Abstract
Division of roles was observed during group hunting by the false cleanerfish, Aspidontus taeniatus (Blenniidae), when they raid the nests of the damselfish (Pomacentridae) and eat their guarded eggs. In this paper, we provide the first description of the collaborative group egg-eating behavior by the false cleanerfish. When raiding the nests of the three-spot dascyllus, Dascyllus trimaculatus, whose eggs are guarded by parents, the false cleanerfish divided roles as follows: “decoy” or “watcher” to draw attention and attract attacks from the parents, and “hider” or “intruder” to avoid detection by the parents and invade the nest. The potential differential costs associated with each role are unique among examples of group hunting strategies in fishes. However, once any individual in the group successfully invaded the nest, all individuals quickly achieved successful predation of the eggs and gained immediate shared benefit. We propose that the group egg-eating behavior of the false cleanerfish not only reinforces the evidence that fish can collaborate with other individuals but also suggests the hypothesis that collaborative hunting can evolve through mutualism even in fishes. Digital video images related to the article are available at http://www.momo-p.com/showdetail-e.php?movieid=momo240411at01a, and http://www.momo-p.com/showdetail-e.php?movieid=momo240411at02a.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group hunting, also known as social predation (see Lang and Farine 2017), is a classic example of cooperative behavior in the animal kingdom (Clutton-Brock 2009). This phenomenon is closely related to the evolution of sociality in animals and has attracted a lot of interest (Packer and Ruttan 1988; Clutton-Brock 2009). Given the significant variability in group hunting strategies among species, various attempts have been made to compare their complexity and explore their evolutionary origins (Boesch and Boesch 1989; Ellis et al. 1993; Bailey et al. 2013; Lang and Farine 2017).
Collaboration, which combines different and complementary actions, all directed toward the same prey, is the most complex level of group hunting (Boesch and Boesch 1989). Although group hunting strategies have been observed across a wide range of animal species (Lang and Farine 2017), collaboration with the concept of “division of roles” or “division of labor,” where individuals specialize in specific subtasks (Anderson and Franks 2001), has been documented in only a handful of species (Bednarz 1988; Boesch and Boesch 1989; Gazda et al. 2005; Stander 1992). For example, Taï chimpanzees successfully hunt prey by combining several complementary roles: such as “driver,” “blocker,” “chaser,” and “ambusher” (Boesch 2002, 2005). Traditionally, animals that engage in group hunting have been assumed to have a certain intelligence due to the cognitive demands associated with cooperation (Trivers 1971; Axelrod and Hamilton 1981; Packer and Ruttan 1988). However, recent evidence suggests that even species lacking complex neural networks engage in group hunting, indicating the possibility that these behaviors are underpinned by relatively simple mechanisms (Clutton-Brock 2009; Lang and Farine 2017).
In fishes, the interspecific communicative and coordinated hunting by the grouper, Plectropomus pessuliferus, and the giant moray eel, Gymnothorax javanicus, is a well-known example of collaboration (Bshary et al. 2006). By complementing their hunting behavior without changing their original skills, they achieve higher foraging success compared to solitary hunting. Furthermore, it has been reported that the yellow saddle goatfish, Parupeneus cyclostomus, is the only known fish species to engage in dividing roles during intraspecific group hunting, with individuals assuming two roles: “chasers,” pursuing small prey fish, and “blockers,” blocking their escape path (Strübin et al. 2011). It has been shown that those roles result from a simple decision-making process of choosing actions depending on the distance to conspecifics swimming ahead (Steinegger et al. 2018, 2020).
In this study, we are the first to describe the collaborative group egg-eating behavior of the false cleanerfish, Aspidontus taeniatus (Blenniidae), in which conspecifics divide their several roles within the group to raid the nests of damselfishes (Pomacentridae) to prey on their guarded eggs. Furthermore, we discuss the mechanisms of cooperation maintained within hunting groups.
