Abstract
Background
Literature on issues relating to comprehension during the process of obtaining informed consent (IC) has largely focused on the challenges potential participants can face in understanding the IC documents, and the strategies used to enhance comprehension of those documents. In this review, we set out to describe the factors that have an impact on comprehension and the strategies used to enhance the IC process in sub-Saharan African countries.
Methods
From November 2021 to January 2022, we conducted a literature search using a PRISMA tool. We searched electronic databases (PubMed, EMBASE, EBSCOHOST) to identify relevant peer reviewed studies. We then reviewed the references of these articles to find additional literature that might have been missed through the initial search. We were particularly interested in full text articles in English that focused on the IC process in SSA published between 2006 and 2020. We included systematic reviews, and studies from Western and Asian countries that included data about SSA. We excluded articles that focused on medical interventions and studies that did not require IC.
Results
Out of the 50 studies included most were multi-country (n = 13) followed by single country studies in South Africa (n = 12); Kenya, Tanzania, Uganda (n = 5) each; Gambia, Ghana and Nigeria (n = 2)each ; and one each for Botswana, Malawi, Mali, Mozambique. We identified three areas of focus: (1) socio-cultural factors affecting IC; (2) gaps in the ethical and legal frameworks guiding the IC process; and (3) strategies used to improve participants’ understanding of IC.
Conclusion
Our review showed wide recognition that the process of achieving IC in SSA is inherently challenging, and there are limitations in the strategies aimed at improving comprehension in IC. We suggest that there is a need for greater flexibility and negotiation with communities to ensure that the approach to IC is suited to the diverse socio-cultural contexts. We propose moving beyond the literal translations and technical language to understanding IC comprehension from the participants’ perspectives and the researchers’ views, while examining contextual factors that impact the IC process.
Similar content being viewed by others
Background
The achievement of valid IC is universally recognized as central to the ethical conduct of scientific research [1,2,3]. However, ensuring valid consent is complex for a number of reasons, including, balancing the differing interests of the participants, researchers and sponsors in a single document [1,2,3], facilitating informed choices by participants [1,2,3], meeting ethical and legal obligations and applying them in local contexts [4] and addressing the impact of real world settings.
These issues can be clustered around two key problems, disjuncture between the national and international legal-ethical stipulations [4], and the limited guidance on obtaining informed consent in social and cultural contexts where decision-making is not solely in the hands of an individua [1, 4,5,6]. The use of poorly designed IC documents, misunderstanding in local languages and terms used in IC documents, and low literacy levels result in poor understanding during the IC process [7, 8]. Although poorly designed IC materials is a global problem, low-and middle income countries, (LMIC) including sub-Saharan (SSA) countries face unique challenges. These range from how to address beliefs about health and decision making to views about autonomy, and low functional literacy levels in English. The literature increasingly shows that alternative approaches are needed to address this issue in LMIC. Some work has been done in this regard with attention being placed on the translation of IC documents into local languages, the development of tools designed to enhance participants’ understanding [1, 4], and tailoring the IC information to suit local contexts [5].
Against this background we set out to explore topics focusing on comprehension of the IC process across SSA countries. We also describe and highlight the challenges of strategies used to enhance the process of understanding IC in SSA. Although there are many ways in which consent processes can fail to achieve valid consent, our focus in this review was on comprehension. We propose moving beyond the literal translations and technical language to understanding IC comprehension from the participants’ perspectives and the researchers’ views, while examining contextual factors that impact the IC process.
Objectives
-
1.
Identify studies focusing on exploring obstacles to comprehension and relevant strategies to enhance the understanding of IC documents in SSA, paying particular attention to language and translations.
-
2.
Describe strategies to enhance comprehension during the informed consent process in SSA countries with diverse local settings.
-
3.
Suggest perspectives for researchers to consider to enhance comprehension of the informed consent process in diverse settings in SSA countries.
Methods
Study selection
From November 2021 to January 2022, we conducted a literature search using a PRISMA tool.” We were interested in articles that focused on the IC process in SSA. In the first stage, we searched the electronic databases (PubMed, EMBASE, EBSCOHOST) to identify peer reviewed studies. We then manually reviewed the references of these articles for additional relevant literature that might have been missed through the initial search. We conducted searches using a combination of the following terms: ‘informed consent’ or ‘comprehension’ or’ health research’, or “language” or translation’ or ‘sub-Saharan Africa’. Furthermore, we searched various individual SSA countries to ensure that we included articles which we may have missed when using the term “sub-Saharan Africa” (Table 1: Search strategy and selection criteria).
