Abstract
Background
Mutualistic interactions, which constitute some of the most advantageous interactions among fish species, are highly vulnerable to environmental changes. A key mutualistic interaction is the cleaning service rendered by the cleaner wrasse, Labroides dimidiatus, which involves intricate processes of social behaviour to remove ectoparasites from client fish and can be altered in near-future environmental conditions. Here, we evaluated the neuromolecular mechanisms behind the behavioural disruption of cleaning interactions in response to future environments. We subjected cleaner wrasses and surgeonfish (Acanthurus leucosternon, serving as clients) to elevated temperature (warming, 32 °C), increased levels of CO2 (high CO2, 1000 ppm), and a combined condition of elevated CO2 and temperature (warming and high CO2, 32 °C, and 1000 ppm) for 28 days.
Results
Each of these conditions resulted in behavioural disruptions concerning the motivation to interact and the quality of interaction (high CO2 − 80.7%, warming − 92.6%, warming and high CO2 − 79.5%, p < 0.001). Using transcriptomics of the fore-, mid-, and hindbrain, we discovered that most transcriptional reprogramming in both species under warming conditions occurred primarily in the hind- and forebrain. The associated functions under warming were linked to stress, heat shock proteins, hypoxia, and behaviour. In contrast, elevated CO2 exposure affected a range of functions associated with GABA, behaviour, visual perception, thyroid hormones and circadian rhythm. Interestingly, in the combined warming and high CO2 condition, we did not observe any expression changes of behaviour. However, we did find signs of endoplasmic reticulum stress and apoptosis, suggesting not only an additive effect of the environmental conditions but also a trade-off between physiological performance and behaviour in the cleaner wrasse.
Conclusions
We show that impending environmental shifts can affect the behaviour and molecular processes that sustain mutualistic interactions between L. dimidiatus and its clients, which could have a cascading effect on their adaptation potential and possibly cause large-scale impacts on coral reef ecosystems.
Similar content being viewed by others
Background
Global change is one of the main drivers of marine biodiversity decline [1]. Rapid modifications of temperature and pH in the oceans are accelerating demographic changes in marine species, substantially modifying ecosystem diversity, and affecting inter-specific interactions [2, 3]. In particular, key ecological interactions such as competition, reproduction and mutualism are modified by environmental conditions that consequently generate new social contexts among species [1]. Therefore, there is a strong link between the physical environment and the outcome of ecological processes mediated by animal behaviour. Also, the effect of environmental changes on marine organisms has potentially profound outcomes at higher levels of biological organization [1].
Changes in the marine environment such as elevated levels of CO2 (ocean acidification) and elevated temperature (ocean warming) have a variety of effects on marine organisms, especially in vertebrates such as fish [4,5,6,7]. CO2 changes in the water lead to acid-base regulation in animal tissues influencing ecological traits such as reproduction, larval growth and biological senses that can have consequences on fish boldness, learning, recognition and predator avoidance, among others [1]. For instance, levels of monoamines, such as dopamine and serotonin, can be chemically altered through ion exchangers, and have been shown to contribute to fish aggression and decision-making [8, 9]. However, there are inter-specific and intra-specific variations in the sensitivity to ocean acidification which may allow for adaptive physiological strategies in response to an acidified ocean [10]. At present, behavioural impairment through elevated CO2 has mostly been attributed to the alteration in gamma-aminobutyric acid (GABA) neurotransmission in the brain [11, 12]. However, additional mechanisms such as the alteration of Ca2+/calmodulin protein kinase II (CaMKII) and AMPA glutamate receptors affecting olfactory abilities and neuronal excitability have been reported [13, 14]. Changes in sensory perception, such as reductions of olfactory sensitivity and modifications of the olfactory epithelium to odorants for food detection, have further been attributed to medium-term exposure to ocean acidification [15,16,17].
Further effects of global change, such as elevated temperature, generate changes in the metabolic rates of fish, reducing their aerobic performance and collapsing aerobic capabilities that are required for basic functions such as obtaining food or maintaining symbiosis with other species [4, 18]. Since there is a physiological need to cope with warming conditions to avoid heat shocks, proteins and transcription factors can be activated to protect and maintain cellular functions [19]. In fact, the expression of cellular stress responses with genes associated with antioxidant defense, apoptosis and protein folding are often exhibited with an elevated temperature [20], and the effect of this elevated temperature response is a reduction in fish performance across immune responses, respiration and foraging [21,22,23,24,25]. Furthermore, high temperatures also interact with elevated CO2 and increase, reduce or change the effects on metabolic demands, growth, development, behaviour and survival of marine fish species [25,26,27,28]. For example, with the combination of environmental changes, antipredator behaviour is reduced in damselfishes [29]. On the other hand, anemonefish food consumption increases under exposure to both temperature and CO2, whereas no effects are seen with CO2 exposure alone [30].
Cleaning mutualisms, one of the most beneficial interactions between fish species, is highly susceptible to environmental changes [9, 31,32,33,34]. In particular, cleaning mutualisms are a crucial interaction on coral reef ecosystems consisting of the removal of ectoparasites and dead tissue from the skin of other fishes (known as ‘clients’), enhancing their health and survival [35, 36]. For instance, the bluestreak cleaner wrasse Labroides dimidiatus is known for its cleaning ability that leads to the enhancement of reef fish well-being and diversity [37]. This species also possesses remarkable cognitive abilities of learning and memory [38] which allows the establishment of long-term mutualistic relationships with clients that visit cleaning stations to obtain stress relief [39] and boost their health [40], while the cleaner wrasse obtains food in return. Therefore, changes to the interaction behaviour of L. dimidiatus due to environmental conditions could have consequences for marine fish communities [35, 37]. Disruptions such as habitat degradation and temperature changes disturb mutualistic interactions leading to shifts from mutualism to antagonism, loss of interaction, and unexpected switches to new participants or partners [41]. For instance, ocean acidification and warming impair the motivation to interact of cleaner wrasses, indirectly leading to cascading effects in fish communities and the abundance of clients [9, 42]. In addition, the quality of these interactions can be affected by the interruption of cognitive processes involved in recognition of individual clients, which is essential in establishing cleaning relationships [43]. Consequently, this may affect the functioning of the mesolimbic reward system [44] and lead to mutualism breakdown which indirectly impact coral reef ecosystems by decreasing reef fish diversity [42].
Previously, Paula et al. [31] found that when cleaner wrasses were exposed to warmer temperatures and high CO2 levels for 45 days, they interacted less frequently with surgeonfish clients. Furthermore, the cleaners used more reconciliation strategies, such as providing tactile stimulation, without increasing any dishonest behaviour (i.e. ‘cheating’, behaviour known as cleaner’s preference to eat mucus instead of cleaning ectoparasites; [45]. This suggested that the cleaners were no longer able to anticipate the costs of interacting with the clients. The behavioural disruptions in cleaning interactions are known to be correlated with changes in the levels of dopamine and serotonin in multiple brain regions [31] and to be reverted by the administration of GABAergic antagonists [32]. However, to clearly understand the mechanisms behind these disruptions, a molecular approach is needed.
To systematically study these underlying mechanistic changes in interacting cleaner wrasse L. dimidiatus and its client (Acanthurus leucosternon), we observed the interaction behaviour for fish exposed to (i) present-day environmental ‘control’ conditions (29 °C, pCO2 ~ 400 μatm), (ii) ‘warming’ (32 °C, pCO2 ~ 400 μatm), (iii) ocean acidification ‘high CO2’ (29 °C, pCO2 ~ 1000 μatm) or (iv) elevated ‘warming and high CO2’ (32 °C, pCO2 ~ 1000 μatm) following IPCC’s RCP scenario 8.5 (Fig. 1, Additional file 1: Table S1). We evaluated the fine-scale transcriptional responses across the three main regions of the brain (fore-, mid- and hindbrain) known to harbour the expression of significant neurotransmission, neurohormones and neuropeptides during cleaning interactions [31, 46]. Since cleaning interactions involve the expression of the dopaminergic and glutamatergic pathways [47], we may expect brain molecular drivers in the response to environmental change in the cleaner wrasse and its client to include cellular stress response, changes to GABAergic neurotransmission and in gene expression levels of neuroamines. Due to the importance of this inter-specific and mutualistic interaction, it is necessary to unravel the underlying mechanisms that drive such interactions. Moreover, it is also essential to understand the mechanisms that cause a disruption to these behaviours, which potentially result in mutualism breakdown and wide-ranging effects on the coral reef ecosystem.