Materials and methods
Study species
The false cleanerfish, A. taeniatus closely resembles the bluestreak cleaner wrasse, Labroides dimidiatus (Labridae) in body shape and coloration, an appearance described as the most elaborate mimicry among coral reef fishes (Randall and Randall 1960; Wickler 1968). As a result, false cleanerfish can easily approach other fishes and bite their fins by aggressive mimicry (Wickler 1968; Sato et al. 2023). However, fin biting is an opportunistic foraging strategy when preferred food sources, such as fish eggs, polychaete tentacles, and bivalve mantles, are scarce (Kuwamura 1983; Fujisawa et al. 2020; Kuwamura et al. 2022). In our study area, damselfish eggs are particularly abundant from April to October, during high water temperature season. False cleanerfish that are larger than 70 mm in total length (TL) primarily rely on foraging for damselfish eggs, and do so in groups (Fujisawa et al. 2018; Sato et al. 2022).
Behavioral observation
Behavioral observations of the false cleanerfish were conducted on the coral reefs of Sesoko Island, a marine protected area of approximately 100 × 350 m, in front of Sesoko Station of the Tropical Biosphere Research Center at the University of the Ryukyus (127°52′ E, 26°38′ N), Okinawa, southern Japan. The study site was a fringing reef with a sandy bottom off the reef edge at a depth of 5 m (during high tide). We conducted behavioral observations for a total of 392 30-min observations (196 h total) over 106 survey days from April 12th, 2019, to October 28th, 2019, on randomly encountered individuals or groups of the false cleanerfish. All behavioral observations were made by snorkeling between 07:00 a.m. and 06:00 p.m. The majority of surveys were conducted in the morning (152 h, 77.6% of the total observation time) because egg-eating was frequently observed during this time (see results). All 65 observed individuals were captured once using a screen net and subsequently identified by elastomer fluorescent tags (Northwest Marine Technology) during the survey period (See Sato et al. 2022). The median observation time per individual was 75 min (range: 30–3360 min, N = 38). When the false cleanerfish targeted the damselfish nest, we recorded their egg-eating using an underwater video camera (Olympus Tough TG-5, Japan). Video recordings were usually made from a distance of at least 1 m from the individual to minimize disturbance to their behaviors.
Results
A total of 131 instances of egg-eating behavior by the false cleanerfish, A. taeniatus, were observed during the survey period, targeting 13 damselfish species and the halfmoon triggerfish, Sufflamen chrysopterum (See also Sato et al. 2022). Egg-eating was observed more frequently in the morning (33.6% of morning 30 min-observations) compared to the afternoon (21.6%, Fisher's exact test: p = 0.036). The overall success rate of egg-eating was 77.9% (n = 122, excluding 9 instances with no eggs present in the nest). Notably, specific behaviors involving the temporary division of roles, as described later, were observed in cases targeting pomacentrid species exhibiting lower success rates (Electronic Supplementary Material, Table S1). However, due to the small sample size, the factors contributing to the occurrence of division of roles could not be determined in this study.
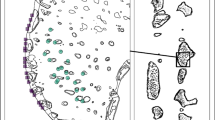
On July 14, 2019, a group of five false cleanerfish individuals (individual IDs #01–05, Table S2) awaited an opportunity to raid a nest of the three-spot dascyllus, Dascyllus trimaculatus. The three-spot dascyllus had laid eggs on the surface of scattered boulders (0.5–2.0 m diameter) on the sandy substrate. The male parent damselfish was observed guarding the eggs, remaining vigilant, patrolling around its nest, and attacking the false cleanerfish by short aggressive chases when they approached within 3.0–5.0 m of the nest. Another individual, probably the female parent damselfish, was in proximity to the nest, but did not participate in the defense at this phase. The largest false cleanerfish (#01: female, 118 mm TL) made the initial attempt to rush into the nest. When the male parent damselfish pursued this individual false cleanerfish, the latter immediately changed direction and swam away from the nest. Meanwhile, the other false cleanerfish individuals closely monitored the interactions between the male parent damselfish and the first false cleanerfish promptly seizing the opportunity to raid the nest; they immediately slipped past the male parent damselfish and rushed into the nest as “intruders.” The first false cleanerfish that made the initial raid attracted the damselfish attack, and consequently acted as a “decoy.” This “decoy” individual returned to the group as soon as the damselfish attack was directed toward another false cleanerfish individual and resumed its raiding. In this event, upon finding no eggs in the nest of the three-spot dascyllus, the false cleanerfish group abandoned the raid. Similar decoy behaviors were also occasionally observed during raids on the nests of other damselfishes, such as Stegastes obreptus in three cases (Fig. 1; http://www.momo-p.com/showdetail-e.php?movieid=momo240411at01a; Video S1; Table S1), and D. trimaculatus in four cases (Table S1).