Inclusion criteria
We included articles focusing on the process of informed consent in (1) multidisciplinary fields including social sciences, medical research and bio banking; (2) studies focusing on broader environmental issues that impact the IC process such as the socio-cultural factors and the ethical and legal frameworks that govern the IC process; and (3) studies focusing on strategies aimed at improving the comprehension of IC process including issues around translations, language and its meaning, and IC comprehension assessments. We also included systematic reviews, and studies from Western, and Asian countries that included data about SSA countries. We included full text English articles published between 2006 and 2020. This period was critical because of growth in clinical research especially HIV and AIDS, as well as increase in global health research which highlighted differences and anomalies during the informed consent process between global south and global north.
Exclusion criteria
We excluded articles that focused on medical interventions and studies that did not require IC. We also excluded studies that were not conducted in SSA.
Study selection
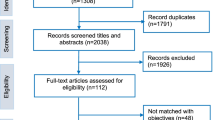
We use the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) tool to guide the selection process. The initial search resulted in a total of 66,310 articles from the electronic search, and (n = 9) from directed search. Records initially identified through the search were screened to exclude studies that were unrelated to the research topic. After these irrelevant studies (n = 66,122) were excluded, the titles and abstracts of 189 articles were screened by authors BN, JS and MP to determine applicability according to inclusion and exclusion criteria. A total of 72 articles were rejected after the titles and abstracts were reviewed. The studies were screened independently, and later as a team.
Differences and discrepancies were resolved by discussion until consensus was reached. Following this level of screening, full text of 117 articles were screened, and 67 articles were excluded using the same process. A total of 50 articles met the criteria and were included for review, Fig. 1: Flow chart of the search process. Of the 50 studies, 44 were primary studies and 6, systematic review studies.
We used the framework thematic analysis approach to develop and organize the themes [9]. Using a matrix (Word document) BN, JS and MP categorised articles that met the criteria into 3 main focus areas, (1) gaps in the ethical and legal frameworks guiding informed consent process); (2) socio-cultural factors; and (3) strategies used to improve participants’ understanding of the IC. (Supplementary Table 1: Thematic analysis of the studies reviewed). Studies that addressed more than one theme were clustered into one category using a consensus approach.
Out of the 50 studies included in the review (Table 2, Summary of reviewed studies) most were multi-country studies (n = 13) followed by single country studies in South Africa (n = 12); Kenya, Tanzania, Uganda (n = 5) respectively; Gambia, Ghana and Nigeria (n = 2) respectively; and Botswana, Malawi, Mali, and Mozambique one each. All studies reviewed show that the IC process is fraught with challenges and complexities across SSA. Most of the studies focused on adults, with two focusing on IC in paediatric research [10, 11]. We identified three areas which impact on the IC process, namely, (1) socio-cultural factors; gaps in the ethical and legal frameworks guiding the IC process); (2) and (3) strategies used to improve participants’ understanding of the IC.
Social and cultural contexts
We found a recognition on the interplay between socio-cultural context and the IC process [9,10,11,12,13,14,15]. Nevertheless, Marshall [13] and Reynolds et al. [5] show that aligning legal-ethical principles and socio-cultural realities remain a challenge. Key issues include challenges to the way the ethical guidance approaches IC. Krogstad et al. [16], suggest that international guidelines place too much emphasis on the importance of the individual in the consent process. Therefore, regulatory IC requirements could violate the core individual ethics principles of participants. Following this type of reasoning in many SSA settings it is submitted that the IC process needs to be multi-layered involving family and community members [17,18,19,20,21]. The review by Krogstad [16], and a study in Tanzania by Palmeirim [22], showed that proper consideration of a social context approach to consent meant communities in rural settings place high value on oral interactions. Verbal consent is commonly obtained often in the presence of a literate witness who is able to read available consent documents. However, Colom [10] notes that this raised concerns that the witnesses may impose their views on the consenting participant or be selective about the information offered rather than encouraging dialogue and acting as a safeguard. Ssali et al. [23] in Uganda, argued that obtaining a volunteer’s signature or thumbprint on a consent form raised issues of trust and did not necessarily enhance IC comprehension [24]. Nevertheless, this approach brings with it its own complexities. Fourthly, studies in Kenya (Boga [23], Ghana (Tindana [25] and South Africa (Zulu [26] report difficulties with the definition of community, competing interests, social and power inequities and the impact these have on participants’ comprehension of the IC process. Fifthly, there was little guidance on how this multi-layered approach could be implemented.
Gaps and inconsistencies in the ethical and legal frameworks guiding the informed consent process
Although there is a recognition that IC must be understood within the specific social and cultural context, the normative ethical-legal framework was found to be lacking. Two main issues emerged from this review. First, there are contradictions between national and international norms in respect to the requirements for comprehension during the IC process [10, 27]. Inconsistent legal norms were compounded by variations between the legal and ethical guidelines within countries. Four studies by Colom [10], Andrews [28] Matimba et al. [29] and Wright et al. [30] highlight these complexities, including a lack of consistency about what information is essential for research participants to know and a lack of regulatory guidance and language for the collection and use of human biospecimens in many SSA countries Barchi [31]. Secondly, the lack of detail in how to operationalise the core aspects of IC. These concerns add a layer of complexity for researchers [10, 27].They have also led to a growing interest in updating and aligning country-specific guidelines with the law to ensure that research participants are adequately protected.