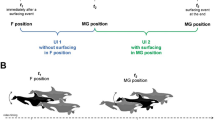
Experimental design where Labroides dimidiatus (N = 24) and Acanthurus leucosternon (N = 24) were allowed to interact after an environmental acclimation period (28 days) in one of the following conditions: Control, warming, high CO2, or a combined condition of warming and high CO2 (aquarium setup parameters and behavioural data can be found on Additional file 1: Table S2-S3a, b)
Results
Behavioural responses
After 28 days of acclimation to one of the different treatment scenarios, we note that the proportion of time spent interacting was significantly affected by the interaction of CO2 and temperature (p = 0.002; Additional file 1: Table S3b). Post hoc comparisons further revealed that time spent in cleaning interactions decreased significantly with high CO2 (− 66.5%, p = 0.01) and warming (− 75.5%, p = 0.01), but not for warming and high CO2 (p = 0.112; Fig. 2a, Additional file 1: Table S3c). Considering cleaners’ motivation to interact, the proportion of interactions started by cleaners was also significantly affected by the interaction of CO2 and temperature (p < 0.001; Additional file 1: Table S3b). Post hoc comparisons indicated a significant decrease under all treatments compared to the control (high CO2 − 80.7%, p < 0.001; warming − 92.6%, p < 0.001; warming and high CO2 − 79.5%, p < 0.001; Fig. 2b, Additional file 1: Table S3c). Contrarily, clients’ motivation (client posing displays ratio, Fig. 2c) was significantly altered by temperature (p < 0.001) but not CO2 (p = 0.771) nor the interaction of CO2 and temperature (p = 0.187, Additional file 1: Table S3b). Post hoc comparisons (Additional file 1: Table S3c) revealed that treatments with high temperature had significantly higher client posing displays ratio than under control temperatures (warming + 246.2%, p = 0.002; warming and high CO2 + 198.7% p = 0.006), but not under high CO2 only (p = 0.268).
Behavioural responses from interaction trials between the cleaner L. dimidiatus and client A. leucosternon. a proportion of time interacting (in seconds), b proportion of interactions started by cleaners, c client posing display ratio, d proportion of time spent in tactile stimulation (in seconds), e number of client jolts per 100 s interaction, f mean interaction duration (in seconds). (*) define significance based on post hoc tests (p < 0.05). Additional details of associated tests are found in Additional file 1: Table S3b-c
When considering the quality of the cleaning interactions, namely the proportion of interaction time spent in tactile stimulation, there was no significant change attributable to temperature, CO2, or the interaction between CO2 and temperature (Fig. 2d, Table S3c). Additionally, while the interaction between CO2 and temperature significantly influenced the ratio of client jolts per 100 s of interaction, subsequent post hoc analysis revealed that none of the treatment conditions showed a significant deviation from the control (Fig. 2e, Table S3c). On the contrary, the mean interaction duration experienced a significant reduction in response to the combined effects of CO2 and temperature (Fig. 2f, p = 0.016). Further post hoc comparisons revealed a marked decrease in interaction duration under all experimental conditions compared to the control group (Additional file 1: Table S3c). Specifically, the high CO2 condition led to a decrease of 57.7% (p = 0.007), the warming condition to a reduction of 72.6% (p = 0.002), and the combination of both warming and high CO2 resulted in a 66.1% decrease (p = 0.002).
Transcriptional response
Whole transcriptional response (number of differentially expressed genes—DEGs) following the interaction trial revealed a similar pattern in both species across treatments: warming > warming and high CO2 > high CO2. Warming showed the largest molecular response for L. dimidiatus (5,804 DEGs) and A. leucosternon (3493 DEGs) (Fig. 3), followed by warming and high CO2 with 4581 DEGs for L. dimidiatus and 505 DEGs for A. leucosternon. Finally, high CO2 alone showed the smallest numbers of DEGs for both species revealing 2508 for L. dimidiatus and 376 for A. leucosternon (Fig. 3). For L. dimidiatus, the 672 DEGs common across all environmental conditions (regardless of brain region) are involved in protein folding processes, positive regulation of endothelial cell proliferation, chemosensory behaviour, and regulation of bone mineralization, among others (Additional file 1: Table S4, Fig. 3a). As for A. leucosternon, the 30 common DEGs were related with biological regulation, regulation of cellular process, and regulation of apoptotic signalling pathway (Additional file 1: Table S5, Fig. 3b).
Number of differentially expressed genes that are unique to each condition and common between elevated temperature, high CO2 and elevated warming and high CO2 conditions of the whole brain for a the cleaner fish Labroides dimidiatus and b the powder-blue surgeonfish Acanthurus leucosternon. Numbers in brackets are proportional to the size of the circle and represent the total differential expressed genes found under each environmental condition
Even though the magnitude of transcriptional reprogramming in the whole brain associated with the conditions was similar for both studied species, differences in gene expression patterns were exhibited across brain regions (Fig. 4, Additional file 2: Figures S1-S3). In particular, for L. dimidiatus, the hindbrain region (HB) displayed 1198 unique DEGs for high CO2, while in A. leucosternon more DEGs were found in the forebrain (204, FB) under the same conditions. Furthermore, the FB region in L. dimidiatus presented the largest molecular reaction under the warming condition (1632), while in A. leucosternon was in the HB (2732). Finally, under warming and high CO2, most differential expression in L. dimidiatus was observed in FB region (471), while in A. leucosternon was in HB (82) (Fig. 4a,b, Additional file 2: Figures S1-S6).
Number of unique differentially expressed genes (DEGs) for each brain region under each environmental condition compared to the control condition for a L. dimidiatus and b A. leucosternon. Further overlapping DEGs between brain regions can be found in Additional file 2: Figures S1-S6
Molecular responses to elevated temperature
Labroides dimidiatus
Exposure to a higher temperature led to unique functional changes (only seen for this treatment). These changes were related to immune response, execution phase of apoptosis, response to hypoxia, and stress-activated MAPK cascade (Additional file 1: Table S6a). Furthermore, elevated temperature elicited specific changes in FB (1450 DEGs) and MB (309 DEGs) (Fig. 4a, Additional file 1: Table S6b-c), characterized by functional enrichments of cellular stress responses and the expression of actin filaments genes, DNA damage, glucose, and glycine receptors, response to environmental stress, cell proliferation and heat shock proteins (Additional file 1: Table S6a-c). In particular, genes underlying functions of stress and hypoxia were evidenced by the upregulation of HSF2 (Heat shock factor protein 2), a specific promoter under conditions of heat stress, processes of insulin metabolism given by IGF1R (insulin growth factor 1), AKT3 (RAC-gamma serine/threonine-protein kinase) and INSR (insulin receptor) and transcriptional co-suppressor functions of hypoxia through HIPK2 (Homeodomain-Interacting Protein Kinase 2). Regarding the HB specifically, we found opioid receptors differentially expressed such as OPRK (Opioid Receptor Kappa 1). Simultaneously with these stress signatures, histones and epigenetic regulation were also differentially expressed under warming. Peptide hormone secretion and thyroid hormone receptor binding were upregulated mainly in the FB, underlined by upregulation of hormone receptors to behavioural responses to stress (Corticotropin Releasing Hormone Receptor 2) and adenylate cyclase (cAMP) inhibition (Cannabinoid Receptor 1, also Differentially Expressed in MB). Furthermore, neurotransmission was differentially regulated with genes related to processes of calcium (CAC1D, KCC2B, SORCN), glutamate (DHE3), GABA (GABR1) and potassium (KCNB1) transport. Finally, molecular signatures of olfactory behaviour, adult locomotory behaviour and social behaviour were evident almost exclusively in FB (Additional file 1: Table S6a-c).
Acanthurus leucosternon
For the client species, stress responses, metabolic functions, and synapse activity were also elicited with elevated temperature (3493 DEGs; Additional file 1: Table S7, Fig. 3b). Although, in contrast to the cleaner wrasse (Fig. 4a), differential gene expression in HB was the largest among the brain regions for A. leucosternon (2,732, Fig. 4b), and similar functional processes were shared with FB. For instance, responses to stimulus, epigenetic regulation, synapse activity, behaviour, and learning were differentially expressed for the client species under warming. On the other hand, some DEGs were common with the cleaner wrasse, such as adult locomotory behaviour, locomotory exploration behaviour and social behaviour. However, their differential expression was significant almost exclusively in the HB (Additional file 1: Table S7). Additionally, the FB and MB revealed changes in molecular signatures involved in synaptic transmission (glutamate and GABA), protein binding, and cellular responses to stimuli (Additional file 1: Table S7) and highlighted a significant alteration of metabolic process and glutamatergic synapses under elevated temperature. Some enriched hormone responses were found related to thyroid hormone receptor binding, regulation of growth hormone secretion, and steroid hormone binding, underlined by upregulation of Thyroid hormone receptor alpha (THRA), Thyroxine 5-deiodinase 3 (IOD3) and several Chromodomain-helicase-DNA-binding proteins (CHD6, 7, 8, 9), found almost exclusively in the HB (Additional file 1: Table S7).