Decoy behavior observed in a group of the false cleanerfish Aspidontus taeniatus raiding the nest of the western gregory Stegastes obreptus. (a) Two false cleanerfish individuals swam in parallel facing toward the damselfish nest. The number indicates individual IDs. (b) The “decoy” swam forward and provoked the damselfish parents’ attack. (c) The “decoy” immediately escaped, drawing the damselfish parents’ attack for a while. (d) The “intruder” rushed into the nest while the “decoy” was attacked. These photographs were cut from the Video S1 taken by H.S. at 02:52 p.m. on May 16, 2019 at a depth of 5 m on the reef of Sesoko Island, Okinawa, Japan (http://www.momo-p.com/showdetail-e.php?movieid=momo240411at01a)
Subsequently, an additional false cleanerfish (#06: female, 108 mm TL) joined the group, which now totaled six individuals. This group then targeted another nest of the three-spot dascyllus. The egg-guarding male parent damselfish, engaged in courtship displays and vocalization with a visiting female, remained vigilant for the false cleanerfish. The false cleanerfish group waited for several minutes at a distance of 2.0–3.0 m from the target nest until the moment when their vigilance was temporarily decreased during egg spawning. The newcomer false cleanerfish (#06) attracted the attention of the egg-guarding male and female three-spot dascyllus. This time, the three-spot dascyllus pair showed a coordinated defense; the male parent chased the false cleanerfish, however the simple decoy behavior did not result in the others getting close to the nest because of the presence of the female parent. Instead, the false cleanerfish group approached the nest by moving swiftly along the rock surface, exhibiting behavior reminiscent of crawling. Additionally, they appeared to use the rock as a means to conceal their presence. Although no video footage was captured at the time, the group successfully raided the nest, dividing their roles as “watcher” and “hider,” as observed later.
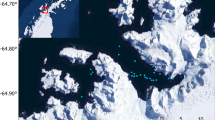
On September 14, 2019, the three-spot dascyllus eggs were attached to part of the sloping surface of a rock (approximately 1.0 m diameter, with a pointed peak in the center) (Fig. 2; http://www.momo-p.com/showdetail-e.php?movieid=momo240411at02a; Video S2). Three large false cleanerfish individuals (#06, 07, 08, Table S2) suddenly swam quickly from a distance of 2.0–3.0 m away in the opposite direction of where the eggs were attached, and then approached the nest by crawling along the rock surface (Fig. 2a) and stopped near the top of the rock. The leading individual monitored the damselfish parents’ behaviors, thus acting as “watcher” (Fig. 2b). The other two followed individuals hid in rock hollows, assuming the role of “hider” (Fig. 2b,c). The male parent damselfish noticed the “watcher” peering from the top of the rock and aggressively chased it (Fig. 2c). As soon as the male parent approached the “watcher,” it quickly escaped, effectively drawing the male parent away from the nest. At that moment, the presumably undetected “hiders” quickly rushed into the nest (Fig. 2d), and were allowed a few seconds of raiding before being noticed by the damselfish, forcing its return (Fig. 2e). However, the male parent damselfish swiftly expelled the “hiders” due to its high swimming ability. In the subsequent attempt, the false cleanerfish adjusted its hiding position slightly backward. Employing a similar behavioral pattern involving the roles of “watcher” and “hider,” the false cleanerfish waited for an opportunity to raid. This time, the male parent attacked the “hider,” allowing the “watcher” to rush into the nest. Although the female parent still defended the eggs, one false cleanerfish individual forcibly intruded into the nest, and raiding was repeated one after another by all three false cleanerfish individuals. Despite the three-spot dascyllus pair’s attempts to fend them off by biting and aggressive chasing, the false cleanerfish continued to consume eggs until their stomachs were visibly distended, completing the raid in 19 s. However, despite their consumption, eggs remained in the nest. A similar behavioral pattern was observed in the two cases described here in D. trimaculatus (Table S1).