Strategies used to improve participants’ understanding of IC
In the papers reviewed, we identified four categories of interventions used to improve comprehension in the consent process. These included translation of consent forms [7, 23,24,25,26,27,28,29,30,31,32], and multimedia medium [21, 33,34,35,36,37,38,39], IC assessment tools and a combination of these strategies [40].
The language used and the translation of IC
Many SSA countries are characterised by multilingual communities. (Supplementary Table 2: Languages spoken in SSA countries reviewed). This led to two inter-related issues with the language in IC forms; the words or terms used by local communities and the translation of them into local languages to make them more accessible. The studies reviewed reported misunderstandings and miscommunication, especially when investigators and participants speak different languages, when IC documents have to be translated, or when scientific research and the notion of IC are unfamiliar to study participants [33, 37, 40, 42, 49–53].
The nature of the words used in IC are critical to understanding. Terms such as ‘understanding’, ‘comprehension’, ‘knowledge’, ‘remembering’, ‘retention’, ‘recall, ‘awareness’ or ‘recognition’ were used interchangeably with the potential to influence apprehension of the information. Interestingly, Bentley et al. [41] attempted to make IC more accessible by the incorporation of culturally appropriate analogies, a method linked to a theory for improving IC comprehension in Malawi.
Although it is accepted that the translation of IC forms into local languages is essential it brings with it a range of changes. Studies by Palmeirim [22, 42], Afolabi et al. [35, 43, 44], Mack et al. [45], Staunton [46], Penn [47], and Moodley et al. [48]reported the challenges of providing information in the participants’ native languages because in many communities, local languages exist only in oral forms and they do not have standardised writing formats. This made written translation and back-translations of informed consent documents not only impractical, but also less precise, and may inadvertently misrepresent the research being conducted. Despite this, studies across SSA countries primarily employ back-translation. To address limitations inherent in translations, Boga and colleagues in Kenya [23], went beyond translations and targeted the broader socio- cultural context by co-developing a dictionary of language with communities to incorporate the socio-cultural nuances and issues that might be missed during the translation. Interestingly, only one study by Baiden and colleagues [49]argued against the appropriateness of translating approved English version ICF into the local language, and instead proposed the development of contextualized informed procedures based on the values and aspirations of the participants in different contexts. In fact, Burgess et al. argued that translating IC documents into unfamiliar local dialects could ironically enhance the vulnerability of the participants [50].
A study by Muzanyi et al. [51] found that the participants’ choice of language was associated with the level of education in Uganda, a preference for English may be influenced by English being one of the national mediums of communication. In Tanzania, Bukini and colleagues [38] reported that low literacy levels had little influence on comprehension of IC, rather, the methods used to provide information, the language, and time spent with the study participants were the key factors influencing understanding.
IC assessment tools
We identified a range of IC assessment tools, strategies and approaches employed to improve comprehension of IC forms. The tools ranged from study quizzes, psycho-metric development and testing tools through to multimedia interventions [33, 37, 52,53,54,55,56]. The assessment methods used differed significantly, ranging from recall and retention of specific elements of the IC, readability of IC forms, overall assessment of language and meaning, as well as participants’ satisfaction about the IC process [57, 50]Morrow et al. [52] focusing on research into paediatric critical care revealed concerns about therapeutic misconceptions in medical research [58]. In this study, Morrow and colleagues showed that most participants in South Africa perceive medical research to be similar to medical care, and may not understand the study purpose and therefore caregivers believed that their infants would be protected from HIV if they joined the research project. Similar findings were reported by Moodley et al. [48]In Ghana, Baiden et al. [49], Malawi, Bentley [59]South Africa, Ndebele et al. [41], and Moodley et al. [60] reported that most clinical trials with complex study procedures or consent forms tended to evaluate the understanding or recall of specific scientific and technical trial terms including randomisation, placebo. In Ghana, O [57] reported varied comprehension levels of disclosed information among participants and variability was also observed among younger and older participants. In Mozambique and South Africa, Ossemane et al. [61] and Fischer et al. [62], indicated that readability of IC was influenced by long sentences, the number of words containing three or more syllables of words per sentence resulting in poor comprehension. In South Africa the study by Fischer et al. [62] showed that two-thirds of the ICFs analysed for readability did not meet recommendations by the national ethical guidelines stipulations, and that the IC documents were hard to read and exceeded the South African national functional literacy level of grade 7, equivalent to end of primary school level education. Afolabi et al. [39] used digitised audio tools in the participants’ local languages to enhance comprehension among clinical trial participants with low-literacy levels in Gambia. While most studies focused on the adult population, in a study in Kenya, Afolabi and colleagues [33] adapted the assessment tool (DICCQ) among a diverse population of adolescents, young adults and parents.
