Abstract
Background
Shared decision-making (SDM) is considered a key component of high quality cancer care and may be supported by patient decision aids (PtDAs). Many patients, however, face multiple social disadvantages that may influence their ability to fully participate in SDM or to use PtDAs; additionally, these social disadvantages are among the determinants of health associated with greater cancer risk, unwarranted variations in care and worse outcomes. The purpose of this systematic review is to describe the extent to which disadvantaged social groups in the United States (US) have been included in trials of cancer-related PtDAs and to highlight strategies, lessons learned and future opportunities for developing and evaluating PtDAs that are appropriate for disadvantaged populations.
Methods
We selected cancer-related US studies from the Cochrane 2014 review of PtDAs and added RCTs meeting Cochrane criteria from searches of PubMed, CINAHL, PsycINFO (January 2010 to December 2013); and reference lists. Two reviewers independently screened titles/abstracts; three reviewers independently screened full text articles, performed data extraction and assessed: 1) inclusion of participants based on seven indicators of social disadvantage (limited education; female gender; uninsured or Medicaid status; non-U.S. nativity; non-White race or Hispanic ethnicity; limited English proficiency; low-literacy), and 2) attention to social disadvantage in the development or evaluation of PtDAs.
Results
Twenty-three of 39 eligible RCTs included participants from at least one disadvantaged subgroup, most frequently racial/ethnic minorities or individuals with limited education and/or low-literacy. Seventeen studies discussed strategies and lessons learned in attending to the needs of disadvantaged social groups in PtDA development; 14 studies targeted disadvantaged groups or addressed subgroup differences in PtDA evaluation.
Conclusions
The diversity of the US population is represented in a majority of cancer-related PtDA RCTs, but fewer studies have tailored PtDAs to address the multiple social disadvantages that may impact patients’ participation in SDM. More detailed attention to the comprehensive range of social factors that determine cancer risk, variations in care and outcomes is needed in the development and evaluation of PtDAs for disadvantaged populations.
Trial registration
Registered 24 October 2014 in PROSPERO International prospective register of systematic reviews (CRD42014014470).
Similar content being viewed by others
Background
The social determinants of health (SDH) are conditions in which people live, learn, work and play that interact with individual-level characteristics (e.g., age, gender, genetics, behavior) to affect a wide range of health risks and outcomes [1–3]. In the cancer care continuum, rates of incidence and death for the most common cancer types vary considerably within and across socioeconomic groups [4]. However, patients in the United States (US) who are members of certain disadvantaged subgroups (e.g., racial/ethnic minorities, the uninsured or underinsured and individuals with limited education, low income or unhealthy living conditions) are more likely than those in advantaged groups to be diagnosed with cancer at later stages [4–9], undergo greater variation in screenings and treatments received [4, 5, 8–11], and experience higher rates of morbidity and mortality [4, 5, 8, 9, 12, 13]. Compared to those in advantaged groups, cancer patients in disadvantaged subgroups are also more likely to report worse patient-provider communication and quality of care [14].
In recent years, the call to address SDH as key drivers of health inequities has gained momentum among health system leaders and policymakers in the US. Objectives outlined in Healthy People 2020 emphasize the importance of addressing SDH as one of four overarching goals for the decade [2]. New research also identifies the need to move beyond simply recognizing the fundamental role upstream SDH play in influencing health toward using this knowledge to develop and evaluate interventions that examine the mechanisms through which SDH influence downstream processes of care and outcomes [1, 15–18]. Interventions involving patient decision aids (PtDAs), developed with attention to SDH, represent important opportunities to influence patient-provider communication and shared decision making (SDM) processes.
PtDAs are tools (e.g., brochures, videos, internet-based programs) designed to help individuals participate in decisions about their healthcare by preparing them to discuss and make informed, values-based decisions in partnership with their providers [19–21]. The Institute of Medicine (IOM) has highlighted the need to support engagement in SDM, using PtDAs when available, as integral to delivering high quality cancer care [22]. In order to accomplish this goal, PtDAs must be appropriate for their intended audiences and present factual, balanced information in easy-to-understand formats that help patients consider the pros and cons of the options available to them. Consensus-based standards offer guidance on what should be included in the PtDAs and how the tools should be developed, including detailed instructions about using plain language to support the needs of people with limited reading skills [23, 24]. The standards, however, are largely silent about addressing the needs of people who face other social disadvantages that may influence health care decision making [1, 25, 26].
The multiple causal pathways through which social factors are theorized to shape health are long and complex [1, 17, 18, 27, 28]. We posit that SDH may influence patient-provider interactions and SDM by limiting the actual and/or perceived options available to patients. For example, limited education, literacy or English proficiency (LEP) may obstruct patient-provider communication, even when translators are available, or limit patients’ perceived ability to act on the information they receive [29–31]. Economic instability (e.g., low income, hourly employment, transient housing) may cause patients to delay or forego needed care because they are unable to afford co-pays/cost-shares, to purchase or appropriately store prescribed therapies, or to take time away from work for longer-term treatments [1, 17]. It may also limit one’s ability or willingness to use interventions, such as PtDAs, that require reliable computer and/or high speed internet access [32, 33]. Limitations of the built environment (e.g. inadequate access to healthy foods, transportation, safe parks) may cause patients not to choose treatment options that require lifestyle and behavior changes or travel to certain areas for treatment [1, 17]. Lack of family/social support may render home- and/or community-based treatments less desirable, while social norms/attitudes (e.g., perceived and/or actual discrimination) may lead patients and their families to distrust the advice of their providers or health system officials [1, 17, 34, 35]. Most certainly, access to healthcare (e.g., access to providers, insurance status) may reduce patients’ actual or perceived access to certain providers/facilities and/or specific treatments not covered by their insurance plans [2, 25, 27, 34]. In other words, when presented with more than one option, patients may not perceive that the full range of options is available to them based on their personal circumstances. We argue that PtDAs for disadvantaged patients should be tailored to address SDH because such tools can then help them think through and, importantly, share with their clinicians concerns about how SDH-related barriers influence their preferences for treatment.