Temporary division of roles in a group of the false cleanerfish Aspidontus taeniatus raiding a nest of the three-spot dascyllus Dascyllus trimaculatus. (a) Three false cleanerfish individuals approached by crawling. The number indicates individual IDs. (b) The leading individual became a “watcher” to monitor the target’s behavior, and the followers became “hiders.” (c) The egg-guarding male parent damselfish noticed and attacked the “watcher.” (d) The “hiders” waited for the male parent to pass by and then jumped out. (e) The “hiders” rushed into the nest. These photographs were cut from the Video S2 taken by H.S. at 7:54 a.m. on September 14, 2019 at a depth of 5 m on the reef of Sesoko Island, Okinawa, Japan (http://www.momo-p.com/showdetail-e.php?movieid=momo240411at02a)
Discussion
Our observation of the false cleanerfish, A. taeniatus, raiding damselfish nests reveals a remarkable example of cooperative behavior in fishes. The group of the false cleanerfish successfully preyed on the eggs by temporarily combining several complementary roles within the group members. This adds to the growing body of evidence for collaborative hunting in aquatic environments (Bshary et al. 2006; Strübin et al. 2011; Steinegger et al. 2018, 2020).
The egg-eating behavior of the false cleanerfish groups offers an excellent opportunity to investigate the mechanisms underlying cooperation among individuals, a subject often explained through hypotheses such as kin selection, reciprocity, mutualism, or manipulation including by-product benefit (Trivers 1971; Axelrod and Hamilton 1981; Clutton-Brock 2009).
In cases where a kin relationship exists within a group of animals engaging in cooperative behavior, assistance to other individuals can be explained by an increase in inclusive fitness (Hamilton 1964). However, false cleanerfish juveniles (less than 60 mm TL) have a floating life stage that disperses in the pelagic zone (Losey 1974; Ohta and Tachihara 2004). Since the recruitment of juveniles into the reef environment is influenced by ocean currents and winds, it is unlikely that there is a high degree of relatedness within the group (Avise and Shapiro 1986). Therefore, the false cleanerfish egg-eating groups probably do not have a kinship, thus ruling out kin selection as a driving force behind their cooperation.
Reciprocity is the most common explanation of cooperation between unrelated individuals, in which individuals incur a temporary cost to cooperate with a partner in anticipation of future returns (Trivers 1971; Axelrod and Hamilton 1981). However, reciprocity requires high cognitive demands, such as the ability to assess costs and distinguish between cooperative and non-cooperative partners, and there is little evidence in nature, except for humans, to support this (Clutton-Brock 2009). In the context of cooperation in group hunting, recent reports and simulation studies suggest that it is more likely to occur through simpler mechanisms that do not require reciprocity, as opposed to what was previously assumed (Muro et al. 2011; Lang and Farine 2017; Steinegger et al. 2018). Simpler mechanisms represent pseudo-reciprocity, such as mutualism, which is an immediate shared benefit to both parties, and by-product benefit, which profits the partner as a result of the selfish behavior (Connor 1986; Clutton-Brock 2009; Carter 2024).
In the case of the false cleanerfish, individuals may incur differential costs depending on the behavioral role adopted during egg-eating. For example, the “decoy” and “watcher” would be first attacked by egg-guarding parents and have to initially flee, and some group members may be bitten by egg-guarding parents when eating eggs. Those variations in costs among individuals within a group may potentially give rise to conflicts among group members. However, once the raid is successful, all individuals benefit from the shared reward of eating eggs, mitigating potential conflicts, and suggesting a mutualistic relationship among group members. The “decoy” and “watcher” roles shown in the false cleanerfish are similar in their function, in terms of both aimed at attracting damselfish attacks to create opportunities for conspecifics to invade the nest. Differences in behavioral patterns may be influenced by target nest rugosity, the aggressiveness of damselfish, the number of parent fish, and other factors. Therefore, we propose that the cooperation among individual false cleanerfish can be explained by simple mutualism, whereby participation in group egg-eating yields greater fitness than solitary situations because all group members may gain immediate shared benefit through eating eggs. The false cleanerfish may have developed mutualistic group egg-eating behavior through trial and error while raiding highly defensive damselfish nests in small groups (around 10 individuals at most: Sato et al. 2022), unlike the other species that opportunistically prey on damselfish eggs in large selfish aggregations (Cheney 2008; Foster 1987).