Findings on assessment tools showed varying degrees of efficacy, and there were suggestions that these tools are inadequate [40, 41, 48]. Ssali et al., [23] working in Uganda pointed out that while the assessment tools are important, the language used may be more important in enhancing IC comprehension [24]. Afolabi et al. [43] note that empirical assessment of consent comprehension in many SSA countries is in its infancy and that the means of assessing understanding may be unfamiliar and confusing for participants. Afolabi and colleagues further highlighted that the paucity of studies on instruments for informed consent comprehension is not surprising, given the cost and highly technical nature of psycho-metric development and testing of a comprehension instrument.
Discussion
Our review showed wide recognition in published studies that the process of achieving IC in SSA is fraught with challenges. We also showed complexities of the languages in multilingual settings and the limitations of translating IC documents to make them accessible to local languages. Furthermore, we underscore the importance of addressing social and cultural contexts in the informed consent process, as well as the complexities of operationalising IC documents in a culturally appropriate manner. For example, the insistence on written communications in settings where communities value oral communication and signing of the IC does not enhance or guarantee comprehension. Rather, these practices are conducted to meet legal requirements. Consequently, most of the studies suggest a need for flexibility and negotiations around the norms to suit the diverse socio-cultural contexts.
The current normative guidelines have gaps and inconsistencies [27, 30]. One of the points of conflict between ethics and law is around obtaining informed consent in social and cultural contexts where decision-making is not solely in the hands of an individual. Following a legal approach ethical guidelines tend to also follow an individualistic approach to consent leaving limited guidance on how to work in social contexts where this notion is foreign. The presence of ambiguity in the legal and ethical frameworks that govern the IC process is not limited to SSA, but exists globally [16]. One gap is the need to develop guidelines that define the most crucial information relevant for comprehension of informed consent in SSA research settings as well as the best way of how this information should be communicated. A further concern is the focus on individual consent is a contested position with varying views on how one can meet the need for individual autonomy with the cultural context in certain communities. Given this lack of consensus on the guidelines, operationalising them is difficult.
Similar findings have been reported across the globe, for example in the Asia-Pacific region [4, 63,64,65], United States [66,67,68,69,70,71], and Europe [72, 73]. However, the complexities tend to be more pronounced in SSA countries due to among others; the socio-cultural context, poverty and power relations. Studies reviewed showed that although high rates of illiteracy and functional illiteracy may contribute to the difficulties of comprehension of IC, the language and delivery of the IC information ranked high in the barriers to IC [3, 39, 74,75,76]. Concerningly, most of the studies reported on the participants’ performance and few focused on the researchers’ communication skills and delivery of IC process, a key factor during the IC process. Our review showed that similar to the study participants, most researchers are equally ill prepared and often have a limited understanding of the legal and clinical terms during the IC process [7, 32, 74,75,76,77,78]. This may represent an asymmetry, with the emphasis on the failure of the participants without focusing on the role of the researcher’ communication and delivery skills. We also showed limitations and challenges inherent in the language used and the translation of IC, and assessment tools aimed at enhancing comprehension of the IC process.
Our review showed that comprehension of IC in paediatric research is under-represented in SSA despite wide support for adolescent participation in health research.
Although we set out to identify strategies to enhance compression of the IC process, our review showed that most the strategies used including translation and backtranslations; tools developed to assess and enhance comprehension of the IC process do not address the structural, systematic and contextual issues that impact the IC process and directly affect understanding. Considering these limitations and complexities inherent in the IC consent process, we suggest alternative approaches moving beyond translations of the literal language and efforts to seek to address contextual and structural factors that impact comprehension of the IC process. This would include locating the participants’ world view at the centre of the IC process and taking account of how they perceive the IC process instead of the top-down approach in which the participants fit in with the process devised by others. This requires researchers, and ethics committee members to reflect and ask questions such as does the signing of the IC documents equate to comprehension, how can linguistic and contextual factors be integrated to ensure valid consent, and what researcher’ factors impact delivery of IC process and what strategies can be implemented to mitigate highlighted gaps in varied contexts.
Strengths ad limitations
One of the strengths of the review is that it advances the discussion regarding IC comprehension beyond the limitations of the assessment and translation of IC documents and suggests perspectives that researchers should consider enhancing the IC process. These include: moving beyond the literal language and translations to understanding IC comprehension from the participants’ perspectives, as well as examining researcher factors that impact the IC process.
One of the limitations is that we did not assess the domains of the various elements such as voluntary participation, compensation, confidentiality, anonymity, risks and benefits. Furthermore, studies which assessed the domains of IC documents varied considerably with little regard to the crucial information that could engender comprehension.