Molecular responses to high CO2 exposure
Labroides dimidiatus
Under high CO2, the differentially expressed molecular signatures in L. dimidiatus also showed to initiate cellular stress responses. The molecular signatures displayed differed from the other treatments, with the HB presenting the largest number of DEGs compared to the other brain regions under high CO2 (HB: 1,198 > MB: 60 > FB: 32, Additional file 2: Figure S2). The functional changes were related to stress, such as positive regulation of stress-activated MAPK cascade and response to osmotic stress in the HB (Additional file 1: Table S8). Molecular responses in apoptosis, osmotic and oxidative stress were also found in high CO2. However, the DEGs involved differed from those in warming (Additional file 1: Table S8, Fig. 5). Unique functional responses to high CO2 were related to synaptic neurotransmission, glutamate and GABA such as positive regulation of synaptic transmission, glutamatergic and gamma-aminobutyric acid secretion. These processes were underlined by several upregulated ionotropic (NMDE1, NMDZ1) and downregulated metabotropic glutamate receptors (GRM8, 2), Gamma-aminobutyric acid associated-genes (GBRAP, GABT) and Sodium-chloride transporters (SC6A1, 13). Further processes related to circadian rhythm and visual perception were found (e.g. camera-type eye photoreceptor cell differentiation, eye photoreceptor cell fate commitment and blue light photoreceptor activity). These processes were differentially expressed mainly in the HB, with a downregulation of phosphodiesterases (PLCB1) and phosphatases (PHR4B) as well as an upregulation of cristallins (CRYAB), cryptochromes (CRY1, 2), sidekick (SDK2) and calcium proteins (CABP1). Furthermore, hormone responses (e.g. dopamine, gonadotropin, oestrogen, thyroid and corticotropin; Additional file 1: Table S8) were also found with functional enrichments in the HB and upregulated DEGs of corticotropin receptor and releasing factors (CRF, CRHBP), dopamine genes (DRD5, TY3H), thyroid hormone receptor 3 (TR150), Thyroxine 5-deiodinase 3 (IOD3) and melatonin receptors (MR1BB). Finally, epigenetic regulations were found, such as histone regulation, acetylation and methylation processes (Additional file 1: Table S8), as well as a variety of enriched behaviours, learning and memory functions (e.g. adult locomotory behaviour, social behaviour, olfactory and vocal learning, short-, medium- and long-term memory). These functions were underlined by upregulated transcription in the HB of glutamate receptors (GRIA2, 3, 4, NMDZ1), dopamine genes (TY3H, DRD5), Isotocin receptor (ITR) and Early growth response protein 1 (EGR1, Additional file 1: Table S8).
Significantly enriched functions in the (whole) brains of L. dimidiatus and A. leucosternon with different near-future environmental treatments (warming, high CO2, and warming and high CO2). The size of the circles is proportional to the number of differentially expressed genes, and the colour of the circles represents the false discovery rate adjusted for enrichment significance level (FDR < 0.05)
Acanthurus leucosternon
High CO2 generated the most variable effect in the molecular reaction of the exposed client individuals compared to the other conditions, revealing differential responses among the biological replicates (Additional file 2: Figure S7). Various genes associated with organ development, stress, diencephalon morphogenesis, behaviour and learning, such as locomotory behaviour and associative learning, were altered in expression, mainly in FB, which presented the largest molecular response (Additional file 1: Table S9, Fig. 4b). In addition, MB and HB had fewer unique DEGs than FB (204, Fig. 4b), and their molecular functions were involved in synapses activation and signalling, peroxidase activity, oxygen carrier activity, gamma-aminobutyric acid signalling pathway, homeostasis and immune responses (Additional file 1: Table S9). Unique functional responses in high CO2 for the client included functions of signalling involved in the determination of organs and tissues, as well as responses to stress. Interestingly, hormone activity was the only enriched function found in this species that had the upregulation exclusively in FB of Isotocin (NEUI), Gonadotropin (GTHB1), Pro-thyrotropin (TRH), Thyrotropin (TSHB) and Somatostatin (SMS1B) hormones. In addition, behavioural processes are altered, including adult locomotory and social behaviour, underlined by upregulated genes of Glutamate decarboxylase (DCE1) and downregulated glutamate receptor GRM5, Isotocin (NEUI) and dopamine-related gene Tyrosine 3-monooxygenase (TY3H), almost exclusively in FB, except for GRM5. Finally, learning processes were altered, but no processes of memory, epigenetic regulation or circadian rhythm were found for this species.
Molecular response to the combined treatment: warming and high CO2
Labroides dimidiatus
The combined treatment of warming and high CO2 triggered the differential expression of 4581 DEGs (Fig. 3a, Additional file 1: Table S10a), from which 60% (2735 DEGs) were shared with warming treatment. These DEGs were mostly related to associative learning, oxidative stress, cell death, insulin activity and immune response. DEGs shared with high CO2 corresponded to only 4.6% (211 DEGs) and were involved in osmotic stress, long-term synaptic potentiation and nervous projection development (Fig. 3a). In warming and high CO2, a total of 963 genes were found to be uniquely differentially expressed (21%, Fig. 3a), mainly in the FB (471 DEGs) and MB (442 DEGs), and only 146 DEGs in HB (Additional file 2: Figure S2). In this unique response, elevated stress response signals are revealed by neuron apoptotic process, response to endoplasmic reticulum stress, and regulation of neuron death (Additional file 1: Table S10b, Fig. 5). According to the brain regions, FB revealed cellular stress response but also neuron development (Additional file 1: Table S10b), while genes involved with the regulation of cell population proliferation and regulation of cell death were differentially expressed in MB (Additional file 1: Table S10b). Concerning HB, genes related to the glutamatergic synapse pathway, calcium/calmodulin-dependent serine protein kinase were differentially expressed (Additional file 1: Table S10). Furthermore, warming and high CO2 unique responses were related to organ development and morphogenesis and the genes were mostly transcribed in FB and MB, underlined by upregulation of DNA-binding transcription of helix-loop-helix processes (ID3A), cysteine glutamate transporters (XCT) and receptors (GRID1, NMDE4, GRM3, 8). Furthermore, there was an upregulation of organ and neuromuscular tissue development (BMR1B, HDAC8, FGF12, TSN2, FZD2-6, AGRIN), synaptic integrity and signalling (GRB2A, PTPRF, CSKP, CBLN1) and histone-related genes (KDM1B, HDAC8). In addition, steroid and phosphatidic acid processes with upregulations of glutamate transporter XCT and downregulation of glutamate receptor GRM3 and insulin (INSI1) were observed, but also by steroid hormone receptor 2 (ERR2), endoplasmic reticulum processes (GORS1, MA1B1), cellular death and repair (DAPK2, RD23A), with downregulated phosphodiesterase activity (PDE3A, PDE7A), calcium/calmodulin genes (CAC1I, PDE1C, PLPL9, RAMP1), adenylate cyclase activity (ADCY2,7,9), lipids and fatty acid processes (ACBG2, DGKD, DGKZ). Furthermore, melanin hormone activity (MCH, MCH2) was the only hormone-related process detected in this condition and exclusively in HB. Finally, associative learning was the only cognitive process found in the combined condition, underlined by immediate early genes (FOS, FOSL2), synapse-regulation genes (NLGNX, NPTX1), long-term potentiation plasticity (NPTX2), osmoregulation and corticotropin releasing factor regulation (UTS1), but dopamine nor serotonin were not differentially expressed in this condition. Unlike warming and high CO2 in isolation, no changes in behaviour or memory-related mechanisms were exhibited in this combined condition.
Acanthurus leucosternon
Client individuals exposed to warming and high CO2 displayed a relatively small molecular response compared to the cleaner wrasse (Figs. 3b and 5). The molecular reprogramming was though mostly shared (72%) with warming. This combined condition elicited unique differential expression of microtubule nucleation, binding, bundle activity and organ and tissue development. Moreover, locomotory behaviour and medium-term memory were found in the client in contrast with the cleaner wrasse, while associative learning was observed in both species. There were several responses to stress, including positive regulation of response to endoplasmic reticulum stress and apoptosis, but no cellular death functions were exhibited. In particular, molecular mechanisms of cell growth, glutamatergic synapses, immune response, immediate early genes, apoptosis and DNA damage were part of the transcriptional processes altered under this condition in the FB and HB (Additional file 1: Table S11). Even though no enriched functions were found significant in MB, the differential gene expression found was related to DNA binding, transcription factor and GABA (Additional file 1: Table S11).
Discussion
Future environmental conditions of high CO2 and warming elicit molecular reprogramming in the brains of the interacting cleaner wrasse L. dimidiatus and its client A. leucosternon. Here, the cleaner wrasse L. dimidiatus, in particular, exhibited large transcriptional alterations and a lower interaction motivation and quality. A disruption to the mutualistic cleaning behaviour in future environmental conditions has also been previously observed [31]. Moreover, the loss of cleaners’ cognitive and strategic sophistication has been associated with increasing CO2 levels [9] and back-to-back extreme environmental disturbances (cyclones, heatwaves, and bleaching; [34]. This combined evidence shows that future environmental conditions will likely disturb this important mutualistic cleaning behaviour. One of the reasons for stressor-led behavioural change may be the large neuromolecular alterations displayed, especially for L. dimidiatus, when exposed to future conditions. This hints at an elevated impact of environmental changes on species with elevated cognitive abilities and suggests a need for more adjustments in physiological functions in such species to ensure their survival. Here we shed light on the expression patterns underlying the cleaning interaction in predicted near-future environmental conditions and reveal transcriptional and functional mechanisms underlying the susceptibility of this vital cleaning mutualism.
