Abstract
Background
Dolomiaea costus (syn: Saussurea costus; Family Asteraceae) occupies an important place in the traditional Chinese medicinal plants and is prescribed for a wide range of disorders. The current study aimed to tentatively identify the phytoconstituents of D. costus extract and to explore antiproliferative activity against human breast cancer cells and its possible apoptotic mechanism along with antiviral activity against human adenovirus 5 (Adv-5).
Methods
The phytoconstituents of 70% ethanol extract of D. costus were assessed using HPLC/ESI-MS/MS technique. The cell viability was investigated against breast cancer cell line (MCF-7) via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Mechanistically, the apoptotic effects on the Bax, Bcl2 and Caspase 3 were determined via quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR). Further, the antiviral activity was assessed against Adv-5 based on virucidal and adsorption mechanisms.
Results
The HPLC/MS analysis of the extract revealed tentative identification of twenty compounds of polyphenolic nature, mainly flavonoids, lignans, coumarins, and anthocyanidins. The plant extract showed a cytotoxic effect against MCF-7 and Vero cells with IC50 values of 15.50 and 44 µg/ml, respectively, indicating its aggressiveness against the proliferation of breast cancer cells as confirmed by apoptotic genes expression which revealed upregulation of Bax and Caspase 3 but further insight analysis is needed to explore exact mechanistic pathway. Antiviral activity against Adv-5 was observed at a non-toxic concentration of the tested extract.
Conclusions
Such observations against human breast cancer and viral replication supported further studies for nanoformulations in drug delivery systems as targeting therapy and in vivo studies before biomedical applications.
Similar content being viewed by others
Introduction
The genus Saussurea (Fam. Asteraceae) comprises about 400 species distributed mainly in the north-temperate zone area including alpine habitats, the Himalayas, and Central Asia [1]. About 30 species have been recorded in Traditional Chinese Medicine (TCM), Tibetan medicines, Uyghur medicine, Mongolian medicine, and Kazakhstan medicine for broad-spectrum applications like anti-inflammatory, analgesic, antifatigue, anti-aging, hormonal-related gynecological disorders, infertility, and immunomodulation [2,3,4]. Among these species, S. costus, S. involucrate, S. eopygmaea, S. obvallata, S. polycolea, S. laniceps, and S. medusa have been used in both indigenous medical applications and current herbal therapies [5, 6].
Dolomiaea costus (syn. Saussurea costus) occupies an important place in the traditional Chinese medicinal plants [7,8,9]. It is well-known as a rich source of different bioactive phytoconstituents with reported interesting activities like flavonoids, phenylpropanoids, lignans, coumarins, monoterpenes, sesquiterpene lactones, steroids and volatile oils [10, 11]. In Arabian countries, D. costus is commonly named costus root, Kust and Qist Hindi. In the folk medicine, D. costus was prescribed for typhus fever, rheumatism, nervous disorders, irregular menstruation, heart diseases, asthma, gastric ulcer, inflammation, liver diseases and for hair to kill lice and to turn grey hair to black [12,13,14]. The essential oil isolated from D. costus possesses very strong aroma and is employed in high-grade perfumes, hair cosmetic formulation, and insect repellents [7]. Scientific evidence efforts proved that the D. costus roots displayed antioxidant, anxiolytic, hepatoprotective, antiulcer, hypolipidemic, anti-inflammatory, neuroprotective, antimicrobial, antiparasitic and antirheumatic properties [15,16,17,18]. Further, the methanol extract showed cardiotonic effects [19], while the petroleum ether extract showed potent anticonvulsant activity against picrotoxin-induced convulsions in mice [15, 20]. Interestingly, the phytochemicals isolated from D. costus like costunolide and dehydrocostuslactone were found to suppress tumor growth as well as metastases of breast cancer cell via tumor necrosis factor-α-(TNF-α)-induced nuclear factor-kappa (NF-κB) activation, leading to inhibition of MDA-MB-231 migration and invasion. Further, these compounds remarkedly reduced matrix metallopeptidase-9 (MMP-9) expression which is a well-known NF-kB-dependent gene and play a vital role to proceed breast cell cancer growth and metastases [21, 22]. Moreover, D. costus was found to be a potent inducer of apoptosis owing to the presence of costunolide as a major phytoconstituent [17, 23, 24].