Conclusion
We conducted a scoping review to examine published studies focusing on improving the IC process and assessing impact of these strategies in sub-Saharan Africa countries. Our review showed that while translations of IC documents and assessment tools improve comprehension of IC documents, these strategies continue to face limitations and challenges, and do little to address the underlying socio-cultural factors that constrain comprehension of the IC process. Our review suggests that there is a need for greater flexibility and negotiations with communities to ensure that the approach to IC is suited to the diverse socio-cultural contexts.
Availability of data and materials
Not applicable.
Abbreviations
- IC:
-
Informed consent
- LMIC:
-
Low and middle income countries
- SSA:
-
Sub-Saharan Africa
References
Marshall PA. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases & World Health Organization. TDR/SDR/SEB/ST/07.1. Special Topics in Social, Economic, and Behavioral Research report; No.5. World Health Organization. 2007. https://apps.who.int/iris/handle/10665/43622.
Laurie G, Postan E. Rhetoric or reality: what is the legal status of the consent form in health related research? Med Law Rev. 2013;21:371–414.
Aderibigbe KS, Chima SC. Knowledge and practice of informed consent by physiotherapists and therapy assistants in Kwazulu-Natal Province, South Africa. South Afr J Physiotherapy. 2019;75(1):1–10.
Adams V, Miller S, Craig S, Nyima, Sonam D, et al. The challenge of cross-cultural clinical trials research: Case Report from the Tibetan Autonomous Region, people’s Republic of China. Med Anthropol Q. 2005;19(3):267–89.
Bull S, Farsides B, Ayele FT. Tailoring information provision and consent processes to research contexts: the value of rapid assessments. J Empir Res Hum Res Ethics. 2012;7(1):37–52.
Nakkash R, Makhoul J, Afifi R. Obtaining informed consent: observations from community research with refugee and impoverished youth. J Med Ethics. 2009;35(10):638–43.
Reynolds L, Cousins T, Newell ML, Imrie J. The social dynamics of consent and refusal in HIV surveillance in rural South Africa. Soc Sci Med. 2013;77(1):118–25.
Ngwenya N, Luthuli M, Gunda R, Gumede NA, Adeagbo O, Nkosi B, et al. Participant understanding of informed consent in a multidisease community-based health screening and biobank platform in rural South Africa. Int Health. 2020;12:560–6.
Appiah R. Gurus and Griots: revisiting the research informed consent process in rural african contexts. BMC Med Ethics. 2021;22(1):1–11.
Colom M, Rohloff P. Cultural considerations for informed consent in paediatric research in low/middle-income countries: a scoping review. BMJ Paediatr Open. 2018;2(1):1–14.
Marshall PA. Informed consent in International Health Research. J Empir Res Hum Res Ethics: Int J. 2006;1(1):25–42.
Hinga AN, Molyneux S, Marsh V. Towards an appropriate ethics framework for Health and Demographic Surveillance Systems (HDSS): learning from issues faced in diverse HDSS in sub-Saharan Africa. BMJ Glob Health. 2021;6(1):e004008. https://doi.org/10.1136/bmjgh-2020-004008.
Marshall PA. Informed consent in international health research. J Empir Res Hum Res Ethics. 2006;1(1):25-42. https://doi.org/10.1525/jer.2006.1.1.25.
Nakalega R, Akello C, Gati B, Nakabiito C, Nolan M, Kamira B, et al. Ethical considerations for involving adolescents in biomedical HIV prevention research. BMC Med Ethics. 2021;22(1):1–7.
Bull S, Cheah PY, Lwin KM, Marsh V, Molyneux S, Parker M, et al. Consent and Community Engagement in diverse research contexts: reviewing and developing research and practice. J Empir Res Hum Res Ethics. 2013;8(4):1–18.
Krogstad DJ, Diop S, Diallo A, Mzayek F, Keating J, Koita OA, et al. Informed consent in international research: the rationale for different approaches. Am J Trop Med Hyg. 2010;83(4):743–7.
Bukini D, Treadwell M, Anie K, Dennis-antwi J, Kamga KK, Mccurdy S, et al. Exploring the role of shared decision making in the consent process for pediatric genomics research in Cameroon, Tanzania and Ghana. AJOB Empir Bioeth. 2020;10(3):182–9.
Folayan MO, Haire B, Harrison A, Fatusi O, Brown B. Beyond informed consent: ethical considerations in the design and implementation of sexual and Reproductive Health Research among Adolescents. 2015;18:118–26.
Torrorey-Sawe R, van der Merwe N, Mining SK, Kotze MJ. Pioneering informed consent for return of Research results to breast Cancer patients facing barriers to implementation of genomic medicine: the kenyan BRCA1/2 testing experience using whole Exome sequencing. Front Genet. 2020;11(March):1–12.
Dawson L, Kass NE. Views of US researchers about informed consent in international collaborative research. Soc Sci Med. 2005;61(6):1211–22.
Tindana P, Bull S, Amenga-Etego L, De Vries J, Aborigo R, Koram K, et al. Seeking consent to genetic and genomic research in a rural ghanaian setting: a qualitative study of the MalariaGEN experience. BMC Med Ethics. 2012;13(1):1.