Hormonal responses are important in the interaction behaviour between the client and the cleaner [48], in particular the client differentially expresses genes involved in the thyroid hormonal pathway when interacting [47]. However, when exposed to warming or high CO2, this hormonal response is altered for both L. dimidiatus and A. leucosternon. The thyroid hormone metabolism and associated hypothalamic–pituitary–thyroid axis (HPT-axis) plays an endocrine role during stress in teleosts [49] by maintaining homeostasis and neuronal activities. In other fish species exposed to high temperatures and acidic conditions, changes to HPT-axis related triiodothyronine (T3) and thyroxine (T4) have been observed [50,51,52,53]. While the cleaner wrasse and client did not change expression of T3 and T4 directly, alterations were seen in other parts of the pathway, such as Thyrotropin subunit beta (TSHB, TR150) and Thyroxine 5-deiodinase (IOD3) known for regulating synthesis [54] and inactivation of T3 and T4 [55]. Additional endocrine adjustments to Gonadotropin (GnRH), which are inhibited by the thyroid hormones [56], highlight the involvement of thyroid hormones in the response to warming and high CO2 in cleaning interactions. As the thyroid hormones are known to control several physiological functions in fish, including stress resilience and adaptation [49, 57], future climate change environmental conditions reveal to be stressful for this essential social interaction between a cleaner wrasse and client fish.
Warming revealed the largest transcription of cellular stress responses for both species involved in the mutualistic interaction. Cellular stress activity is commonly transcribed with repair mechanisms (e.g. immune response, ATP-related processes; [58]. DNA damage and apoptosis initiation genes were expressed at elevated levels along with heat shock proteins, which are important in maintaining cellular activity and preventing DNA degradation [59]. Such processes are costly, and alterations in metabolic actions are expected [60]. For instance, significant reductions of brain monoamine metabolites of dopamine and serotonin (e.g. DOPAC and 5-HIAA) have been previously found underlying L. dimidiatus motivation and quality to interact [31]. Expression changes in glycine and glutamate during warming emphasize osmotic and energetic disruptions that may limit the transportation of glucose and oxygen from the blood to the brain [61]. Genes related to hypoxia-inducible factors and further osmolytes, such as lactate, were highly upregulated uniquely under warming in our cleaner wrasse, suggesting high-stress levels and an alteration of osmolytes and metabolites. This may have the potential to compromise behavioural performance, as seen in reduced activity levels, cognitive performance, muscular activity and lateralization behaviour in other fishes with elevated temperature [26, 61,62,63,64]. The behavioural changes in the cleaning interaction during warming, however, may not be a direct cause of elevated temperature interfering with the capacity to interact in the brain, but more indirect by needing to shift focus on increased metabolism related to cellular stress responses and oxygen demands in the brain [65, 66].
High CO2 altered responses of stimulus reception, ion transport, circadian rhythm, visual perception, dopamine and neurotransmitters (glutamate and GABA) in the brain of L. dimidiatus, indicating an effect of acidification on neurotransmission. While GABA receptors were differentially expressed in all three conditions, only high CO2 showed an effect on most of the pathway, including the upregulation of genes associated with GABA signalling, sodium and chloride-dependent transporter and GABA aminotransferase (GABT). The upregulation of GABT facilitates the degradation of GABA into succinic semialdehyde, which is in charge of regulating the supply of GABA in the brain (‘GABA shunt’; [67]). As a result, succinic semialdehyde is incorporated into the Krebs cycle from which glutamine is formed and subsequently converted into glutamate [68], a major excitatory neurotransmitter already known to be regulating the interaction behaviour in the cleaner wrasse [47]. Our results thus suggest an interplay between the expression of GABAergic and glutamatergic pathways in the cleaner wrasse when faced with ocean acidification. GABA ion channel interference in high CO2 has been attributed to neurotransmission dysfunction [12, 69] in the brain of teleost fish. In fact, administration of a GABAA receptor antagonist (gabazine) led to the recovery of most high CO2-induced cleaning behaviour alterations [32]. Moreover, under control conditions, the administration of a GABAA receptor agonist (muscimol), produced similar cleaning motivational disruptions as high CO2, suggesting a crucial role of the GABA ion channel interference as a mechanism for cleaning behaviour disruption under high CO2. A variety of alterations in lateralization behaviour [70], predator recognition [71] and learning performance [71] are also known to be related to GABAergic neuromodulation. Another important neurotransmitter in the brain, dopamine, is also connected to the interaction behaviour in L. dimidiatus and plays a key role already in normal environmental conditions [47]. Alterations (significant reductions) of dopamine activity have been observed under high CO2 and connected to a reduction in motivation to interact as well as the reward and risk perception system [31, 72,73,74,75]. The gene expression changes of dopamine receptors under high CO2 suggest transcriptional modifications that are being altered in the dopaminergic pathway, which is vital for L. dimidiatus success in consolidating cleaning behaviour. Thus, changes in glutamatergic/GABAergic and dopaminergic neurotransmission and ion transport under acidification could explain the disruption of the mutualistic interaction with ocean acidification.
Alterations in GABA neurotransmission and ion transport have also been associated with visual functions [70, 76]. Changes in ion gradients over neuronal membranes through GABA receptor activity under high CO2 were linked with reduced retinal reaction time (and reduced speed in light response) in another coral reef fish (Acanthochromis polyacanthus). L. dimidiatus upregulated genes involved in visual perception, including key photoreceptors, retinal development genes and GABA receptors, under high CO2. These changes may suggest an alteration in retinal reaction timedue to a disruption of retinal action potentials (e.g. V-ATPase pump; [77, 78]), as fish vision is regulated by changes in the intra- and extra-cellular pH of the retina modulated by the vacuolar adenosine triphosphatase and the expression of photoreceptors (V-ATPase; [78]). Consequently, we hypothesize that this transcriptional modification may alter stimuli reception in ganglion cells and the retina reducing its normal reaction time [76]. This response may also be related to changes in the circadian rhythm found altered for L. dimidiatus. High CO2 commonly alters the transcriptional regulation of the circadian pathway in fish brains across many species, including coral reef fishes [10, 79,80,81]. Although further studies are needed to corroborate the link and its mechanism under ocean acidification, pH changes have the potential to alter the visual perception of cleaners, including the speed of processing images, which is a crucial ability to recognize clients as well as consolidate long-standing cleaning relationships [43].
While it is important to understand the mechanisms underlying the responses to near-future conditions in isolation, revealing antagonism and synergism of multiple drivers is crucial for identifying molecular processes in more realistic future environmental conditions [1, 11]. We found that much of the transcriptional changes were shared with warming, including metabolic genes and immune response, whereas ion regulation, GABA/glutamatergic activity and synaptic transmission were processes shared with high CO2. Despite sharing a transcriptional response with the single treatments, the combination of the future conditions revealed enhanced stress signatures underlined by endoplasmic reticulum (ER) stress response in the cleaner wrasse, suggesting the transcription of protein folding regulation and homeostasis. This type of stress can alter synaptic function and memory storage due to exposure to harmful stimuli, such as hypoxia and oxidative stress caused by environmental stress [82]. Cellular stress responses have been observed in other fish, suggesting increasing mortality and failure of acclimation through phenotypic plasticity [83, 84]. Thus, the elevated stress signatures observed in the cleaner wrasse show increased demands of protein folding and homeostasis repair, signalling, synaptic function and regulation of organ development imposed by both combined environmental changes. As these modifications are not detected in conditions in isolation, it seems this may pose an additive effect in response to future environmental conditions and has the potential to disrupt important biological functions.
Interestingly, while signs of stress increased in the combined condition, there were no expression changes in behaviour-related genes. The proportion of time interacting did not significantly decrease during warming and high CO2 compared to control. This observation suggests that fish may prioritize maintaining biological performance (e.g. protein folding and repair) while making fewer adjustments to the transcriptional response underlying the interaction behaviour. Trade-offs between biological performance and behaviour have been documented in other fish species revealing limits in the adaptive potential to climate change [27, 31, 85, 86]. Combined environmental stressors have been shown to modify correlations between physiological function and behavioural traits through differential phenotype sensitivities and environmental contexts [87]. In the cleaner wrasse, molecular mechanisms underlined by increased ER stress, organ development and morphogenesis compared to reduced glutamatergic neurotransmission and an absence of changes in behavioural genes and dopamine suggest a trade-off between these two processes (i.e. stress and behaviour). As such, the trade-offs between physiological performance related to stress and behaviour in the context of mutualistic interactions could compromise the establishment of future social interactions in L. dimidiatus, and its ability to adapt to climate change.