Future research should delve deeper into the quantitative analysis of the costs and benefits at the individual level for testing the mutualism hypothesis, and decision-making process for the behavioral rules employed by each individual during egg-eating; this entails analyzing role specialization or alternation. Additionally, we predict that specific behaviors, such as “decoy” behavior, are more likely to occur in situations where the defensive capabilities of the target damselfish are high. Furthermore, the roles of individuals would be determined by factors such as egg-eating experience, individual hunger level, or personality traits like boldness. Understanding the individual-level mechanisms driving cooperative behavior in the false cleanerfish will provide valuable insights into the evolutional implications of collaborative hunting strategies in reef fishes.
Data availability
All data for this study are available in the Electronic Supplementary Materials.
References
Anderson C, Franks NR (2001) Teams in animal societies. Behav Ecol 12:534–540
Avise JC, Shapiro DY (1986) Evaluating kinship of newly settled juveniles within social groups of the coral reef fish Anthias squamipinnis. Evolution 40:1051–1059
Axelrod R, Hamilton WD (1981) The evolution of cooperation. Science 211:1390–1396
Bailey I, Myatt JP, Wilson AM (2013) Group hunting within the Carnivora: physiological, cognitive and environmental influences on strategy and cooperation. Behav Ecol Sociobiol 67:1–17
Bednarz JC (1988) Cooperative hunting harris’ hawks (Parabuteo unicinctus). Science 239:1525–1527
Boesch C (2002) Cooperative hunting roles among taï chimpanzees. Hum Nat 13:27–46
Boesch C (2005) Joint cooperative hunting among wild chimpanzees: taking natural observations seriously. Behav Brain Sci 28:692–693
Boesch C, Boesch H (1989) Hunting behavior of wild chimpanzees in the Taï National Park. Am J Phys Anthropol 78:547–573
Bshary R, Hohner A, Ait-el-Djoudi K, Fricke H (2006) Interspecific communicative and coordinated hunting between groupers and giant moray eels in the red sea. PLoS Biol 4:e431
Carter GG (2024) Reciprocity versus pseudo-reciprocity: a false dichotomy. Ethology 130:e13431
Cheney KL (2008) Non-kin egg cannibalism and group nest-raiding by Caribbean sergeant major damselfish (Abudefduf saxatilis). Coral Reefs 27:115
Clutton-Brock T (2009) Cooperation between non-kin in animal societies. Nature 462:51–57
Connor RC (1986) Pseudo-reciprocity: investing in mutualism. Anim Behav 34:1562–1566
Ellis DH, Bednarz JC, Smith DG, Flemming SP (1993) Social foraging classes in raptorial birds: highly developed cooperative hunting may be important for many raptors. Bioscience 43:14–20
Foster SA (1987) Acquisition of a defended resource: a benefit of group foraging for the neotropical wrasse, Thalassoma lucasanum. Environ Biol Fishes 19:215–222
Fujisawa M, Sakai Y, Kuwamura T (2018) Aggressive mimicry of the cleaner wrasse by Aspidontus taeniatus functions mainly for small blennies. Ethology 124:432–439
Fujisawa M, Sakai Y, Kuwamura T (2020) The false cleanerfish relies on aggressive mimicry to bite fish fins when benthic foods are scarce in their local habitat. Sci Rep 10:8652
Gazda SK, Cornnor RC, Edgar RK, Cox F (2005) A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc R Soc B Biol Sci 272:135–140
Hamilton WD (1964) The genetical evolution of social behaviour II. J Theor Biol 7:17–52
Kuwamura T (1983) Reexamination on the aggressive mimicry of the cleaner wrasse Labroides dimidiatus by the blenny Aspidontus taeniatus (Pisces; Perciformes). J Ethol 1:22–33
Kuwamura T, Sato H, Sakai Y (2022) Comparison of mortality and feeding behavior of the false cleanerfish Aspidontus taeniatus and the lance blenny A. dussumieri regarding the effects of mimicry. J Ethol 41:73–77
Lang SDJ, Farine DR (2017) A multidimensional framework for studying social predation strategies. Nat Ecol Evol 1:1230–1239
Losey GS (1974) Aspidontus taeniatus: Effects of increased abundance on cleaning symbiosis with notes on pelagic dispersion and A. filamentosus (Pisces, Blenniidae). Z Tierpsychol 34:430–435