Palmeirim MS, Ross A, Obrist B, Mohammed UA, Ame SM, Ali SM, et al. Informed consent procedure in a double blind randomized anthelminthic trial on Pemba Island, Tanzania: do pamphlet and information session increase caregivers knowledge? BMC Med Ethics. 2020;21(1):1–9.
Boga M, Davies A, Kamuya D, Kinyanjui SM, Kivaya E, Kombe F, et al. Strengthening the informed consent process in international health research through community engagement: the KEMRI-Wellcome Trust Research Programme experience. PLoS Med. 2011;8(9):1–4.
Ssali A, Poland F, Seeley J. Exploring informed consent in HIV clinical trials: a case study in Uganda. HLY. 2016;(September).
Tindana P, Campbell M, Marshall P, Littler K, Vincent R, Seeley J, et al. H3Africa Community Engagement Working Group. Developing the science and methods of community engagement for genomic research and biobanking in Africa. Glob Health Epidemiol Genom. 2017;2:e13. https://doi.org/10.1017/gheg.2017.9.
Zulu JM, Sandøy IF, Moland KM, Musonda P, Munsaka E, Blystad A. The challenge of community engagement and informed consent in rural Zambia: an example from a pilot study. BMC Med Ethics. 2019;20(1):1–9.
Britz R, le Roux-Kemp A. Voluntary informed consent and good clinical practice for clinical research in South Africa: ethical and legal perspectives. S Afr Med Journal. 2016;102(July):1–23.
Andrews S. Legal and ethical issues: informed consnet and HIV, a review of the topic with reference to the particular problems posed by the HIV pandemic. SAJHIV.
Matimba A, Chimatira A, Kuguyo O, January J, Mupambireyi Z, Marimbe-Dube B, et al. Understanding ethical, legal and societal issues (ELSIs) in Human Biobanking and Genomics for Research and Healthcare in Zimbabwe: the Genomics inheritance Law Ethics and Society GILES initiative. AAS Open Res. 2019;2:1. https://doi.org/10.12688/aasopenres.12917.2.
Wright GEB, Koornhof PGJ, Adeyemo AA, Tiffin N. Ethical and legal implications of whole genome and whole exome sequencing in african populations. BMC Med Ethics. 2013;14(1):1.
Barchi F, Little MT. National ethics guidance in Sub-Saharan Africa on the collection and use of human biological specimens: a systematic review Ethics in Biomedical Research. BMC Med Ethics. 2016;17(1).
Afolabi MO, Okebe JU, Mcgrath N, Larson HJ, Bojang K, Chandramohan D. Informed consent comprehension in African research settings. Trop Med Int Health. 2014;19(6):625–42. https://doi.org/10.1111/tmi.12288.
Afolabi MO, Rennie S, Hallfors DD, Kline T, Zeitz S, Odongo FS, et al. An adapted instrument to assess informed consent comprehension among youth and parents in rural western Kenya: a validation study. BMJ Open. 2018;8(7):e021613.
Staunton C, de Roubaix M, Baatjies D, Black G, Hendricks M, Rossouw T, et al. Ethical challenges in developing an educational video to empower potential participants during consent processes in HIV cure research in South Africa. J Virus Erad. 2018;4(2):99–102.
Afolabi OM, Bojang K, D’Alessandro U, Imoukhuede EB, Ravinetto RM, Larson HJ, et al. Multimedia Informed Consent Tool for a low literacy African Research Population: Development and Pilot-Testing. J Clin Res Bioeth. 2014;05(03):1–18.
Molyneux CS, Peshu N, Marsh K. Understanding of informed consent in a low-income setting: three case studies from the kenyan coast. Soc Sci Med. 2004;59(12):2547–59.
Gikonyo C, Bejon P, Marsh V, Molyneux S. Taking social relationships seriously: Lessons learned from the informed consent practices of a vaccine trial on the kenyan coast. Soc Sci Med. 2008;67(5):708–20.
Bukini D, Mbekenga C, Nkya S, Purvis L, McCurdy S, Parker M, et al. A qualitative study on aspects of consent for genomic research in communities with low literacy. BMC Med Ethics. 2020;21(1):1–7.
Afolabi MO, Bojang K, D’Alessandro U, Ota MOC, Imoukhuede EB, Ravinetto R et al. Digitised audio questionnaire for assessment of informed consent comprehension in a low-literacy african research population: development and psychometric evaluation. BMJ Open 2014;4(6).
Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. J Am Med Assoc. 2004;292(13):1593–601. https://doi.org/10.1001/jama.292.13.1593.
Ndebele PM, Wassenaar D, Munalula E, Masiye F. Improving understanding of clinical trial procedures among low literacy populations: an intervention within a microbicide trial in Malawi. BMC Med Ethics. 2012;13(1):29.