Despite the increasing number of PtDAs being developed, little is known about the availability of cancer-related PtDAs evaluated in the US that address the pathways through which SDH may influence the decision making processes of disadvantaged patients. The purpose of this review is to address this gap in the literature. We specifically aim to answer the questions: 1) To what extent have disadvantaged social groups been included in randomized controlled trials (RCTs) of cancer-related PtDAs conducted in the US; and 2) What are the strategies and lessons learned about developing and evaluating PtDAs that address one or more SDH or otherwise support the decision making needs of disadvantaged audiences?
Methods
Eligibility criteria, search methods, study selection and data extraction
We examined all RCTs of PtDAs that were found in the 2014 updated Cochrane systematic review of patient decision aids [20] plus 6 other trials identified in an independent search of PubMed, CINAHL and PsycINFO (January 2010 through December 2013) using terms similar to the Cochrane protocol (Additional file 1). We included studies that met the Cochrane criteria; that is, they used a RCT design and evaluated a PtDA as part of the intervention. We eliminated duplicate studies and excluded studies that were not conducted in the US, did not target cancer-related decisions, or did not focus on active decision making. We focus our review on the US because there are major differences between the US and other high income countries in the way healthcare is financed and delivered that may impact patient-provider interactions and SDM processes. These differences are particularly relevant when examining socially disadvantaged patients. Neither study quality nor the neutrality of the PtDA was considered in judging eligibility for inclusion; those assessments are available elsewhere for studies included in the Cochrane review [20]. Reference lists from the RCTs were also searched (and references of those references, if necessary) to identify related articles reporting quantitative and/or qualitative information relevant to development of the PtDAs. Two authors (KRE, GRK) independently reviewed article titles/abstracts to assess eligibility based on our inclusion/exclusion criteria. Three authors (KRE, GRK, NMD) independently reviewed the full text of potentially eligible articles and extracted specified data using an electronic form, and disagreements were resolved by consensus; KRE aggregated the results. When available, multiple reports from the same study were reviewed for relevant data but counted as a single study.
Measures
Inclusion of disadvantaged subgroups in RCTs
We evaluated inclusion of disadvantaged subgroups in the selected RCTs based on documentation of seven sample characteristics related to social disadvantage: limited education; female gender; uninsured or Medicaid status; non-US nativity; non-White race or Hispanic ethnicity; limited English proficiency (LEP); and low-literacy. These population characteristics are associated with inequities in healthcare access and, because they are recommended in reporting guidelines for systematic reviews focused on health equity [36], they were expected to be readily available in published studies. For disadvantaged subgroups other than those with LEP or low-literacy, we classified studies as inclusive when a proportion of the sample met or exceeded US national averages for the specified subgroup [37, 38]. Studies in which individuals with LEP were eligible to participate and studies that targeted low-literacy populations were also considered inclusive. Subgroup definitions for each criterion are given here:
-
1.
Limited Education: ≥ 13 % less than high school (HS), ≥ 30 % HS or general educational development (GED) certificate, cumulatively, ≥ 43 % HS or less;
-
2.
Female Gender: ≥ 50 % female, unless gender-specific condition;
-
3.
Uninsured or Medicaid Status: ≥ 16 % uninsured, ≥ 16 % Medicaid beneficiaries or, cumulatively, ≥ 32 % uninsured or Medicaid beneficiaries;
-
4.
Non-US Nativity: ≥ 12 % born outside the US;
-
5.
Non-White Race or Hispanic Ethnicity: ≥ 13 % Black, ≥ 16 % Hispanic, ≥ 5 % Asian/Native Hawaiian or Pacific Islander (Asian/NHPI) or < 63 % non-Hispanic White;
-
6.
LEP: study facilitated participation using ≥ 1 language other than English;
-
7.
Low-Literacy: study measured and reported participants’ literacy or health literacy as low, or described participants as being from low-literacy populations.
We coded studies as “Yes” (met a criterion), “No” (did not meet a criterion or did not report data related to a criterion) or “Not Applicable” (criterion was not applicable to the cancer context; e.g., breast cancer studies limited to female patients; prostate cancer studies limited to male patients).
Attention to SDH in PtDA development
To address this aim, we examined the included RCTs and related articles that we were able to identify through cited and citing references for documentation of a systematic process that explicitly investigated the needs of one or more disadvantaged subgroups in the development of the PtDA. We distinguish this from the previous measure (inclusion) because an RCT may have included disadvantaged groups in the samples but provided no evidence of attention to social disadvantage in how the aid was developed. We also reviewed sources for study context; eligibility criteria; identification of a conceptual framework; descriptions of the PtDA format; intended delivery mode/setting/timing; subgroups targeted in the development process; and development strategies used to inform PtDA content and to test the usability/feasibility of the intervention. We defined usability/feasibility testing as activities intended to assess participants’ ability to understand and complete intervention-related tasks; time required to complete such tasks; satisfaction with (acceptability) and estimated or actual use of the intervention (demand); and other activities related to the assessment of intervention feasibility [37].
Attention to SDH in PtDA evaluation
We examined articles for any discussion of the effectiveness of the PtDA in a disadvantaged subgroup based on: 1) the authors’ stated a priori objectives to evaluate the effectiveness of the PtDA in the targeted subgroup(s), 2) use of evaluation tools specifically designed for disadvantaged subgroups (e.g., low-literacy version of a validated questionnaire), or 3) stratified subanalyses and/or PtDA-by-subgroup interactions. Here, we distinguish attention to SDH in PtDA evaluation from our measure of inclusion because studies may have failed to meet our criteria for including socially disadvantaged groups in the sample but still considered social disadvantage in the analytic plan. In contrast, studies may have met our criteria for including disadvantaged groups in the samples but failed to consider subgroup differences in the analytic plan. In the latter case, inferences about disadvantaged groups cannot be made.