Breast cancer remains the most common cause of cancer deaths among women globally, its treatment has many drawbacks and several adverse effects [25]. Recent studies have suggested many medicinal plants act as anti-cancer agents by inducing apoptosis in cancer cells [25,26,27,28,29,30]. Costus showed to have anti-hepatotoxic, anti-diabetic, antifungal, anthelmintic, anti-tumor, anti-inflammatory, anti-ulcer, antimicrobial, and immunostimulant effects. Previously, this species demonstrated its protective role against Ehrlich Solid Tumor (EST)-induced cardiac toxicity, injury, and alterations in apoptotic p53, pro-apoptotic Bax, and vascular endothelial growth factor (VEGF) expression [25]. Moreover, it demonstrated its ability to induce apoptosis in the breast and colon cancers [31]. This information encouraged us to do our research on this plant to investigate our own plant to obtain optimum concentration before proceeding nanotechnology-based drug delivery approach and in vivo study to manage such chronic disease.
Human adenovirus 5 (Adv-5) can affect multiple human organs such as gastrointestinal tract, respiratory tract, and ocular surface. The children and immunocompromised adults are more susceptible to adenoviral infections [32], also no FDA approved antivirals for such virus [33]. To our knowledge, there is no published data available for the utilization of D. costus root extract as antiviral agent against replication of human adenovirus type 5 in cell culture.
HPLC/ESI-MS/MS is well-known as a comprehensive analytical technique used for the identification of plant metabolites [34, 35]. In the literature of D. costus, we found extensive studies accomplished on its essential oil, while the other plant phytoconstituents have not been investigated in depth. Therefore, the current study was designed to tentatively identify the phytochemical constituents of 70% ethanol extract of D. costus using HPLC/ESI-MS/MS technique. In addition, we aimed to explore the in vitro antiproliferative activity against human breast cancer cells and its possible underlying mechanism along with its possible antiviral activity against replication of human adenovirus in Vero cells, this will open new era of biomedical application based on both anti-cancerous and antiviral activities.
Materials and methods
Plant material and extraction
D. Costus roots were purchased from local herbal market, Cairo, Egypt, in May 2021. The plant was authenticated by Professor Usama K. Abdel Hameed, Department of Botany, Faculty of Science, Ain Shams University, Cairo, Egypt. A voucher specimen (Number: PHG-P-SC-435) is deposited at the Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. The plant roots (1 kg) were chopped into small pieces and extracted with 70% ethanol (3 × 10 L). The pooled extracts were evaporated under reduced pressure at 55 °C until complete dryness to obtain 37 g of a brown material.
HPLC-ESI/MS-MS conditions
The phytochemical analysis of D. Costus extract were assessed according to previously reported method using high-performance liquid chromatographic (HPLC) analysis joined with an ESI-MS/MS spectrometer detector [35]. This technique allowed tentative identification of phytoconstituents based on the molecular weights. The plant extract (100 µg/ml) was dissolved in methanol (HPLC-grade), then filtered via membrane disc (0.20 μm). Then, the filtrate (10 µL) was injected to HPLC/ESI-MS/MS. The used HPLC instrument has the following specifications: Waters® stocked with a reversed phase C-18 column (ACQUITY UPLC-BEH C-18, particle size ~ 1.7 μm, dimensions = 2.1 × 50 mm). Prior to injection, the mobile phase was filtered through membrane disc filter (0.2 μm) and sonicated. The elution run took 35 min using gradient elution (water and methanol acidified with 0.1% formic acid) with flow rate of 0.2 mL/min. On an XEVO TQD triple quadruple instrument, positive and negative ions were acquired using ESI-MS. Waters® Corporation, Milford, MA01757, U.S.A supplied the HPLC unit and mass spectrometer. The vacuum pump was provided by Edwards®, U.S.A. at desolvation temperatures of 150 and 440° C. The mass spectra were obtained using the software Maslynx 4.1 at ESI range m/z of 100–1000. In order to tentatively identify the obtained mass spectra, the peak retention time (tR) and their fragmentation pattern were compared with the reported data in the literature.