Palmeirim MS, Mohammed UA, Ross A, Ame SM, Ali SM, Keiser J. Evaluation of two communication tools, slideshow and theater, to improve participants’ understanding of a clinical trial in the informed consent procedure on Pemba Island, Tanzania. PLoS Negl Trop Dis. 2021;15(5):1–13.
Afolabi MO, Rennie S, Hallfors DD, Kline T, Zeitz S, Odongo FS, et al. An adapted instrument to assess informed consent comprehension among youth and parents in rural western Kenya: a validation study. BMJ Open. 2018;8(7):e021613.
Afolabi MO, Okebe JU, Mcgrath N, Larson HJ, Bojang K, Chandramohan D. Informed consent comprehension in african research settings. Trop Med Int Health. 2014;19(6):625–42.
Mack N, Ramirez CB, Friedland B, Nnko S. Lost in translation: assessing effectiveness of Focus Group Questioning Techniques to develop Improved translation of terminology used in HIV Prevention clinical trials. PLoS ONE. 2013;8(9).
Staunton C. Informed consent for HIV cure research in South Africa: issues to consider. BMC Med Ethics. 2015;16(3):1–7. https://doi.org/10.1186/1472-6939-16-3.
Penn C, Evans M. Assessing the impact of a modified informed consent process in a south african HIV/AIDS research trial. Patient Educ Couns. 2010;80(2):191–9.
Moodley K, Pather M, Myer L. Informed consent and participant perceptions of influenza vaccine trials in South Africa. J Med Ethics. 2005;31(12):727–32.
Baiden F, Akazili J, Chatio S, Achana FS, Oduro AR, Ravinetto R, et al. Should consent forms used in clinical trials be translated into the local dialects? A survey among past participants in rural Ghana. Clinical Trials. 2016;13(2):234–9. https://doi.org/10.1177/1740774515609290.
Minnies D, Hawkridge T, Hanekom W, Ehrlich R, London L, Hussey G. Evaluation of the quality of informed consent in a vaccine field trial in a developing country setting. BMC Med Ethics. 2008;9:1–9.
Muzanyi G, Sekitoleko I, Johnson JL, Lunkuse J, Nalugwa G, Nassali J, et al. Level of education and preferred language of informed consent for clinical research in a multi-lingual community. Afr Health Sci. 2020;20(2):955–9.
Burgess LJ, Gerber B, Coetzee K, Terblanche M, Agar G, Kotze TJ. An evaluation of informed consent comprehension by adult trial participants in South Africa at the time of providing consent for clinical trial participation and a review of the literature. Open Access J Clin Trials. 2019;11:19–35.
Chaisson LH, Kass NE, Chengeta B, Mathebula U, Samandari T. Repeated assessments of informed consent comprehension among HIV-infected participants of a three-year clinical trial in Botswana. PLoS ONE. 2011;6(10):1–10.
Sugarman J, Corneli A, Donnell D, Liu T, Rose S, Celentano D, et al. Are there adverse Consequences of quizzing during informed consent for. HIV Research? 2012;37(11):693–7.
Campbell MM, Susser E, Mall S, Mqulwana SG, Mndini MM, Ntola OA, et al. Using iterative learning to improve understanding during the informed consent process in a south african psychiatric genomics study. PLoS ONE. 2017;12(11):1–11.
Kiguba R, Kutyabami P, Kiwuwa S, Katabira E, Sewankambo NK. Assessing the quality of informed consent in a resource-limited setting: a cross-sectional study. BMC Med Ethics. 2012;13(1):1.
Oduro AR, Aborigo RA, Amugsi D, Anto F, Anyorigiya T, Atuguba F, et al. Understanding and retention of the informed consent process among parents in rural northern Ghana. BMC Med Ethics. 2008;9:1–9.
Morrow BM, Argent AC, Kling S. Informed consent in paediatric critical care research – a south african perspective. BMC Med Ethics. 2015;1–13.
Corneli AL, Bentley ME, Sorenson JR, Henderson GE, van der Horst C, Moses A, Nkhoma J, et al. Using formative research to develop a context-specific approach to informed consent for clinical trials. 2011;1(4):45–60. https://doi.org/10.1525/jer.2006.1.4.45.
Moodley K, Staunton C, Rossouw T, De Roubaix M, Duby Z, Skinner D. The psychology of “cure” - unique challenges to consent processes in HIV cure research in South Africa. BMC Med Ethics. 2019;20(1):1–11.
Ossemane EB, Moon, Try D, Sacarlal J, Sevene E, Kenga D, Heitman E. Assessment of Parents’/Guardians’ initial comprehension and One-Day Recall of elements of informed consent within a mozambican study of Pediatric Bacteremia. J Empir Res Hum Res Ethics. 2016;176(1):139–48.
Fischer AE, Venter WDF, Collins S, Carman M, Lalla-Edward ST. The readability of informed consent forms for research studies conducted in South Africa. South Afr Med J. 2021;111(2):180–3.