Results
Characteristics of the RCTs
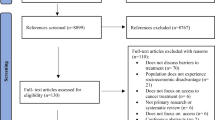
The initial literature search returned 223 unique references; 106 were selected for full-text review (Fig. 1). Of these references, 39 RCTs of PtDAs reported in 46 articles [38–83] published between 1995 and 2013 met our inclusion criteria (Table 1). The RCTs addressed three cancers: breast (BCa, k = 12), colorectal (CRC, k = 7) and prostate (PCa, k = 20); and primarily supported screening or prevention decisions (k = 32). The BCa studies focused on genetic testing/counseling (k = 5), high risk prevention (k = 3), reconstruction surgery (k = 1), and breast conserving therapy (BCT) versus mastectomy (k = 3). Six CRC studies addressed screening (fecal occult blood testing, flexible sigmoidoscopy, colonoscopy, double contrast barium enema); one addressed microsatellite instability (MSI) genetic testing. Seventeen studies evaluated PtDAs designed to support PCa screening decisions (prostate specific antigen testing or digital rectal exam); and three studies addressed PCa treatment (active surveillance, prostatectomy, radiation therapy or medication). Nearly all studies were conducted in urban/suburban areas; only two studies were conducted in rural areas. Regional variation was relatively evenly distributed.
Inclusion of disadvantaged subgroups
Twenty-three of 39 studies met one or more of our a priori criteria for inclusion of disadvantaged subgroups: HS or less education (k = 12); females (6-of-7 CRC screening studies); Medicaid or uninsured status (k = 7); non US nativity (k = 2); non-white race or Hispanic ethnicity (k = 18); LEP (k = 4); and low-literacy (k = 3), Fig. 2.
Frequency of Disadvantaged Subgroups Included in Randomized Trials of Cancer-Related Patient Decision Aids (k = 39 studies). Study samples met (Yes) or did not meet or did not report (No) criteria for being inclusive of specified subgroup, or criterion was not applicable (NA) to the cancer context (e.g., gender specific studies)
One-third of trials addressing decision making in BCa prevention or treatment targeted a socially disadvantaged group, but none of the three on BCa prevention in high risk groups included disadvantaged groups. One of five studies about BRCA genetic testing included representation of non-White patients (Black women). All six studies about CRC screening included at least one disadvantaged group; the sole study on CRC genetic testing did not. Most trials addressing PCa screening included at least one disadvantaged group, and one of these studies addressed five disadvantaged subgroups. Two of three PCa treatment trials included one disadvantaged group.
Attention to SDH in PtDA development
We conducted a secondary search for additional qualitative and quantitative reports about the development of the PtDAs and identified clear evidence of attention to social disadvantage in the development of the PtDAs evaluated in 17 RCTs. This evidence (k = 12) plus the articles reporting the results of the included RCTs are referenced in Table 2. Nearly all of the developmental work used theoretical models to guide PtDA development, most frequently the Ottawa Decision Support Framework (ODSF). Several studies employed two or more PtDA formats and/or delivery modes, including coaching or counseling sessions, or utilized non-physician members of the health care team to deliver or assist with delivery of the PtDA. Racial/ethnic minorities and individuals with limited education and/or low health literacy were the subgroups most frequently targeted in PtDA development.
Development strategies included focus groups and/or key informant interviews, cognitive interviews or questionnaires to guide content modification, concept mapping, and expert assessments of plain language readability and/or cultural appropriateness. Several PtDAs featured characters and/or narrators similar to the race/ethnicity or social status of target subgroups. Two studies adapted the PtDA to the users’ preferred language (Spanish). The development process for several of the PtDAs employed usability and/or feasibility testing, such as acceptability or comprehensibility assessments, to explore and modify design features (e.g., language, narration, video, animation, touch screen formats) that would make the material more accessible for participants with limited health literacy, numeracy or computer/internet skills [50, 51, 56, 58–60, 73, 74, 77, 78, 81–83]. As part of the development process, several studies conducted activities associated with tracking estimated and/or actual use of the PtDA (demand testing). The average time patients spent using the PtDAs ranged from 10 to 207 min.
Attention to SDH in PtDA evaluation
We identified 14 studies with clear evidence of attention to social disadvantage in evaluating the PtDA, according to our stated criteria (Table 2). Most were included based on the authors’ a priori objectives to evaluate the effectiveness of PtDAs in specific populations (e.g., Black men, Hispanic men, low-literacy populations), but we also found articles that stratified analyses by or discussed low versus high education/income, low versus adequate literacy or race. Three studies also utilized a low-literacy version of O’Connor’s Decisional Conflict Scale (DCS) to reduce measurement error in limited literacy populations. Although several studies classified participants as “low-literacy”, only one study measured literacy using standard criteria (the Rapid Estimate of Adult Literacy in Medicine, REALM).
Discussion
This review assessed the extent to which disadvantaged subgroups have been included in US RCTs of cancer-related PtDAs and summarized the strategies employed in developing and evaluating PtDAs that address one or more SDH. Our findings revealed that nearly 60 % of studies were inclusive of disadvantaged participants based on at least one criterion associated with health inequities [36], most frequently gender, race/ethnicity (required reporting statistics for research projects funded by the US government), and education. Inclusion of subgroups disadvantaged by their insurance status, LEP, low-literacy or non US-nativity was far less common. The processes used to develop the PtDAs, as well as the features of the tools that promoted or hindered decision making in disadvantaged subgroups, were not well-described in most studies. Our purpose was to glean key lessons from those studies in which SDH were prominent in the development or evaluation of cancer-related PtDAs and to highlight the need for more attention to the comprehensive range of social factors that may influence decision making among disadvantaged patients. Below, we outline our observations and present opportunities for future research.