Assessment of cell viability
The cytotoxic effect of plant extract was assessed against in vitro model of human breast cancer cells (MCF-7) and Vero cells as a model of normal cells using colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to previously published protocol [36]. Vero and MCF-7 ATCC origin were purchased commercially from a holding company for biological products &vaccines (VACSERA) in Cairo, Egypt. Tissue culture reagents and required staffs are obtained from analysis company in Egypt (Gibco- Merelbeke, Belgium). MTT dye was purchased from Serva Electrophoresis GmbH, Heidelberg, Germany. The cell lines were maintained at virology & Immunology laboratory by subculturing at standard conditions, 37 °C with 5% CO2. The assay relies on reduction of tetrazolium salts into their insoluble formazan crystals that can be solubilized and measured spectrophotometrically. First, MCF-7cells were seeded in a 96-well plate with a density of 104 cell/well and incubated overnight at 37 °C with 5% CO2. After that, the cells were treated with serial concentrations of the plant extract. After 48 h of incubation, 30 µl of MTT was added into each well and then incubated at 37 °C for 3 h. About 200 µl of dimethyl sulfoxide was added to each well to dissolve the insoluble formazan crystals. The absorbance was read at 570 nm using a multimode microplate reader. The CC50 was calculated using non-linear regression analysis, CC50 was determined with GraphPad Prism software version 6 [37].
\(Cell\,viability\,(\% ) = \frac{{{A_{test}}}}{{{A_{control}}}} \times 100\)
Whereas (A test) is the mean absorbance of the tested sample, and (A control) is the mean absorbance of the control sample. In each assay negative control (untreated cells) and positive control (DMSO) was included.
Assessment of apoptotic effect against MCF-7 using quantitative RT-qPCR assay
The expression level of apoptotic genes on a mRNA transcriptional level was investigated according to previously published protocol [38, 39]. The data obtained from quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) were analyzed using the comparative CT method [40]. RT-qPCR was performed using Power SYBR Green PCR Master Mix, which was obtained from Thermo Scientific, USA. The cells were seeded at a density of 5 × 104 cells/ml into a six-well plate. After 24 h, the cells were treated with the polymeric materials and incubated for 24 h [41]. The cells were trypsinized and harvested, then cell pellets were collected and stored at -80 °C. Briefly, the difference in cycle threshold, ΔCT, was determined as the difference between the tested gene and human GAPDH. Then, we obtained ΔΔCT by finding the difference between the two groups. The fold change (FC) was calculated as 2−ΔΔCT.
Assessment of antiviral activity against human adenovirus type 5 (Adv-5)
The antiviral activity of the plant extract was assessed based on two different antiviral mechanisms including virucidal mechanism and adsorption mechanism. AdenoVirus was obtained from Virology Unit, School of Medicine in Kuwait. The virus was propagated into vero cells and titrated using PCR assay and used in the antiviral assay. Initially, cells were seeded in a 6-well plate with a density of 5 × 105 cell/well and incubated overnight at 37 °C with 5% CO2. For investigation of virucidal mechanism, the Adv-5 was pre-incubated with 0.5 CC50 of plant extract for 1 h at 4 °C. After 24 h of seeding, the cells were infected with the pre-incubated virus. For investigation of adsorption mechanism, the cells were seeded for 24 h with 0.5 CC50 of plant extract. After that, the cells were infected with Adv-5 and incubated for 24 h at 37 °C and 5% CO2. Viral copies/ml was determined using real time PCR assay, and the results were compared with the viral control [33, 42]. Prior starting the antiviral activity assay, ADV-5 was propagated in Vero cells and investigated under an inverted microscope until 80–90% of CPE and cell lysis [43]. After that, the virus culture was subjected to viral nucleic acid extraction using QIAmp RNA extraction kit, (Qiagen,Valencia, USA). The extraction was done according to the manufacturer’s instructions. Then, 100 ng of DNA template was used in the PCR assays. Detection and quantification of Adv-5 viral load was done by real time detection system (Applied Biosystems 7500 Fast Real-time PCR) according to previously published protocol [33, 42, 43].