Muro C, Escobedo R, Spector L, Coppinger RP (2011) Wolf-pack (Canis lupus) hunting strategies emerge from simple rules in computational simulations. Behav Processes 88:192–197
Ohta I, Tachihara K (2004) Larval development and food habits of the marbled parrotfish, Leptoscarus vaigiensis, associated with drifting algae. Ichthyol Res 51:63–69
Packer C, Ruttan L (1988) The evolution of cooperative hunting. Am Nat 132:159–198
Randall JE, Randall HA (1960) Example of mimicry and protective resemblances in tropical marine fishes. Bull Mar Sci 10:444–480
Sato H, Sakai Y, Kuwamura T (2022) Effects of group behavior in the predatory raid on damselfish nests by the false cleanerfish Aspidontus taeniatus. Ethology 128:77–84
Sato H, Sakai Y, Kuwamura T (2023) Cleaner fish coloration does not always reduce predation risk: testing the effect of protective mimicry in the false cleanerfish Aspidontus taeniatus. Biol J Linn Soc. https://doi.org/10.1093/biolinnean/blad163
Stander PE (1992) Cooperative hunting in lions: the role of the individual. Behav Ecol Sociobiol 29:445–454
Steinegger M, Roche DG, Bshary R (2018) Simple decision rules underlie collaborative hunting in yellow saddle goatfish. Proc R Soc B Biol Sci 285:20172488
Steinegger M, Sarhan H, Bshary R (2020) Laboratory experiments reveal effects of group size on hunting performance in yellow saddle goatfish, Parupeneus cyclostomus. Anim Behav 168:159–167
Strübin C, Steinegger M, Bshary R (2011) On group living and collaborative hunting in the yellow saddle goatfish (Parupeneus cyclostomus). Ethology 117:961–969
Trivers RL (1971) The evolution of reciprocal altruism. Q Rev Biol 46:35–57
Wickler W (1968) Mimicry in plants and animals. McGraw-Hill, New York
Acknowledgements
We thank the staff of Sesoko Station, Tropical Biosphere Research Center at the University of the Ryukyus for supporting our fieldwork. We dedicate this work to the memories of the late Dr. Tetsuo Kuwamura, co-author, with deep gratitude and sincere respect for his contributions to the present study.
Funding
Open Access funding provided by Hiroshima University. This study was supported by the Sasakawa Scientific Research Grant from the Japan Science Society and Grant-in-Aid for JSPS Fellows JP23KJ1628 to HS, JSPS KAKENHI Grant Number JP19K06845 to TK, and JSPS KAKENHI Grant Number JP23H03868 to YS.
Author information
Authors and Affiliations
Contributions
HS and TK made behavioral observations; HS drafted the original manuscript; HS, YS, and TK substantially contributed to the revision of the manuscript drafts.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
All procedures performed in this study followed the Guidelines for the Proper Conduct of Animal Experiments and related activities laid down by the Hiroshima University Animal Research Committee (No. 020A170410 certified on April 10, 2017), the ASAB/ABS Guidelines for the Use of Animals in Research (Guidelines for the Treatment of Animals in Behavioral Research and Teaching; https:// doi.org/https://doi.org/10.1016/j.anbehav.2019.11.002).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 Decoy behavior observed in a group of the false cleanerfish Aspidontus taeniatus raiding the nest of the western gregory Stegastes obreptus. This video is available at the Movie Archives of Animal Behavior: http://www.momo-p.com/showdetail-e.php?movieid=momo240411at01a (MP4 35224 KB)
Supplementary file4 Temporary division of roles in a group of the false cleanerfish Aspidontus taeniatus raiding a nest of the three-spot dascyllus Dascyllus trimaculatus. This video is available at the Movie Archives of Animal Behavior: http://www.momo-p.com/showdetail-e.php?movieid=momo240411at02a (MP4 92976 KB)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sato, H., Sakai, Y. & Kuwamura, T. Temporary division of roles in group hunting for fish eggs by a coral reef fish. J Ethol (2024). https://doi.org/10.1007/s10164-024-00812-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10164-024-00812-w