Inclusion of disadvantaged subgroups
Although diversity in the US is represented in a majority of the studies reviewed, disadvantaged subgroups were not included in some PtDA trials in numbers proportional to their burden of disease for the decision making contexts being targeted. A key example is our finding that disadvantaged subgroups were not included in most studies that focused on BRCA genetic testing or BCa prevention in high risk populations. These represent important targets for PtDA studies given current research that demonstrates significant disparities experienced by Black and Hispanic women in awareness and use of BRCA testing/genetic counseling [84–87]. Another important example is our finding that few cancer treatment trials were inclusive of Black men and women, despite their disproportionately high rates of incidence and mortality for PCa or mortality for BCa, respectively [4].
These findings stimulate important questions about how to establish an inventory of PtDAs that is most relevant to disadvantaged social groups and their clinicians. For example, we identified no PtDAs evaluated in US RCTs that addressed the decision to give the hotly debated human papillomavirus (HPV) vaccination [88, 89] to adolescents and young women to prevent cervical cancer. Cervical cancer morbidity and mortality rates are highest among Hispanic, Black, American Indian and Alaskan Native women in the US [4], while disparities in awareness of and knowledge about HPV persist among these populations [90]. Lung cancer morbidity and mortality are highest among Black men in the US [4], and lung cancer screening, in combination with smoking cessation interventions, is now recommended for high risk populations [91]. PtDAs to support prevention and screening decisions for these conditions may be useful to disadvantaged populations. PtDAs for cancer surveillance among disadvantaged populations is also an important but understudied area. One study of Hispanic and immigrant men found that not all patients fully recognized that watchful waiting, or opting not to be screened, can also be responsible choices related to PCa [63]. Other racial/ethnic differences in treatments discussed, preferred and received for localized PCa (surgery, radiation therapy, and active surveillance) have also been reported [92].
Attention to SDH in PtDA development and evaluation
Education and health literacy
Education and health literacy were among the most widely addressed disadvantages in the studies reviewed. Most studies that focused on addressing limited education in PtDA development targeted 7th to 8th grade readability as the threshold for ease-of-use. The studies that identified low-literacy patients as an intended audience for the PtDA did not consistently report how this construct was defined and/or measured. Focus group and individual interviews were frequently used to determine whether the PtDAs were accessible to limited education/low literacy patients. Myers et al., for example, engaged a literacy expert from a community-based health promotion organization in Philadelphia to facilitate this process. The expert conducted face-to-face interviews to ascertain whether Black men recognized the purpose of a PCa screening information booklet and understood its related language, terms and concepts [68]. Through this process, the authors learned that the medical terms should be simplified and more pictures should be included. They found that an increased emphasis on the issue of PCa screening as a decision to be made in partnership with a physician was also needed – many men thought the purpose of the PtDA was to promote PCa screening rather than to support decision making.
Several studies found that the acceptability and demand for PtDAs varied between advantaged and disadvantaged subgroups based on PtDA format and other design attributes. Volk et al. found that low compared to high literacy users were willing to spend more time viewing the PtDA [77]. Marcus et al. [51] and Taylor et al. [74] found that participants were more likely to use print-based versus web-based PtDAs (web-based PtDA use was highest among White participants who reported frequent internet use overall) [74]. Other studies acknowledged that disadvantaged patients needed more help utilizing PtDAs and facilitated delivery of the intervention using non-physician members of the healthcare team. Like the use of patient navigators to provide psychosocial and logistical support for disadvantaged cancer patients [93, 94], the use of health coaches to guide decision making [95] for disadvantaged populations may also be needed. Appropriate consideration should be given to both PtDA design and the other factors that may influence disadvantaged participants’ exposure to the intervention. By engaging patients early in the development process, the most appropriate PtDA formats and delivery strategies can be identified.
The influence of PtDAs on facilitating SDM via improved patient-provider communication, or even patients’ interest in participating in SDM, was discussed rarely in identified studies. By definition, being health literate includes being able to act on health information [96]. Several factors may influence whether patients engage in SDM with their physicians (some are highlighted below as part of our discussion regarding social norms and attitudes). Although LEP, the use of translators in explaining treatment options, and the lack of available educational materials in a patient’s preferred language, for example, are factors known to influence patients’ reliance on physician recommendations over more active participation in decision making [29], PtDAs designed for disadvantaged populations may play a role in improving these aspects of patient-provider interactions. More information is needed to better understand this issue, particularly in light of a recent systematic review finding that SDM interventions were more beneficial to disadvantaged, compared to advantaged subgroups [97].
Another notable finding is the limited consideration of numeracy in relation to health literacy. Two studies described addressing numeracy as part of the PtDA development process [59, 83]; one study measured and reported participants’ baseline numeracy skills [74]. As reported in a recent study [98], numeracy and health literacy have largely been treated as separate concepts in the literature despite indications that PtDAs designed for patients with different levels of health literacy may not support the needs of patients with disparate numeracy skills. This is another important area for future research.