Results and discussion
Characterization of D. costus phytoconstituents using HPLC/ESI-MS/MS technique
The phytoconstituents of the 70% ethanol extract of D. costus were tentatively identified by HPLC/ESI-MS/MS and the total ion chromatogram (TIC) of metabolic profile is shown in supporting information (Fig. S1). The HPLC/ESI-MS/MS analysis of D. costus extract revealed the structural information for twenty peaks, identified according to the previous reported data. The retention times, pseudomolecular ions, molecular formula and fragments observed in MS/MS chromatograms are listed in Table 1. These compounds are distributed in four different main categories (flavonoids, lignans, coumarins, anthocyanidins, and sesquiterpene lactone). The identification of these compounds was based on matching the accurate mass (m/z) of the pseudomolecular [M-H]− and [M+H]− ions for the peaks and their fragmentation pattern with the previous reported data of literature. The chemical structures of the identified compounds are illustrated in Fig. 1.
Flavonoids
As demonstrated in Table 1, flavonoids constituted the predominant category of identified compounds in the investigated extract. A total of thirteen flavonoids were identified by comparison and matching their fragmentation pattern with those described previously: chrysoeriol-O-hexouronide (5) [7], nepetin-O-hexoside (6) [44], isorhamnetin-O-hexoside-O-deoxyhexoside (9) [7, 44], diosmetin-O-hexoside (11) [1, 7], apigenin-O-hexoside (12) [45], gossypetin-O-hexoside (13) [7], hispidulin (14) [46], acacetin-O-hexoside-O-deoxyhexoside (15) [7], apigenin (16) [7], quercetin-O-hexoside-O-deoxyhexoside (17) [45], hesperetin (18) [7], and luteolin-di-O-hexoside (19) [7]. We noticed that the hexose and rutinose moieties of identified flavonoids were the most common. The MS/MS fragmentation analysis showed loss of a hexose moiety (m/z 180), followed by loss of rhamnose moiety (m/z 164) to produce an intense peak corresponding to aglycone with high abundance in their fragmentation pattern. Different phytochemical studies have proven isolation of identified flavonoid aglycones and their hexosides, as well rutinosides from different Saussurea extracts such as S. involucrate, S. Lappa, S. tridactyla and S. stella [44, 47]. The number and structure of bound sugar moieties, as well glycosidic linkages, affect the MS and MS-MS spectra of flavonoid glycosides [48]. The loss of well-defined mass fragments from the pseudomolecular ion may provide detailed data about the related saccharide. The cleavage of the sugar bond initiates fragmentation of glycoside, and this cleavage identifies the aglycone.
Lignans
According to the previous research reports, different lignan structures with various biological activities were previously reported in Saussurea extracts [1]. Similarly, the current study revealed identification of three lignans namely, medioresinol (2), syringaresinol (7), pinoresinol-O-hexoside (8) based on HPLC-MS/MS of the observed deprotonated pseudomolecular ions.
Coumarins
Two compounds of coumarin class were identified in the investigated extract. First compound is isopimpinellin (1) which was previously isolated from S. involucrate [3]. The other compound is coumarin glycoside identified as fraxetin-O-hexoside (20), previously obtained from S. eopygmaea [49].