Conclusions
In conclusion, the molecular signatures underlying the mutualistic interaction of L. dimidiatus and a client species under predicted future climate change conditions provoked an array of effects that compromised cleaning interactions, ranging from cellular stress responses, alteration of neurotransmission and high metabolic demands. In both warming and high CO2, alterations in thyroid hormone-related genes were notable with potential endocrine disruptions revealing a key stress response to the environmental condition in these interacting fishes. During warming, the upregulation of hypoxia, osmolyte, and metabolite alterations suggested homeostatic disruption and aerobic constraints due to high temperatures. Furthermore, exposure to high CO2 altered gene expression in ion transport and neurotransmission driven by GABAergic and glutamatergic neurotransmission. This suggests that an interplay between these two processes also affects motivation to interact in L. dimidiatus. In addition, under high CO2, visual perception genes were altered in expression, potentially disrupting social relationships as cleaner wrasses strongly rely on client recognition. Finally, the more realistic future combined condition of elevated warming and high CO2 created ER and apoptotic stress on the one hand but also the absence of molecular signatures related to behaviour and less behavioural impairments on the other hand, suggesting a trade-off between these physiological functions and behaviour in L. dimidiatus. A. leucosternon revealed similar molecular signatures across the environmental conditions, but the differential gene expression was considerably less, suggesting less necessity to adjust to environmental changes of temperature and CO2. Altogether the molecular and behavioural responses to climate change-related future environments can compromise the establishment of future social interactions in the cleaner wrasse and its ability to adapt to increased ocean acidification and warming. Overall, these changes exhibit major implications in the future for cleaning interactions and the key ecosystem services they provide.
Methods
Experimental setup
To identify the functional molecular basis of the interaction between two fish species under ocean acidification and warming conditions, 24 female adult individuals of L. dimidiatus and 24 females of A. leucosternon were collected by TMC Iberia in the Maldives islands and transported to the aquatic facilities of Laboratório Marítimo da Guia (MARE) in Cascais, Portugal. We selected the fish species A. leucosternon as a client since it is one of the most frequent clients for the genus Labroides [43]. For L. dimidiatus, female individuals (~ 7 cm) were used to reduce the effect of sex as this species is protogynous [88]. Furthermore, fish were deparasitized with a freshwater bath upon arrival, cleaner wrasses were kept separately in individual tanks (20 L) to avoid aggressive behaviours and surgeonfish (A. leucosternon) were kept in groups. All fish were fed ad libitum once per day, and further information on size and weight can be found in Additional file 1: Table S1. Each individual was first laboratory-acclimated for 5 days in seawater conditions similar to their native site which were based on native site meteorological data and pCO2 values were chosen according to mean values reported for time-series BOBOA moored buoy deployed in the Indian Ocean. The conditions were as follows: salinity at 35.0 ± 0.5 ppt, temperature 29 °C (Maldives 2013–2014 average SST, NOAA), pH 8.1 and pCO2 400 μatm (2014 BOBOA Ocean Acidification mooring, NOAA). Following acclimation, each fish was exposed to one of four experimental treatments for 28 days, namely: (1) present-day scenario (control) (29 °C, pH 8.1, pCO2 ~ 400 μatm), (2) warming (32 °C, pH 8.1, pCO2 ~ 400 μatm), (3) high CO2 (29 °C, pH 7.7, pCO2 ~ 1000 μatm) and (4) warming and high CO2 (32 °C, pH 7.7, pCO2 ~ 1000 μatm), following IPCC’s RCP scenario 8.5 [89], Fig. 1, Additional file 1: Table S1-S2).
Experimental tanks had a semi-open flow-through aquatic system to maintain alkalinity levels, dissolved carbon and pH. Natural seawater was UV-irradiated with a Vecton V2 300 Sterilizer before being passed to each experimental tank. Photoperiods of 12 h/12 h with light and dark cycles were maintained. Ammonia and nitrate levels were checked daily using colourimetric tests (Salifert Profi Test, Holland), pH levels were automatically monitored and adjusted every 2 s (Profilux 3.1 N, GLH, Germany), regulated by injection and aeration of certified CO2 gas and filtered atmospheric air, respectively (Air Liquide, Portugal; soda lime, Sigma-Aldrich). Seawater temperature was regulated using underwater heaters 300 W, TMC Iberia (Portugal). Additional equipment was used to complement the daily monitoring of seawater temperature (VWR pH 1100H pH metre, Avantor, US), salinity (V2 refractometer TMC Iberia, Portugal) and pH (826 mobile pH metre, Metrohm, Germany). Additional quantifications of pH were done using a pH metre connected to a glass electrode (Schott IoLine, Si analytics, ±0.001), calibrated with TRIS-HCl (TRIS) and 2-aminopyridine-HCl (AMP) seawater buffers. Finally, Seawater carbonate system speciation was calculated twice a week from total alkalinity (spectrophotometrically at 595 nm) and pH measurements. Bicarbonate and pCO2 values were calculated using CO2SYS software (Additional file 1: Table S1).
Behavioural analysis
Following the 28-day period of exposure to the treatment conditions, we initiated the behavioural tests. These tests were conducted in specially set up observation tanks (40 L) situated in a designated observation room. Before the interaction, both cleaners and clients underwent a 24-h fasting period. The behavioural trial entailed placing one cleaner wrasse and one client into an observation tank that replicated the environmental conditions of their respective treatments. Each trial was recorded for a duration of 40 min, discounting the initial 5 min allocated for acclimation. The analysis of the behavioural trials was carried out following Paula et al. [31]. Cleaning behaviour was divided into two primary components: (i) motivation to interact and (ii) interaction quality. To characterize motivation to interact, we measured the proportion of time spent in interaction (close body inspection and removal of damaged tissue or scales), the proportion of interactions initiated by the cleaners, and the ratio of the client ‘posing’ displays (i.e. client ‘posing’ displays/time of no interaction; ‘posing’ displays are conspicuous signals used by clients seeking cleaning interactions).
We evaluated the quality of the interactions based on the duration of the interaction, the frequency of client jolts per 100 s of interaction (these jolts are notable signals that imply cheating or dishonesty on the part of the cleaner), and the proportion of interaction time that was spent on tactile stimulation events (touches with pectoral fins known to alleviate stress and prolong interaction duration). We used the event-logging software ‘Boris’ to analyse all behavioural videos, with the behavioural catalogue detailed in supplementary Additional file 1: Table S3a as a reference. Both the cleaner fish and the client were considered as focal subjects in the analysis [90]. Following the behavioural trial, each fish was euthanized. We then measured the body length and extracted the three main regions of the brain for further investigation. The extracted tissues were stored at − 80 °C for subsequent processing.
Statistical analysis of behavioural data
Data exploration was performed according to [91], which promotes a protocol for data exploration. We analysed behavioural data using generalized linear models (GLM) with a Gaussian distribution. These models used CO2 treatment (factor with two levels: Control; high CO2) and temperature treatment (factor with two levels: 29°C and 32°C) as categorical fixed factors, according to [92]. The full models, with all possible interactions, were tested using the function ‘glm’ and the function ‘Anova’ from the package ‘car’ [93] in R, version 3.4.3 [94]. Post hoc multiple comparisons were performed using the package ‘emmeans’ [95] with Benjamini-Hochberg corrections. Model assumptions and performance were validated using the package ‘performance’ [96]. Data exploration used the HighstatLibV10 R library from Highland Statistics [97].
RNA extraction and sequence processing
Total RNA was extracted using the RNAeasy Mini Kit protocol (Qiagen). Homogenization with sterile silicon beads was performed at maximum speed for 30 s in a Tissuelyzer (Qiagen), and Dnase I treatment (Qiagen) was performed according to the manufacturer’s protocol to remove DNA contaminants. The quantity and quality of RNA were tested using a Qubit fluorometer and an Agilent Bioanalyzer for RNA Integrity Numbers (RIN). Samples with a RIN > 8 were retained only. mRNA-focused sequencing libraries were designed with Illumina TruSeq v3 kits and sequenced for paired-end reads of 150 bp length on an Ilumina Hiseq4000 at the King Abdullah University of Science and Technology corelab facility.
To assess the differential gene expression of species interactions under different environmental conditions, raw read quality was examined using FastQC v. 0.11.9 [98]. Further, poor-quality sequences were trimmed, and adapters were removed using the software Trimmomatic v.0.36 (ILLUMINACLIP:TruSeq3-PE.fa:2:30:10 LEADING:4 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:40; [99]). A de novo transcriptome assembly from a previous study [47] was used for each species separately, and the functional annotation of transcripts was conducted using BLAST + 2.10.0, Swissprot/Uniprot protein database (November 29, 2019) and Zebrafish (Danio rerio, Apr 2018). In addition, the Ballan wrasse genome annotation (Labrus bergylta, March 2020, GCA_900080235.1) was used for L. dimidiatus assembly only as it is the closest species to the cleaner wrasse with a reference available. Omicsbox v. 1.3 [100] was used to functionally annotate the transcripts with Gene Ontology (GO terms) and KEGG pathways.