Race, ethnicity and related social norms and attitudes
Matching the race/ethnicity of the actors/models to that of the intended audience is a commonly used strategy in tailoring PtDAs for disadvantaged social groups. Jibaja-Weiss et al. produced six versions of PtDA soap opera segments so that users were able to receive information about BCa treatment from female characters similar to them in race/ethnicity, preferred language and age [50]. Volk and colleagues employed a multiethnic, blue-collar cast and tailored the main character to the viewer through a series of questions completed upon entering the program [77]. The program included a values clarification, social-matching exercise that asked the viewer to “pick who is most like you” with regard to his feelings about PCa screening. The strategy of promoting racial/ethnic concordance to improve processes of care and outcomes for minorities has been extensively studied, with mixed results [99, 100]. The underlying assumption is that people are able to better identify with others who look like them or share similar language or culture. In turn, this may improve their interactions (e.g., greater satisfaction, trust, willingness to ask questions on the part of patients; less uncertainty, bias, stereotyping on the part of providers; better patient-provider communication overall). However, the influence of patient-provider race-concordance on health care interactions varies within and across race/ethnicity based on related SDH (e.g., income, citizenship status, language) [99–101]. These differences may be present in other norms and attitudes related to race/ethnicity and culture (e.g. attitudes about surgery; opinions of spouse, family and friends; concerns about body image) [102]. Reflecting the racial/ethnic diversity of target audiences in PtDAs is most certainly an important patient engagement strategy. However, it is also important to recognize that heterogeneity exists within racial/ethnic groups. More research is needed to better understand whether PtDAs can help to improve decisional outcomes that may be influenced by race/ethnicity and related social norms and attitudes.
Economic instability
Although few studies indicated that patients were asked about economic issues during PtDA development, the need to disentangle the various ways in which economic instability may influence patient decision making is an important area for future research. As noted, Jibaja-Weiss et al. [50] found that PtDA exposure was associated with increased uptake of mastectomy, rather than breast conserving therapy, among low-literacy Black and Hispanic women with early stage BCa. This finding differed from published studies linking PtDAs with the choice to undergo more conservative surgical options [20, 103]. The reason for this difference remains unclear, but other studies of low income women in the US suggest that economic factors (e.g. uninsured or Medicaid status, insurance co-payments, out-of-pocket expenses, concerns about missed work) influence their treatment choices [29, 104, 105].
Other SDH
Multiple factors affect the health and health care received by disadvantaged populations, including many SDH that were not addressed in this research. In some decision contexts, for example, it may be important to use PtDAs to help patients consider how attributes of their neighborhoods and built environments (e.g., housing, transportation, public safety, other public services, environmental noise and pollutants) figure into their decision making. We do not mean to suggest that all SDH can be practically addressed in every PtDA for disadvantaged patients. However, if the purpose of PtDAs is to support high quality SDM in the cancer care continuum, we believe that addressing only education and/or health literacy when developing tools for patients who face multiple social disadvantages falls short of that goal.
Limitations and strengths
This review has several potential limitations. First, despite our efforts to identify all US RCTs of cancer-related PtDAs, it is possible that our search missed relevant RCTs that fit our inclusion criteria. We attempted to minimize this possibility by comparing our list of eligible studies with those in recently published systematic reviews of PtDAs using similar search and eligibility criteria [20, 106, 107]. By limiting our review to RCTs, we may have missed studies that were more inclusive of disadvantaged subgroups or that employed PtDA development and evaluation strategies other than those described in the articles identified for this review. Yet, given the large number of RCTs of cancer-related PtDAs that have been conducted [20], and given the importance of the Cochrane review, limiting this review to the Cochrane criteria of RCTs seems appropriate. We did include citing and cited publications related to the RCTs that cast a larger net for information on PtDA development. We did not, however, contact the study authors for additional information, which may have potentially caused us to miss relevant information not consistently reported. We attempted to address this problem by focusing our data collection on socioeconomic and cultural constructs most widely and consistently reported in the literature. We acknowledge that other constructs related to social disadvantage, such as social capital, place of residence, physical and mental disability, sexual orientation or religion, are important characteristics that should be more consistently reported and evaluated in future PtDA research. We were unable to identify a cancer-related PtDA trial that addressed social disadvantages related to these characteristics. Limitations present in all systematic reviews, including publication bias and potential researcher bias in establishing study inclusion criteria, may also have been present in this study.
Conclusions
The number of cancer-related PtDAs will continue to proliferate in the coming years as such tools are advocated as a strategy to facilitate patient engagement in SDM. While it may be tempting to evaluate or utilize PtDAs developed without attention to SDH and argue that the tools are effective based on improvements in knowledge measured broadly or other decisional outcomes, we believe such an approach misses many important considerations in providing decision support that may ultimately undermine quality decision-making processes. This review addresses a gap in the literature by summarizing the extent to which cancer-related PtDAs have been used by disadvantaged patients in the US and by highlighting the strategies and lessons learned in the development and evaluation of the handful of PtDAs specifically tailored for disadvantaged social groups. Such information may be used to guide clinicians as they attempt to identify among a large and expanding inventory of available PtDAs those tools that that were designed to support the decision making needs of their socially disadvantaged patients. It may also be used to inform the development of new or complementary interventions to support high quality SDM processes among disadvantaged populations.
Abbreviations
BCa, breast cancer; BCT, breast-conserving therapy; BRCA1/BRCA2, breast cancer genes 1 and 2; COL, colonoscopy; CRC, colorectal cancer; DCBE, double contrast barium enema; DCS, decisional conflict scale; DRE, digital rectal exam; FOBT, fecal occult blood testing; FS, flexible sigmoidoscopy; GED, general education development certificate; HS, high school diploma; HPV, human papillomavirus; LEP, limited English proficiency; MSI, microsatellite instability; MW, Midwest; NA, not applicable; NE, northeast; ODSF, Ottawa Decision Support Framework; PCa, prostate cancer; PSA, prostate-specific antigen; PtDA, patient decision aid; RCT, randomized controlled trial; REALM, rapid estimate of adult literacy in medicine; SDM, shared decision making; SDH, social determinants of health; US, United States.
References
Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98.
US Department of Health and Human Services. Healthy People 2020: An Opportunity to Address Societal Determinants of Health in the United States. 2010. http://www.healthypeople.gov/2010/hp2020/advisory/societaldeterminantshealth.htm. Accessed 30 Nov 2015
Centers for Disease Control and Prevention. Social Determinants of Health: Know What Affects Health. 2015. http://www.cdc.gov/socialdeterminants/index.htm. Accessed 30 Nov 2015.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Walker GV, Grant SR, Guadagnolo BA, Hoffman KE, Smith BD, Koshy M, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32:3118–25.
Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–13.
Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57.
Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–23.
National Cancer Institute. About Cancer Health Disparities. http://www.cancer.gov/about-nci/organization/crchd/about-health-disparities. Accessed 25 Apr 2016.
Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516–23.
Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–9.
DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–66.
Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62:283–98.
Palmer NRA, Kent EE, Forsythe LP, Arora NK, Rowland JH, Aziz NM, et al. Racial and ethnic disparities in patient-provider communication, quality-of-care ratings, and patient activation among long-term cancer survivors. J Clin Oncol. 2014;32:4087–94.
Gorin SS, Badr H, Krebs P, Das IP. Multilevel interventions and racial/ethnic health disparities. J Natl Cancer Inst Monogr. 2012;44:100–11.
Yano EM, Green LW, Glanz K, Ayanian JZ, Mittman BS, Chollette V, et al. Implementation and spread of interventions into the multilevel context of routine practice and policy: implications for the cancer care continuum. J Natl Cancer Inst Monogr. 2012;44:86–99.
Braveman PA, Egerter SA, Mockenhaupt RE. Broadening the focus: the need to address the social determinants of health. Am J Prev Med. 2011. doi:10.1016/j.amepre.2010.10.002.
Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014;129 Suppl 2:19–31.
Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making. 2010;30:701–11.
Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014. doi:10.1002/14651858.CD001431.pub4.
Volk RJ, Llewellyn Thomas H, Stacey D, Elwyn G. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S1.
Levit L, Balogh E, Nass S, Ganz PA, editors. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington DC: National Academies Press; 2013.
Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, Van Der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S2.
McCaffery KJ, Holmes-Rovner M, Smith SK, Rovner D, Nutbeam D, Clayman ML, et al. Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S10.
Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4). doi:10.1111/j.1468-0009.2005.00428.x.
Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. 2006;27:167–94.
Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21:60–76.
Woolf SH, Braveman P. Where health disparities begin: the role of social and economic determinants--and why current policies may make matters worse. Health Aff (Millwood). 2011;30:1852–9.
Campesino M, Koithan M, Ruiz E, Glover JU, Juarez G, Choi M, et al. Surgical treatment differences among Latina and African American breast cancer survivors. Oncol Nurs Forum. 2012;39:e324–31.
Marcus EN, Koru-Sengul T, Miao F, Yepes M, Sanders L. How do breast imaging centers communicate results to women with limited English proficiency and other barriers to care? J Immigr Minor Health. 2013;16:401–8.
Sentell T, Braun KL, Davis J, Davis T. Colorectal cancer screening: low health literacy and limited English proficiency among Asians and Whites in California. J Health Commun. 2013;18:242–55.
Bailey SC, O’Conor R, Bojarski EA, Mullen R, Patzer RE, Vicencio D, et al. Literacy disparities in patient access and health-related use of Internet and mobile technologies. Health Expect. 2015;18:3079–87.
Gordon NP, Hornbrook MC. Differences in access to and preferences for using patient portals and other eHealth technologies based on race, ethnicity, and age: a database and survey study of seniors in a large health plan. J Med Internet Res. 2016;18:e50.
Smedley BD, Stith AY. Nelson AR (eds.): Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003.
Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47.
Welch V, Petticrew M, Tugwell P, Moher D, O’Neill J, Waters E, et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012. doi:10.1371/journal.pmed.1001333.
Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452–7.
Green MJ, Biesecker BB, McInerney AM, Mauger D, Fost N. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet. 2001;103:16–23.
Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA. 2004;292:442–52.
Lerman C, Biesecker B, Benkendorf JL, Kerner J, Gomez-Caminero A, Hughes C, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Natl Cancer Inst. 1997;89:148–57.
Miller SM, Fleisher L, Roussi P, Buzaglo JS, Schnoll R, Slater E, et al. Facilitating informed decision making about breast cancer risk and genetic counseling among women calling the NCI’s Cancer Information Service. J Health Commun. 2005;10 Suppl 1:119–36.
Schwartz MD, Benkendorf J, Lerman C, Isaacs C, Ryan-Robertson A, Johnson L. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer. 2001;92:932–40.
Fagerlin A, Dillard AJ, Smith DM, Zikmund-Fisher BJ, Pitsch R, McClure JB, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127:681–8.
Banegas MP, McClure JB, Barlow WE, Ubel PA, Smith DM, Zikmund-Fisher BJ, et al. Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer. Patient Educ Couns. 2013;91:364–71.
Korfage IJ, Fuhrel-Forbis A, Ubel PA, Zikmund-Fisher BJ, Greene SM, McClure JB, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Res. 2013;15:R74.
Ozanne EM, Annis C, Adduci K, Showstack J, Esserman L. Pilot trial of a computerized decision aid for breast cancer prevention. Breast J. 2007;13:147–54.
Schwartz MD, Valdimarsdottir HB, DeMarco TA, Peshkin BN, Lawrence W, Rispoli J, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11–9.
Hooker GW, Leventhal KG, DeMarco T, Peshkin BN, Finch C, Wahl E, et al. Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial. Med Decis Making. 2011;31:412–21.
Heller L, Parker PA, Youssef A, Miller MJ. Interactive digital education aid in breast reconstruction. Plast Reconstr Surg. 2008;122:717–24.
Jibaja-Weiss ML, Volk RJ, Granchi TS, Neff NE, Robinson EK, Spann SJ, et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Educ Couns. 2011;84:41–8.
Marcus AC, Diefenbach MA, Stanton AL, Miller SM, Fleisher L, Raich PC, et al. Cancer patient and survivor research from the cancer information service research consortium: a preview of three large randomized trials and initial lessons learned. J Health Commun. 2013;18:543–62.
Street Jr RL, Voigt B, Geyer Jr C, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer. 1995;76:2275–85.
Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22:125–39.