Other compounds
Anthocyanidin compound was identified as procyanidin B2 (4) in the extract [7]. Another compound of sesquiterpene lactone, namely costunolide (10) was identified and previously isolated from the same extract [45].
Assessment of cytotoxic activity using MTT assay
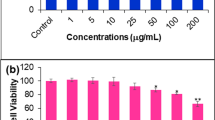
The cytotoxic effect of various concentrations of plant extract (100, 50, 25, 12.5, and 6.25 µg/mL) revealed that its CC50 (the highest dilution of the tested material that kills 50% of the cells) was detected at concentration of 15.50 and 44 µg/mL, on MCF-7 and Vero cells, respectively, after 48 h of cell exposure (Fig. 2). According to the National Cancer Institute guidelines, the plant extract and/or a compound with CC50 values < 20 µg/ml is considered as cytotoxic active agent [27]. Accordingly, the tested extract is considered as anti-cancer agent. In agreement with our results, the ethanol plant extract was found to inhibit proliferation of gastric carcinoma using gastric AGS cancer cells in a dose and time-dependent manner [50]. Grippingly, some of the phytoconstituents identified in the extract (Fig. 1) have already been confirmed to possess anticancer activities for instance, isorhamnetin glycoside isolated from Opuntia Ficus-indica pads was reported to inhibit two different human colon cancer cells (HT-29 and Caco2) by 4.90 and 8.20 µg/mL, respectively [51]. In breast cancer tissues, Lysine-specific demethylase 1 (LSD1) has emerged as a therapeutic target for cancer as it is highly expressed in oestrogen receptor and progesterone receptor-negative tumours initiating tumour differentiation, proliferation, metastasis, and invasion [52]. Among identified compounds in our study, diosmetin glycoside and hesperetin were found to inhibit LSD1 by IC50 values of 21.83 and 78.76 µM, respectively [53]. Costunolide was reported to suppress tumor growth as well as metastases of human breast cancer cells via TNF-α-induced NF-κB activation, leading to inhibition of triple-negative breast cancer cell line (MDA-MB-231) migration and invasion. Further, it resulted in reduced expression of matrix metallopeptidase (MMP)-9 which is a well-known NF-κB-dependent gene and play a vital role to proceed breast cell cancer growth and metastases [21, 22]. In another study, costunolide showed inhibitory effect on a human monocyte cell line THP-1 using reporter gene assay which was induced by a tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) used for tracing out the promoter activity of the inducible nitric oxide synthase (iNOS) gene [54]. iNOS activity was boosted by TPA which in turn inhibited by costunolide (IC50 = 2 mM). Also, costunolide exhibited inhibitory effects on the telomerase activity of MCF-7, and MDA-MB-231 cells in a concentration and time-dependent behaviour and such inhibition was demonstrated in the expression of hTRET mRNA [55]. Rutin, as another extract component, was found to possesses anti-tumor activities by triggering apoptosis in triple-negative breast cancer and upregulation of apoptosis signal-regulating kinase-1 (ASK1) and c-Jun N-terminal kinase (JNK) [56]. Luteolin-O-glucoside as main constituent of Cuminum cyminum demonstrated potent inhibitory effect against MCF-7 cell line with IC50 value of 3.98 µg/mL and selectivity index (SI) of 8.0 [57].