Differential expression analyses
Quantification of transcript abundance for each species was obtained using the script align_and_estimate_abundance.pl from Trinity software. RSEM v1.3.3 [101] was set as quantification method and Bowtie2 as mapping tool [102] using –gene_trans_map to receive gene-level counts. Gene expression matrices for both species were built with the script abundance_estimates_to_matrix.pl [103], while low expression transcripts (< 10 read counts) were filtered out with the filter_low_expr_transcripts.pl script. The command –highest_iso_only was used to retain the most highly expressed isoforms for each gene.
To statistically evaluate differential gene expression, we used DESeq2-package v. 1.26.0 [104] in R with a Wald test statistic using the model design = ~ brain_region + treatment to evaluate the expression differences for each treatment (Control; high CO2; warming; warming and high CO2) factoring in the different brain regions (Forebrain; Midbrain; Hindbrain). As brain regions show large differences in gene expression, further models were used (design = ~ treatment) where each brain region was examined separately. Resulting outliers were examined using principal component analysis (PCA), and two individuals of A. leucosternon from the control condition were removed (Additional file 2: Figures S7-S8). For each species, pair-wise comparisons were computed by comparing environmental conditions to the control: control vs warming, control vs high CO2, and control vs warming and high CO2. We accepted a gene to be differentially expressed with an FDR p-adjusted value of 0.05 and an absolute log2fold change threshold of 0.3. Functional enrichment was performed using Fisher’s exact test by testing the resulting subsets of differentially expressed genes against all transcripts in the transcriptome using an FDR significance value of 0.05 in Omicsbox v. 1.3.
Availability of data and materials
The data that support the findings of this study containing the raw sequencing files and de novo transcriptome assemblies (Labroides dimidiatus and Acanthurus leucosternon) are openly available under the NCBI BioProject PRJNA726349 [47] and TSA accession number GJED00000000.
References
Nagelkerken I, Munday PL. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Chang Biol. 2016;22(3):974–89.
Nagelkerken I, Goldenberg SU, Coni EOC, Connell SD. Microhabitat change alters abundances of competing species and decreases species richness under ocean acidification. Sci Total Environ. 2018;645:615–22. https://doi.org/10.1016/j.scitotenv.2018.07.168.
Burrows MT, Bates AE, Costello MJ, Edwards M, Edgar GJ, Fox CJ, et al. Ocean community warming responses explained by thermal affinities and temperature gradients. Nat Clim Chang. 2019;9(12):959–63.
Milazzo M, Mirto S, Domenici P, Gristina M. Climate change exacerbates interspecific interactions in sympatric coastal fishes. J Anim Ecol. 2013;82(2):468–77.
Clements JCJ, Hunt HLH. Marine animal behaviour in a high CO2 ocean. Mar Ecol Prog Ser. 2015;536:259–79.
Santos CP, Sampaio E, Pereira BP, Pegado MR, Borges FO, Wheeler CR, et al. Elasmobranch responses to experimental warming, acidification, and oxygen loss— A meta-analysis. Front Mar Sci. 2021;8:735377.
Rosa R, Rummer JL, Munday PL. Biological responses of sharks to ocean acidification. Biol Lett. 2017;13(3). https://royalsocietypublishing.org/doi/10.1098/rsbl.2016.0796.
Demin KA, Lakstygal AM, Alekseeva PA, Sysoev M, de Abreu MS, Alpyshov ET, et al. The role of intraspecies variation in fish neurobehavioral and neuropharmacological phenotypes in aquatic models. Aquat Toxicol. 2019;210:44–55.
Paula J, Baptista M, Carvalho F, Repolho T, Bshary R, Rosa R. The past, present and future of cleaner fish cognitive performance as a function of CO2 levels. Biol Lett. 2019;15:10–4. https://doi.org/10.1098/rsbl.2019.0618.
Kang J, Nagelkerken I, Rummer JL, Rodolfo-Metalpa R, Munday P, et al. Rapid evolution fuels transcriptional plasticity to ocean acidification. Glob Change Biol. 2022;28:3007–22.
Munday PL, Jarrold MD, Nagelkerken I. Ecological effects of elevated CO2 on marine and freshwater fishes: From individual to community effects. In: Grosell M, Munday P, Farrell A, Brauner C, editors. 1st ed. Cambridge: Academic Press Elsevier Inc.; 2019. p. 323–68.
Schunter C, Ravasi T, Munday PL, Nilsson GE. Neural effects of elevated CO2 in fish may be amplified by a vicious cycle. Conserv Physiol. 2019;7(1):coz100. https://doi.org/10.1093/conphys/coz100.
Porteus CS, Hubbard PC, Uren Webster TM, van Aerle R, Canário AVM, Santos EM, et al. Near-future CO2 levels impair the olfactory system of a marine fish. Nat Clim Chang. 2018;8(8):737–43.
Williams CR, Dittman AH, McElhany P, Shallin Busch D, Maher MT, Bammler TK, et al. Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob Chang Biol. 2018;25(3):963–77. https://onlinelibrary.wiley.com/doi/10.1111/gcb.14532.
Esbaugh AJ. Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J Comp Physiol B. 2018;188:1–13.
Heuer RM, Grosell M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol. 2014;307:1061–84.
Velez Z, Roggatz Christina C, Benoit David M, Hardege Jörg D, Hubbard Peter C. Short-and medium-term exposure to ocean acidification reduces olfactory sensitivity in gilthead seabream. Front Physiol. 2019;1:731.
Payne NL, Smith JA, van der Meulen DE, Taylor MD, Watanabe YY, Takahashi A, et al. Temperature dependence of fish performance in the wild: Links with species biogeography and physiological thermal tolerance. Funct Ecol. 2016;30(6):903–12.
Bernal MA, Schunter C, Lehmann R, Lightfoot DJ, Allan BJM, Veilleux HD, et al. Species-specific molecular responses of wild coral reef fishes during a marine heatwave. Sci Adv. 2020;6(12):3423–41.
Somero GN. The cellular stress response and temperature: Function, regulation, and evolution. J Exp Zool A Ecol Integr Physiol. 2020;333:379–97. John Wiley & Sons, Ltd.
LeBlanc S, Middleton S, Gilmour KM, Currie S. Chronic social stress impairs thermal tolerance in the rainbow trout (Oncorhynchus mykiss). J Exp Biol. 2011;214(Pt 10):1721–31.
Rendell JL, Fowler S, Cockshutt A, Currie S. Development-dependent differences in intracellular localization of stress proteins (HSPS) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comp Biochem Physiol Part D Genomics Proteomics. 2006;1(2):238–52.
Samaras A, Papandroulakis N, Lika K, Pavlidis M. Water temperature modifies the acute stress response of European sea bass, Dicentrarchus labrax L. (1758). J Therm Biol. 2018;78:84–91.
Tomalty KMH, Meek MH, Stephens MR, Rincón G, Fangue NA, May BP, et al. Transcriptional response to acute thermal exposure in juvenile chinook salmon determined by RNAseq. G3 (Bethesda). 2015;5(7):1335–49.
Pörtner HO, Farrell A. Physiology and climate change. Science (1979). 2008;322(5902):690–2.
Biro PA, Beckmann C, Stamps JA. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc Lond B Biol Sci. 2010;277(1678):71–7.
Allan BJM, Domenici P, Watson SA, Munday PL, McCormick MI. Warming has a greater effect than elevated CO2 on predator–prey. Proc R Soc Lond B Biol Sci. 1857;2017(284):20170784.
Rosa R, Baptista M, Lopes VM, Pegado MR, Paula JR, Trübenbach K, et al. Early-life exposure to climate change impairs tropical shark survival. Proc R Soc Lond B Biol Sci. 2014;281(1793):20141738. https://royalsocietypublishing.org/doi/10.1098/rspb.2014.1738.
Ferrari MCO, Munday PL, Rummer JL, Mccormick MI, Corkill K, Watson SA, et al. Interactive effects of ocean acidification and rising sea temperatures alter predation rate and predator selectivity in reef fish communities. Glob Chang Biol. 2015;21(5):1848–55.
Nowicki JP, Miller GM, Munday PL. Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol. 2012;412:46–51.
Paula J, Repolho T, Pegado MR, Thörnqvist PO, Bispo R, Winberg S, et al. Neurobiological and behavioural responses of cleaning mutualisms to ocean warming and acidification. Sci Rep. 2019;9(12728):1–10.
Paula J, Cascalheira L, Oliveira R, Otjacques E, Frazão-Santos C, Beldade R, et al. GABAergic role in the disruption of wild cleaner fish behaviour under high CO2. Anim Behav. 2023;195:77–84.
Paula JR, Repolho T, Grutter AS, Rosa R. Access to cleaning services alters fish physiology under parasite infection and ocean acidification. Front Physiol. 2022;13:859556.
Triki Z, Wismer S, Levorato E, Bshary R. A decrease in the abundance and strategic sophistication of cleaner fish after environmental perturbations. Glob Chang Biol. 2018;24(1):481–9. http://doi.wiley.com/10.1111/gcb.13943.
