IIA, IIB, and select IIIA [T3N1-2, T4N0-2] resected non-small cell lung cancer (NSCLC) and the clearance of circulating tumor DNA (ctDNA). https://www.clinicaltrials.gov/ct2/show/NCT04367311. Accessed on 17 May 2022.
Adjuvant durvalumab for early stage NSCLC patients with ctDNA minimal residual disease. https://www.clinicaltrials.gov/ct2/show/NCT04585477. Accessed on 17 May 2022.
Personalized escalation of consolidation treatment following chemoradiotherapy and immunotherapy in stage III NSCLC in stage III NSCLC. https://www.clinicaltrials.gov/ct2/show/NCT04585490. Accessed on 17 May 2022.
Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, Marrone K, Sivakumar IKA, Bruhm DC, Rosner S, et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res. 2019;79(6):1214–25.
Acknowledgements
The authors sincerely thank all the authors of the original articles.
Funding
This study was supported by Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, Chinese Academy of Medical Sciences (2021RU002), National Natural Science Foundation of China (No.92059203, No.82072566 and No.81602001), and Peking University People's Hospital Research and Development Funds (RS2019-01 and RZ2022-03). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
KC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: KC, HS, YJ, FY, JW. Acquisition, analysis, or interpretation of data: KC, HS, YJ, HZ, ZW1 (Zihan Wei), XW, ZW2 (Ziyang Wang). Drafting of the manuscript: KC, HS, YJ. Critical revision of the manuscript for important intellectual content: KC, YL, FY, JW. Statistical analysis: HZ, MW, KZ, ZW1, XW, ZW2. Obtained funding: KC, JW. Administrative, technical, or material support: KC, YL, FY, JW. Supervision: KC, YL, FY, JW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA 2020 checklist.
Additional file 2: Table S1.
Example of search strategy as used for the PubMed database. Table S2. Description of included studies. Table S3. Risk of bias assessment of included studies using QUADAS-2 tool. Table S4. Assessment of bias by Newcastle-Ottawa scale. Table S5. Diagnostic performance of different biomarkers in early-stage NSCLC. Table S6. Analysis of concentration of cell-free DNA. Table S7. Concordance between ctDNA and pathological response to neoadjuvant therapy.
Additional file 3: Figure S1.
PRISMA flow diagram. Figure S2. Deeks’ funnel plot in diagnostic analysis. Figure S3. Funnel plot in prognostic analysis of RFS at (A) preoperative and (B) postoperative time point; and OS at (C) preoperative and (D) postoperative time point.
Additional file 4: Table S1.
Summary of concentration of cfDNA in eligible cohorts. Table S2. Predictive value analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shen, H., Jin, Y., Zhao, H. et al. Potential clinical utility of liquid biopsy in early-stage non-small cell lung cancer. BMC Med 20, 480 (2022). https://doi.org/10.1186/s12916-022-02681-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02681-x