Assessment of apoptotic effect against MCF-7 using quantitative RT-qPCR assay
To confirm that the extract inhibits the proliferation of MCF-7 cells, we investigated the apoptotic effect using RT-qPCR analysis. The results showed that mRNA expressions of Caspase 3, BCl2 and Bax genes were upregulated after 24 and 96 h of cell exposure to the plant extract (Table 2). This observation indicated that the apoptotic effect might be mediated through classical intrinsic pathway but further insightful analysis on other apoptotic genes profile and cell cycle analysis is needed to identify the exact possible targets which affect proliferation of human breast cancer cells. There are three major apoptosis-linked pathways such as mitochondrial/apoptosome pathway, the death receptor pathway, and the CTL/NK-derived granzyme B-dependent pathway; all these pathways resulted in caspase activation [58]. Caspase activation terminates during cell apoptosis and the inflammatory response [58]. Mitochondrial apoptosome-driven caspase activation represents the key to caspase-activating mechanism initiated by cytotoxic drugs and leads to caspase-2, -3, -6, -7, -8, -9, and -10 activation during apoptosis [58]. Caspases-3 and -9, are considered the most important parameters during mitochondrial apoptosome-driven caspase activation. The intrinsic mitochondrial pathway of apoptosis is strictly controlled by Bcl-2 family proteins, incorporating Bcl-2, Bcl-XL and MCL-1 [59]. There is strong association between Bcl-2 and Bax to regulate apoptosis [60].
Assessment of antiviral activity against human adenovirus type 5
The results showed that the plant extract (25 µg/ml) exerted antiviral activity via adsorption, and virucidal mechanisms, as evidenced by undetected levels of viral copies using qRT-PCR assay. It could prevent viral entry into host cells, as well as preventing viral infectivity after incubation with the virus for 1 h at 4 °C. In the line of our results, medioresinol (as one of identified compounds of extract) isolated from Crataegus cuneate, was found to suppress hepatitis C virus (HCV) production in a dose-dependent manner via inhibition of HCV-RNA replication but not viral entry or translation [61]. Procyanidin B2 obtained from Cassia fistula potentially inhibited coronavirus disease protease [62] and human immunodeficiency virus (HIV) [63]. Apigenin selectively blocks Enterovirus-71 infection by disrupting viral RNA association with hnRNP A1 and A2 proteins with IC50 and CC50 values of 10.3 and 79.0 µM, respectively [64]. Further, apigenin was found to have anti-adenoviral activity against three viral types including Adv3, Adv-8 and Adv-11 with EC50 values in the range of 8.0 to 26.4 mg/L [65]. This exploratory data will encourage further in vitro study to obtain therapeutic index and then initiate in vivo study.
Conclusions
We identified the chemical profile of D. costus root extract through HPLC/ESI-MS/MS analysis. Twenty phytoconstituents were tentatively identified and characterized as belonging to flavonoids being the major category along with lignans, coumarins, and anthocyanidin. Biological evaluation showed that D. costus is a promising candidate against proliferation of human breast cancer cells, but such observation needs further insights on the molecular level to elucidate the interplay between apoptotic genes expression and their role in cancer cell death and in vivo studies before its application on a clinical setting. In addition, its antiviral activity against one of the common respiratory infections like adenovirus increases its value in biomedical application and inspires its targeting application in a nano-based formulations.
Data Availability
Data are available upon request from the first author, Heba A. S. El-Nashar; heba_pharma@pharma.asu.edu.eg.
Abbreviations
- Adv-5:
-
Human Adenovirus Type 5
- BAX:
-
Bcl-2-associated X protein
- ELISA:
-
Enzyme-linked immunosorbent assay
- HPLC-ESI-MS/MS:
-
High-performance liquid chromatography/electrospray ionization mass spectrometry
- MCF-7:
-
Breast cancer cells
- MMP-9:
-
Matrix metallopeptidase 9
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB:
-
Nuclear factor-kappa
- RT-qPCR:
-
Quantitative reverse transcriptase-polymerase chain reaction
- TIC:
-
Total ion chromatogram
- TNF-α:
-
Tumor necrosis factor-alpha
References
Wang TM, Wang RF, Chen HB, Shang MY, Cai SQ. Alkyl and phenolic glycosides from Saussurea stella. Fitoterapia. 2013. https://doi.org/10.1016/j.fitote.2013.03.027.
Fan CQ, Yue JM. Biologically active phenols from Saussurea medusa. Bioorg Med Chem. 2003. https://doi.org/10.1016/s0968-0896(02)00470-4.