Grutter AS. Cleaner fish really do clean. Nature. 1999;398(6729):672–3.
Bshary R, Côté IM. New perspectives on marine cleaning mutualism. In: Magnhagen C, Braithwaite V, Forsgren E, Kapoor BG, editors. 1st Ed. Florida: CRC Press; 2008. p. 563–92.
Waldie PA, Blomberg SP, Cheney KL, Goldizen AW, Grutter AS. Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS One. 2011;6(6):e21201.
Kohda M, Hotta T, Takeyama T, Awata S, Tanaka H, Asai JY, et al. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? Plos Biology. 2019;17(2):e3000021. https://doi.org/10.1371/journal.pbio.3000021.
Soares M, Oliveira RF, Ros AFH, Grutter AS, Bshary R. Tactile stimulation lowers stress in fish. Nat Commun. 2011;2(1):534–5.
Ros AFH, Lusa J, Meyer M, Soares M, Oliveira RF, Brossard M, et al. Does access to the bluestreak cleaner wrasse Labroides dimidiatus affect indicators of stress and health in resident reef fishes in the Red Sea? Horm Behav. 2011;59(1):151–8.
Toby Kiers E, Palmer TM, Ives AR, Bruno JF, Bronstein JL. Mutualisms in a changing world: an evolutionary perspective. Ecol Lett. 2010;13(12):1459-74.42.
Waldie PA, Blomberg SP, Cheney KL, Goldizen AW, Grutter AS. Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS One. 2011;6(6):99–114.
Tebbich S, Bshary R, Grutter A. Cleaner fish Labroides dimidiatus recognise familiar clients. Anim Cogn. 2002;5(3):139–45.
Soares M. The Neurobiology of Mutualistic Behavior: The Cleanerfish swims into the Spotlight. Front Behav Neurosci. 2017;11(October):1–12.
Grutter AS, Bshary R. Cleaner wrasse prefer client mucus: Support for partner control mechanisms in cleaning interactions. Proc R Soc Lond B Biol Sci. 2003;270((suppl_2)):S242-4.
Soares M, Bshary R, Fusani L, Goymann W, Hau M, Hirschenhauser K, et al. Hormonal mechanisms of cooperative behaviour. Phil Trans R Soc B. 2010;365(1553):2737–50.
Ramírez-Calero S, Paula JR, Otjacques E, Rosa R, Ravasi T, Schunter C. Neuro-molecular characterization of fish cleaning interactions. Sci Rep. 2021;12:8468.
O’Connell LA, Hofmann HA. Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Front Neuroendocrinol. 2011;32(3):320–35. https://doi.org/10.1016/j.yfrne.2010.12.004.
Peter MCS. The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol. 2011;172(2):198–210. https://doi.org/10.1016/j.ygcen.2011.02.023.
Subhash Peter MC, Rejitha V. Interactive effects of ambient acidity and salinity on thyroid function during acidic and post-acidic acclimation of air-breathing fish (Anabas testudineus Bloch). Gen Comp Endocrinol. 2011;174(2):175–83. https://doi.org/10.1016/j.ygcen.2011.08.018.
Brown SB, Evans RE, Majewski HS, Sangalang GB, Klaverkamp JF. Responses of plasma electrolytes, thyroid hormones, and gill histology in Atlantic salmon (Salmo salar) to acid and limed river waters. Can J Fish Aquat Sci. 1990;47(12):2431–40.
Staurnes M, Sigholt T, Åsgård T, Baeverfjord G. Effects of a temperature shift on seawater challenge test performance in Atlantic salmon (Salmo salar) smolt. Aquaculture. 2001;201(1–2):153–9.
Arjona FJ, Ruiz-Jarabo I, Vargas-Chacoff L, del Río MPM, Flik G, Mancera JM, et al. Acclimation of Solea senegalensis to different ambient temperatures: Implications for thyroidal status and osmoregulation. Mar Biol. 2010;157(6):1325–35.
Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1–2):11–53.
Sanders JP, Van Der Geyten S, Kaptein E, Darras VM, Kühn ER, Leonard JL, et al. Cloning and Characterization of Type III Iodothyronine Deiodinase from the Fish Oreochromis niloticus. Endocrinology. 1999;140(8):3666–73. https://doi.org/10.1210/endo.140.8.6902.
Khar A, Taverny-Bennardo T, Jutisz M. Effect of thyroid hormones on gonadotrophin release and biosynthesis using rat pituitary cell cultures. J Endocrinol. 1980;85(2):229–35.
Subhash Peter MC. Understanding the adaptive response in vertebrates: the phenomenon of ease and ease response during post-stress acclimation. Gen Comp Endocrinol. 2013;181(1):59–64. https://doi.org/10.1016/j.ygcen.2012.09.016.
Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–10.
Logan CA, Buckley BA. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J Exp Biol. 2015;218:1915–24.
Liu QN, Xin ZZ, Chai XY, Jiang SH, Li CF, Zhang HB, et al. Characterization of immune-related genes in the yellow catfish Pelteobagrus fulvidraco in response to LPS challenge. Fish Shellfish Immunol. 2016;56:248–54.
Schmidt M, Windisch HS, Ludwichowski KU, Seegert SLL, Pörtner HO, Storch D, et al. Differences in neurochemical profiles of two gadid species under ocean warming and acidification. Front Zool. 2017;14(1):1–13.
Maille A, Schradin C. Ecophysiology of cognition: How do environmentally induced changes in physiology affect cognitive performance? Biol Rev. 2017;1102:1101–12.
Pörtner H, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2006;315(5808):95–7.
Jarrold MD, Munday PL. Elevated temperature does not substantially modify the interactive effects between elevated CO2 and diel CO2 cycles on the survival, growth and behavior of a coral reef fish. Front Mar Sci. 2018;5(DEC):458.
Mattiasen EG, Kashef NS, Stafford DM, Logan CA, Sogard SM, Bjorkstedt EP, et al. Effects of hypoxia on the behavior and physiology of kelp forest fishes. Glob Chang Biol. 2020;26(6):3498–511.
Song M, Zhao J, Wen HS, Li Y, Li JF, Li LM, et al. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii). PLoS One. 2019;14(5):e0217133.
Deutch AY, Roth RH. Pharmacology and biochemistry of synaptic transmission: classic transmitters. In: Byrne J, Heidelberger R, Waxham MN, editors. Cambridge: Academic Press Elsevier Inc.; 2004. p. 245–78.
Olsen RW, Li GD. GABA. In: Brady ST, editor. Siegel GJ. Wayne Albers R: Price DL. Accademic Press; 2012. p. 367–76.
Schunter C, Welch MJ, Nilsson GE, Rummer JL, Munday PL, Ravasi T. An interplay between plasticity and parental phenotype determines impacts of ocean acidification on a reef fish. Nat Ecol Evol. 2018;2(2):334–42.
Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson SA, et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Change. 2012;12:201–4.
Chivers DP, Mccormick MI, Nilsson GE, Munday PL, Watson SA, Meekan MG, et al. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Chang Biol. 2014;20(2):515–22.
de Abreu MS, Maximino C, Cardoso SC, Marques CI, Pimentel AFN, Mece E, et al. Dopamine and serotonin mediate the impact of stress on cleaner fish cooperative behavior. Horm Behav. 2020;125(June):104813. https://doi.org/10.1016/j.yhbeh.2020.104813.
Soares MC, Santos TP, Messias JPM. Dopamine disruption increases cleanerfish cooperative investment in novel client partners. R Soc Open Sci. 2017;4(5):1–7. https://royalsocietypublishing.org/doi/abs/10.1098/rsos.160609.
Ferrari MCO, Mccormick MI, Watson SA, Meekan MG, Munday PL, Chivers DP. Predation in High CO2 Waters: prey fish from high-risk environments are less susceptible to ocean acidification. Integr Comp Biol. 2017;57(1):55–62.
Heuer RM, Welch MJ, Rummer JL, Munday PL, Grosell M. Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci Rep. 2016;6:33216.
Chung WS, Marshall NJ, Watson SA, Munday PL, Nilsson GE. Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J Exp Biol. 2014;217(3):323–6.
Hirasawa H, Yamada M, Kaneko A. Acidification of the synaptic cleft of cone photoreceptor terminal controls the amount of transmitter release, thereby forming the receptive field surrounding the vertebrate retina. J Physiol Sci. 2012;62:359–75.
Damsgaard C, Lauridsen H, Harter TS, Kwan GT, Thomsen JS, Funder AMD, et al. A novel acidification mechanism for greatly enhanced oxygen supply to the fish retina. Elife. 2020;9:1–20.
Schunter C, Jarrold MD, Munday PL, Ravasi T. Diel pCO2 fluctuations alter the molecular response of coral reef fishes to ocean acidification conditions. Mol Ecol. 2021;30(20):5105–18.
Schunter C, Welch MJ, Ryu T, Zhang H, Berumen ML, Nilsson GE, et al. Molecular signatures of transgenerational response to ocean acidification in a species of reef fish. Nat Clim Chang. 2016;6(11):1014–8.