Taber C, Warren J, Day K. Improving the quality of informed consent in clinical research with information technology. Stud Health Technol Inform. 2016;231:135–42.
An I, Harman M, Ibiloglu I. Informed consent in biomedical research: Scopes and challenges. Indian Dermatol Online J. 2017;10(4):481–5.
Al-Amer R, Ramjan L, Glew P, Darwish M, Salamonson Y. Language translation challenges with arabic speakers participating in qualitative research studies. Int J Nurs Stud. 2016;54:150–7.
Mukherjee A, Livinski AA, Millum J, Chamut S. Informed consent in dental care and research for the older adult population: a systematic review. Physiol Behav. 2018;176(5):139–48.
Mccabe ML, Morgan F, Curley H, Begay R, Gohdes DM. Informed consent: the impact of intersexuality. Ethn Dis. 2001;9(1998):16–20.
Bristol ST, Hicks RW. Protecting boundaries of consent in clinical research: implications for improvement. Nurs Ethics. 2014;21(1):16–27.
Campbell LM, Paolillo EW, Bryan R, Marquie-Beck J, Moore DJ, Nebeker C, et al. Informing informed consent for HIV Research. J Empir Res Hum Res Ethics. 2020;15(4):235–43.
Cortés DE, Drainoni ML, Henault LE, Paasche-Orlow MK. How to achieve informed consent for research from spanish-speaking individuals with low literacy: a qualitative report. J Health Commun. 2010;15(SUPPL 2):172–82.
Davidow AL, Katz D, Reves R, Bethel J, Ngong L. The challenge of multisite epidemiologic studies in diverse populations: design and implementation of a 22-site study of tuberculosis in foreign-born people. Public Health Rep. 2009;124(3):391–9.
Hamnes B, Van Eijk-Hustings Y, Primdahl J. Readability of patient information and consent documents in rheumatological studies. BMC Med Ethics. 2016;17(1).
O’ Sullivan L, Feeney L, Crowley RK, Sukumar P, McAuliffe E, Doran P. An evaluation of the process of informed consent: views from research participants and staff. Trials. 2021;22(1):1–15. https://doi.org/10.1186/s13063-021-05493-1.
Nembaware V, Johnston K, Dialli, Alpha A, Kotze, Maitha J, Matimba A, Moodley K, et al. A Framework for Tiered Informed Consent for Health Genomics Research in Africa. Physiol Behav. 2016;176(1):139–48.
Villafranca A, Kereliuk S, Hamlin C, Johnson A, Jacobsohn E. The appropriateness of language found in research consent form templates: a computational linguistic analysis. PLoS ONE. 2017;12(2):1–10.
Chapman KN, Pevzner E, Mangan JM, Breese P. Evaluation of the informed consent process of a Multicenter Tuberculosis Treatment Trial. AJOB Empir Bioeth. 2017;176(3):139–48.
Krieger JL, Neil JM, Strekalova YA, Sarge MA. Linguistic strategies for improving informed consent in clinical trials among low health literacy patients. J Natl Cancer Inst. 2017;109(3):1–7.
Krosin MT, Klitzman R, Levin B, Cheng J, Ranney ML. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clin Trails. 2006;3(3):306–13.
Acknowledgements
This work was inspired by the AHRI staff community, and we would like to thank the AHRI community for the support.
Funding
This research was funded by the Wellcome Trust [Grant number 201433/Z/16/A] and [096527]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The funding body played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
BN. JS. and MP. conceptualized and designed the study. BN. coordinated the data search process. BN. JS. and MP. contributed to the analysis of results and drafted the manuscript. AS. critically reviewed the manuscript. All authors contributed towards the revision of the manuscript and approved the final manuscript.
Authors’ information
BN is a senior research associate at the Africa Health Research Institute. Her research focuses on the ethical-legal frameworks that guide research.
JS is Professor of Anthropology and Health at the London School of Hygiene and Tropical Medicine and faculty member covering Social Science and Research Ethics at the Africa Health Research Institute in KwaZulu-Natal in South Africa.
Ann Strode is a Law Professor at the School of Law, University of KwaZulu Natal, South Africa. Her inter-disciplinary research focuses on the ethical-legal complexities of adolescent participation in health research.
MP is Professor of Bioethics at the University of Oxford. He has special interests in ethical questions relating to global health justice, and conceptions of consent, privacy and confidentiality.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
Thematic analysis of studies reviewed.
Additional file 2: Table 2.
Languages spoken in SSA countries reviewed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Busisiwe, N., Seeley, J., Strode, A. et al. Beyond translations, perspectives for researchers to consider to enhance comprehension during consent processes for health research in sub-saharan Africa: a scoping review. BMC Med Ethics 24, 43 (2023). https://doi.org/10.1186/s12910-023-00920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12910-023-00920-1