Chik WI, Zhu L, Fan LL, Yi T, Zhu GY, et al. Saussurea involucrata: a review of the botany, phytochemistry and ethnopharmacology of a rare traditional herbal medicine. J Ethnopharmacol. 2015. https://doi.org/10.1016/j.jep.2015.06.033.
Mostafa NM, Abd El-Ghffar EA, Hegazy HG, Eldahshan OA. New Methoxyflavone from Casimiroa sapota and the Biological Activities of its Leaves extract against lead acetate Induced Hepatotoxicity in rats. Chem Biodivers. 2018. https://doi.org/10.1002/cbdv.201700528.
Xu Y, Zhao D, Fu C, Cheng L, Wang N, et al. Determination of flavonoid compounds from Saussurea involucrata by liquid chromatography electrospray ionisation mass spectrometry. Nat Prod Res. 2009. https://doi.org/10.1080/14786410802187742.
El-Nashar HA, Mostafa NM, Abd El-Ghffar EA, Eldahshan O A,Singab. A N B. The genus Schinus (Anacardiaceae): a review on phytochemicals and biological aspects. Nat Prod Res. 2021. https://doi.org/10.1080/14786419.2021.2012772.
El Gizawy HA, El-Haddad AE, Saadeldeen AM, Boshra SA. Tentatively Identified (UPLC/T-TOF–MS/MS) Compounds in the Extract of Saussurea costus Roots Exhibit In Vivo Hepatoprotection via Modulation of HNF-1α, Sirtuin-1, C/ebpα, miRNA-34a and miRNA-223. Molecules. 2022. https://doi.org/10.3390/molecules27092802.
Ghoneim AI, Eldahshan OA. Anti-apoptotic effects of tamarind leaves against ethanol-induced rat liver injury. J Pharm Pharmacol. 2012. https://doi.org/10.1111/j.2042-7158.2011.01418.x.
Gamal El-Din MI, Youssef FS, Ashour ML, Eldahshan O, A,Singab ANB. Comparative analysis of volatile constituents of Pachira aquatica Aubl. And Pachira glabra Pasq., their anti-mycobacterial and anti-helicobacter pylori activities and their metabolic discrimination using chemometrics. J Essent Oil Bearing Plants. 2018. https://doi.org/10.1080/0972060X.2019.1571950.
Yi T, Chen H-B, Zhao Z-Z, Jiang Z-H, Cai S-Q, et al. Identification and determination of the major constituents in the traditional Uighur medicinal plant Saussurea involucrata by LC-DAD-MS. Chromatographia. 2009. https://doi.org/10.1365/s10337-008-0923-9.
Abdelghffar EA, Mostafa NM, El-Nashar HA, Eldahshan O, A,Singab ANB. Chilean pepper (Schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Ind Crops Prod. 2022. https://doi.org/10.1016/j.indcrop.2022.114953.
Zahara K, Tabassum S, Sabir S, Arshad M, Qureshi R, et al. A review of therapeutic potential of Saussurea lappa-An endangered plant from Himalaya. Asian Pac J Trop Med. 2014. https://doi.org/10.1016/s1995-7645(14)60204-2.
Rao KS, Babu G, V,Ramnareddy YV. Acylated flavone glycosides from the roots of Saussurea lappa and their antifungal activity. Molecules. 2007. https://doi.org/10.3390/12030328.
El-Shawi OE, El-Nashar HA, Abd El-Rahman S, Eldahshan S, A,Singab O. A N B. Protective effect of Acrocarpus fraxinifolius extract against hepatic Fibrosis Induced by Gamma Irradiation and Carbon Tetrachloride in albino rats. Int J Radiat Biol. 2022. https://doi.org/10.1080/09553002.2022.2087926.