Suresh S, Mirasole A, Ravasi T, Vizzini S, Schunter C. Gobies inhabiting natural CO2 seeps reveal acclimation strategies to long-term acidification. Evol Appl. 2023;16(7):1345–58.
Díaz-Hung ML, Martínez G, Hetz C. Emerging roles of the unfolded protein response (UPR) in the nervous system: a link with adaptive behavior to environmental stress? Int Rev Cell Mol Biol. 2020;350:29–61.
Shi KP, Dong SL, Zhou YG, Li Y, Gao QF, Sun DJ. RNA-seq reveals temporal differences in the transcriptome response to acute heat stress in the Atlantic salmon (Salmo salar). Comp Biochem Physiol Part D Genomics Proteomics. 2019;30:169–78.
Feidantsis K, Pörtner HO, Antonopoulou E, Michaelidis B. Synergistic effects of acute warming and low pH on cellular stress responses of the gilthead seabream Sparus aurata. J Comp Physiol B. 2015;185(2):185–205.
Domenici P, Allan BJM, Watson SA, McCormick MI, Munday PL. Shifting from right to left: The combined effect of elevated CO2 and temperature on behavioural lateralization in a coral reef fish. PLoS One. 2014;9(1):e87969.
Laubenstein TD, Rummer JL, McCormick MI, Munday PL. A negative correlation between behavioural and physiological performance under ocean acidification and warming. Sci Rep. 2019;9(1):1–10.
Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P. Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol. 2013;28(11):651–8.
Kuwamura T, Tanaka N, Nakashima Y, Karino K, Sakai Y. Reversed sex-change in the protogynous reef fish Labroides dimidiatus. Ethology. 2002;108(5):443–50.
Bindoff NL, Cheung WWL, Kairo JG, Arístegui J, Guinder VA, Hallberg R, et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In: Pörtner HO, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, et al., editors. Cambridge. United Kingdom and New York, NY: Cambridge University Press: Intergovernmental Panel on Climate Change; 2019. p. 447–587.
Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. 2016. https://besjournals.onlinelibrary.wiley.com/doi/10.1111/2041-210X.12584.
Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1(1):3–14. https://onlinelibrary.wiley.com/doi/full/10.1111/j.2041-210X.2009.00001.x.
Zuur AF, Ieno EN. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol. 2016;7(6):636–45. https://onlinelibrary.wiley.com/doi/full/10.1111/2041-210X.12577.
Fox J, Weisberg S. An R Companion to Applied Regression - John Fox, Sanford Weisberg. Second. Sage, editor. California; 2011. http://cran.r-project.org/web/packages/car/citation.html.
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. 2017. http://www.r-project.org.
Lenth R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 144. 2020.
Lüdecke D, Ben-Shachar M, Patil I, Waggoner P, Makowski D. Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw. 2021;6(60):3139.
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. 2009. http://link.springer.com/10.1007/978-0-387-87458-6.
Andrews S. Babraham Bioinformatics - FastQC: A Quality Control tool for High Throughput Sequence Data. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(323):1–16.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9.
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Acknowledgements
We would like to acknowledge Lígia Cascalheira and Dr. Tiago Repolho for their help with the maintenance of aquatic systems throughout the experiment. We thank Celia Schunter’s lab members at HKU Sneha Suresh, Jingliang Kang, Natalia Petit-Marty, Arthur Chung and Jade M. Sourisse for their help in improving the bioinformatic pipeline and data analysis, and for engaging in stimulating discussions and comments to this work.
Funding
This work was supported by the Hong Kong Research Grant Committee Early Career Scheme fund 27107919 (CS) and FCT—Fundação para a Ciência e Tecnologia, I.P., within the project grant PTDC/BIA-BMA/0080/2021—ChangingMoods, the scientific employment stimulus program 2021.01030. CEECIND, the strategic project UIDB/04292/2020 granted to MARE, project LA/P/0069/2020 granted to the Associate Laboratory ARNET, and the PhD scholarship UI/BD/151019/2021 awarded to EO.
Author information
Authors and Affiliations
Contributions
JRP built the experimental setup with input from RR. JRP & CS designed the project. JRP provided maintenance of aquarium setups, performed the behavioural assays, and sampled the fish brains. EO and JRP analysed the behavioural videos and data. CS, with support from TR, conducted laboratory work and prepared samples for sequencing. SRC analysed data with input from CS. SRC, CS and JRP wrote the first draft, and all author edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research was conducted under approval of Faculdade de Ciências da Universidade de Lisboa animal welfare body (ORBEA – Statement 01/2017) and Direção-Geral de Alimentação e Veterinária (DGAV – Permit 2018–05-23–010275) in accordance with the requirements imposed by the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. This work does not include human samples or data from human patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Seawater physicochemical parameters in the experimental setups for (N=24) L. dimidiatus and (N=24)A. leucosternon individuals exposed to four conditions: one i) control with present day environmental conditions (29 °C, pH 8.1, pCO2 ~ 400 μatm), ii) warming scenario referred to as elevated ‘warming (32 °C, pH 8.1, pCO2 ~400 μatm), iii) ocean acidification referred to as ‘high CO2’ (29 °C, pH 7.7, pCO2 ~1000 μatm) and a combined condition of iv)‘warming & high CO2’ (32 °C, pH 7.7, pCO2 ~1000 μatm). These parameters follow the IPCC’s RCP scenario 8.5. Table S2. Individual brain dissections data for L. dimidiatus and A. leucosternon for each environmental condition. Brain regions dissected: forebrain (FB), midbrain (MB) and hindbrain (HB). Standard length (SL) and Weight (W) is provided for each individual used in the study. Table S3a. Behavioural trial data for each aquarium setup for individuals of L. dimidiatus and A. leucosternon assigned for control, warming, high CO2 and warming & high CO2. NCBI Biosample accession numbers of behavioural trials are provided. Table S3b. Analysis of Deviance Table (Type II tests) for each behavioural attribute tested during behavioural trials between L. dimidiatus and A. leucosternon. Significant values (*) considered with a p<0.05.Table S3c. Contrast of the post hoc multiple comparisons based on environmental conditions tested: Control, high CO2, warming and warming & high CO2. Significant values (*) considered with a p<0.05. Table S4. Enriched Gene Ontology (GO) terms for the differentially expressed genes shared among conditions of warming, high CO2 and warming & high CO2 for L. dimidiatus (N=672). Table S5. Enriched Gene Ontology (GO) terms for the differentially expressed genes shared among conditions of warming, high CO2 and warming & high CO2 for A. leucosternon (N=30). Table S6a. Specific Enriched Gene Ontology (GO) terms expressed for L. dimidiatus during the warming condition. Table S6b. Enriched Gene Ontology (GO) terms expressed the Forebrain region (FB) of L. dimidiatus during the warming condition. Table S6c. Enriched Gene Ontology (GO) terms for the Midbrain region (MB) of L. dimidiatus during the warming condition. Table S7. Specific Enriched Gene Ontology (GO) terms expressed for A. leucosternon during the warming condition. Table S8. Specific Enriched Gene Ontology (GO) terms expressed for L. dimidiatus during the high CO2 condition. Table S9. Specific Enriched Gene Ontology (GO) terms expressed for A. leucosternon during the high CO2 condition. Table S10a. Differential expressed genes found for L. dimidiatus during the warming &high CO2 condition (4581). Table S10b. Specific Enriched Gene Ontology (GO) terms expressed for L. dimidiatus during the warming & high CO2 condition. Table S11. Specific Enriched Gene Ontology (GO) terms expressed for A. leucosternon during the warming & high CO2 condition.
Additional file 2: Figure S1.
Unique and overlapping differentially expressed genes (DEGs) present in the warming condition for L. dimidiatus. Figure S2. Unique and overlapping differentially expressed genes (DEGs) present in the high CO2 condition for L. dimidiatus. Figure S3. Unique and overlapping differentially expressed genes (DEGs) present in the warming& high CO2 condition for L. dimidiatus. Figure S4. Unique and overlapping differentially expressed genes (DEGs) present in the warming condition for A. leucosternon. Figure S5. Unique and overlapping differentially expressed genes (DEGs) present in the high CO2 condition for A. leucosternon. Figure S6. Unique and overlapping differentially expressed genes (DEGs) present in warming & high CO2 condition for A. leucosternon. Figure S7.PCA’s of normalized gene counts using the design ~brain_region + treatment and a rlog transformation (rld) for each environmental treatment: a) warming, b) high CO2 and c) warming & high CO2) for A. leucosternon. Figure S8. PCA’s of normalized gene counts using the design ~brain_region + treatment and a rlog transformation (rld) for each environmental treatment: a) warming, b) high CO2 and c) warming & high CO2 for L, dimidiatus.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramírez-Calero, S., Paula, J.R., Otjacques, E. et al. Neuromolecular responses in disrupted mutualistic cleaning interactions under future environmental conditions. BMC Biol 21, 258 (2023). https://doi.org/10.1186/s12915-023-01761-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-023-01761-5