Manne SL, Meropol NJ, Weinberg DS, Vig H, Ali-Khan Catts Z, Manning C, et al. Facilitating informed decisions regarding microsatellite instability testing among high-risk individuals diagnosed with colorectal cancer. J Clin Oncol. 2010;28:1366–72.
Hall MJ, Manne SL, Winkel G, Chung DS, Weinberg DS, Meropol NJ. Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing. Cancer Epidemiol Biomarkers Prev. 2011;20:249–54.
Miller Jr DP, Spangler JG, Case LD, Goff Jr DC, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40:608–15.
Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133:761–9.
Ruffin 4th MT, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45:267–73.
Schroy 3rd PC, Emmons K, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31:93–107.
Schroy 3rd PC, Emmons KM, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43:573–83.
Wolf AM, Schorling JB. Does informed consent alter elderly patients’ preferences for colorectal cancer screening? Results of a randomized trial. J Gen Intern Med. 2000;15:24–30.
Allen JD, Othus MK, Hart Jr A, Tom L, Li Y, Berry D, et al. A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the take the wheel trial. Cancer Epidemiol Biomarkers Prev. 2010;19:2172–86.
Chan EC, McFall SL, Byrd TL, Mullen PD, Volk RJ, Ureda J, et al. A community-based intervention to promote informed decision making for prostate cancer screening among Hispanic American men changed knowledge and role preferences: a cluster RCT. Patient Educ Couns. 2011;84:e44–51.
Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. J Gen Intern Med. 2003;18:781–7.
Frosch DL, Bhatnagar V, Tally S, Hamori CJ, Kaplan RM. Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Arch Intern Med. 2008;168:363–9.
Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5:112–9.
Lepore SJ, Wolf RL, Basch CE, Godfrey M, McGinty E, Shmukler C, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Ann Behav Med. 2012;44:320–30.
Myers RE, Daskalakis C, Cocroft J, Kunkel EJ, Delmoor E, Liberatore M, et al. Preparing African-American men in community primary care practices to decide whether or not to have prostate cancer screening. J Natl Med Assoc. 2005;97:1143–54.
Myers RE, Daskalakis C, Kunkel EJ, Cocroft JR, Riggio JM, Capkin M, et al. Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counseling. Patient Educ Couns. 2011;83:240–6.
Partin MR, Nelson D, Radosevich D, Nugent S, Flood AB, Dillon N, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19:835–42.
Rubel SK, Miller JW, Stephens RL, Xu Y, Scholl LE, Holden EW, et al. Testing the effects of a decision aid for prostate cancer screening. J Health Commun. 2010;15:307–21.
Schapira MM, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract. 2000;49:418–24.
Sheridan SL, Golin C, Bunton A, Lykes JB, Schwartz B, McCormack L, et al. Shared decision making for prostate cancer screening: the results of a combined analysis of two practice-based randomized controlled trials. BMC Med Inform Decis Mak. 2012;12:130.
Taylor KL, Williams RM, Davis K, Luta G, Penek S, Barry S, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013;173:1704–12.
Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8:333–40.
Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1:22–8.
Volk RJ, Jibaja-Weiss ML, Hawley ST, Kneuper S, Spann SJ, Miles BJ, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73:482–9.
Williams RM, Davis KM, Luta G, Edmond SN, Dorfman CS, Schwartz MD, et al. Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ Couns. 2013;91:329–36.
Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996;156:1333–6.
Barry MJ, Cherkin DC, YuChiao C, Fowler F, Skates S. A randomized trial of a multimedia shared decision-making program for men facing a treatment decision for benign prostatic hyperplasia. Disease Manage Clin Outcomes. 1997;1:5–14.
Berry DL, Wang Q, Halpenny B, Hong F. Decision preparation, satisfaction and regret in a multi-center sample of men with newly diagnosed localized prostate cancer. Patient Educ Couns. 2012;88:262–7.
Bosco JL, Halpenny B, Berry DL. Personal preferences and discordant prostate cancer treatment choice in an intervention trial of men newly diagnosed with localized prostate cancer. Health Qual Life Outcomes. 2012;10:123.
Berry DL, Halpenny B, Hong F, Wolpin S, Lober WB, Russell KJ, et al. The Personal Patient Profile-Prostate decision support for men with localized prostate cancer: a multi-center randomized trial. Urol Oncol. 2013;31:1012–21.
Cragun D, Bonner D, Kim J, Akbari MR, Narod SA, Gomez-Fuego A, et al. Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast Cancer Res Treat. 2015;151:169–76.
Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med. 2011;13:349–55.
Mai PL, Vadaparampil ST, Breen N, McNeel TS, Wideroff L, Graubard BI. Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 National Health Interview Surveys. Am J Prev Med. 2014;46:440–8.
Pagan JA, Su D, Li L, Armstrong K, Asch DA. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37:524–30.
Gollust SE, Attanasio L, Dempsey A, Benson AM, Fowler EF. Political and news media factors shaping public awareness of the HPV vaccine. Womens Health Issues. 2013;23:e143–51.
Gollust SE, Dempsey AF, Lantz PM, Ubel PA, Fowler EF. Controversy undermines support for state mandates on the human papillomavirus vaccine. Health Aff (Millwood). 2010;29:2041–6.
Blake KD, Ottenbacher AJ, Finney Rutten LJ, Grady MA, Kobrin SC, Jacobson RM, et al. Predictors of human papillomavirus awareness and knowledge in 2013: gaps and opportunities for targeted communication strategies. Am J Prev Med. 2015;48:402–10.
Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8.
Hosain GM, Sanderson M, Du XL, Chan W, Strom SS. Racial/ethnic differences in treatment discussed, preferred, and received for prostate cancer in a tri-ethnic population. Am J Mens Health. 2012;6:249–57.
Paskett ED, Harrop JP, Wells KJ. Patient Navigation: An Update on the State of the Science. CA Cancer J Clin. 2011;61:237–49.
Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The Role of Patient Navigators in Eliminating Health Disparities. Cancer. 2011;117 Suppl 15:3543–52.
Stacey D, Kryworuchko J, Belkora J, Davison BJ, Durand MA, Eden KB, et al. Coaching and guidance with patient decision aids: A review of theoretical and empirical evidence. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S11.
Sørensen K, Van den Broucke S, Fullam J, Doyle G, Pelikan J, Slonska Z, et al. Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health. 2012;12:80.
Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9:e94670.
Malloy-Weir LJ, Schwartz L, Yost J, McKibbon KA. Empirical relationships between numeracy and treatment decision making: A scoping review of the literature. Patient Educ Couns. 2015. doi:10.1016/j.pec.2015.10.002.
Meghani SH, Brooks JM, Gipson-Jones T, Waite R, Whitfield-Harris L, Deatrick JA. Patient–provider race-concordance: does it matter in improving minority patients’ health outcomes? Ethn Health. 2009;14:107–30.
Blanchard J, Nayar S, Lurie N. Patient-provider and patient-staff racial concordance and perceptions of mistreatment in the health care setting. J Gen Intern Med. 2007;22:1184–9.
Campesino M, Saenz DS, Choi M, Krouse RS. Perceived discrimination and ethnic identity among breast cancer survivors. Oncol Nurs Forum. 2012;39:e91–e100.
Hawley ST, Griggs JJ, Hamilton AS, Graff JJ, Janz NK, Morrow M, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101:1337–47.
Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25:1067–73.
Blinder VS, Patil S, Thind A, Diamant A, Hudis CA, Basch E, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118:1664–74.
Jagsi R, Pottow JA, Griffith KA, Bradley C, Hamilton AS, Graff J, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32:1269–76.
Jimbo M, Rana GK, Hawley S, Holmes-Rovner M, Kelly-Blake K, Nease Jr DE, et al. What is lacking in current decision aids on cancer screening? CA Cancer J Clin. 2013;63:193–214.
Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33:428–34.
Jibaja-Weiss ML, Volk RJ, Friedman LC, Granchi TS, Neff NE, Spann SJ, et al. Preliminary testing of a just-in-time, user-defined values clarification exercise to aid lower literate women in making informed breast cancer treatment decisions. Health Expect. 2006;9:218–31.
Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14:432–7.
Kim J, Whitney A, Hayter S, Lewis C, Campbell M, Sutherland L, et al. Development and initial testing of a computer-based patient decision aid to promote colorectal cancer screening for primary care practice. BMC Med Inform Decis Mak. 2005;5:36.
Fetters MD, Ivankova NV, Ruffin MT, Creswell JW, Power D. Developing a web site in primary care. Fam Med. 2004;36:651–9.
Weinrich SP, Seger RE, Rao GS, Chan EC, Hamm RM, Godley PA, et al. A decision aid for teaching limitations of prostate cancer screening. J Natl Black Nurses Assoc. 2008;19:1–11.
Liberatore MJ, Myers RE, Nydick RL, Steinberg M, Brown ER, Gay R, et al. Decision counseling for men considering prostate cancer screening. Comput Oper Res. 2003;30:1421–34.
Dorfman CS, Williams RM, Kassan EC, Red SN, Dawson DL, Tuong W, et al. The development of a web- and a print-based decision aid for prostate cancer screening. BMC Med Inform Decis Mak. 2010;10:12.
Taylor KL, Turner RO, Davis 3rd JL, Johnson L, Schwartz MD, Kerner J, et al. Improving knowledge of the prostate cancer screening dilemma among African American men: an academic-community partnership in Washington, DC. Public Health Rep. 2001;116:590–8.
Kassan EC, Williams RM, Kelly SP, Barry SA, Penek S, Fishman MB, et al. Men’s use of an Internet-based decision aid for prostate cancer screening. J Health Commun. 2012;17:677–97.
Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21:93–100.
Berry DL, Halpenny B, Wolpin S, Davison BJ, Ellis WJ, Lober WB, et al. Development and evaluation of the personal patient profile-prostate (P3P), a web-based decision support system for men newly diagnosed with localized prostate cancer. J Med Internet Res. 2010;12:e67.
Diefenbach MA, Butz BP. A multimedia interactive education system for prostate cancer patients: development and preliminary evaluation. J Med Internet Res. 2004;6:e3.
Funding
There was no direct funding source for this study (PROSPERO: CRD42014014470). The authors were supported in part by the W.K. Kellogg Foundation (P0117943) and the National Cancer Institute (NCI) at the National Institutes of Health (2 R25 CA57712 and R21CA132669). Neither organization had a role in designing, conducting or synthesizing the results of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the W.K. Kellogg Foundation, the National Cancer Institutes or the National Institutes of Health.
Availability of data and materials
The data supporting the conclusions of this article are included within the article in Tables 1 and 2.
Authors’ contributions
KRE is the lead author of the paper: she co-led conceptualization/design of the study, was one of three primary reviewers of the literature, and had primary responsibility for drafting the manuscript. RJV is the senior author of the paper: he developed the initial idea, co-led conceptualization/design of the study, and contributed to drafting the manuscript. PMD assisted with conceptualization/design of the study and contributed to drafting the manuscript. GRK was one of three primary reviewers of the literature and contributed to drafting the manuscript. NMD was one of three primary reviewers of the literature. All authors analyzed and interpreted the data, reviewed it critically for intellectual content, and provided their final approval.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Electronic search strategy used for PubMed. (PDF 8 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Enard, K.R., Dolan Mullen, P., Kamath, G.R. et al. Are cancer-related decision aids appropriate for socially disadvantaged patients? A systematic review of US randomized controlled trials. BMC Med Inform Decis Mak 16, 64 (2016). https://doi.org/10.1186/s12911-016-0303-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-016-0303-6