Ambavade SD, Mhetre NA, Muthal A, P,Bodhankar SL. Pharmacological evaluation of anticonvulsant activity of root extract of Saussurea lappa in mice. Eur J Integr Med. 2009. https://doi.org/10.1016/j.eujim.2009.08.159.
Tag HM, Khaled HE, Ismail HA, El-Shenawy NS. Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. J Basic Clin Physiol Pharmacol. 2016. https://doi.org/10.1515/jbcpp-2015-0044.
Pandey MM, Rastogi S, Rawat AKS. Saussurea costus: botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J Ethnopharmacol. 2007. https://doi.org/10.1016/j.jep.2006.12.033.
Kim DY, Choi BY. Costunolide-A bioactive sesquiterpene lactone with diverse therapeutic potential. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122926.
Akhtar MS, Bashir S, Malik MN, H,Manzoor R. Cardiotonic activity of methanolic extract of Saussurea lappa Linn roots. Pak J Pharm Sci. 2013.
Younis IY, El-Hawary SS, Eldahshan OA, Abdel-Aziz MM, Ali ZY. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci Rep. 2021. https://doi.org/10.1038/s41598-021-96377-6.
Choi YK, Cho SG, Woo SM, Yun YJ, Jo J et al. Saussurea lappa Clarke-Derived Costunolide prevents TNF α -Induced breast Cancer Cell Migration and Invasion by inhibiting NF-κB activity. Evidence-based complementary and alternative medicine: eCAM. 2013. https://doi.org/10.1155/2013/936257.
Hassan R, Masoodi MH. Saussurea lappa: a comprehensive review on its pharmacological activity and phytochemistry. Curr Traditional Med. 2020. https://doi.org/10.2174/2215083805666190626144909.
El-Nashar HAS, Adel M, El-Shazly M, Yahia IS, El Sheshtawy HS, et al. Chemical Composition, Antiaging Activities and Molecular Docking Studies of essential oils from Acca sellowiana (Feijoa). Chem Biodivers. 2022. https://doi.org/10.1002/cbdv.202200272.
El-Nashar HAS, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, et al. GC/MS analysis of essential oil and enzyme inhibitory activities of Syzygium cumini (pamposia) grown in Egypt: Chemical characterization and molecular Docking Studies. Molecules. 2021. https://doi.org/10.3390/molecules26226984.
El-Nashar HAS, El-Labbad EM, Al-Azzawi MA, Ashmawy NS. A New Xanthone Glycoside from Mangifera indica L.: Physicochemical Properties and in Vitro Anti-Skin Aging Activities. Molecules (Basel, Switzerland). 2022. https://doi.org/10.3390/molecules27092609.
Elgharabawy RM, El Sayed TE, Abd-Allah Rezk I, Tousson N. E. Therapeutic impact of Costus (Saussurea lappa) Against Ehrlich Solid Tumor-Induced Cardiac toxicity and DNA damage in female mice. Frontiers in pharmacology. 2021. https://doi.org/10.3389/fphar.2021.708785.
El-Nashar HAS, Mostafa NM, El-Badry MA, Eldahshan O, A,Singab ANB. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (cav.) cabrera leaf and bark grown in Egypt. Nat Prod Res. 2021. https://doi.org/10.1080/14786419.2020.1765343.
El-Nashar HAS, Sayed AM, El-Sherief HAM, Rateb ME, Akil L, et al. Metabolomic profile, anti-trypanosomal potential and molecular docking studies of Thunbergia grandifolia. J Enzyme Inhib Med Chem. 2023. https://doi.org/10.1080/14756366.2023.2199950.
Ishaq AR, El-Nashar HAS, Younis T, Mangat MA, Shahzadi M, et al. Genus Lupinus (Fabaceae): a review of ethnobotanical, phytochemical and biological studies. J Pharm Pharmacol. 2022. https://doi.org/10.1093/jpp/rgac058.
Jamaddar S, Sarkar C, Akter S, Mubarak MS, El-Nashar HA et al. Brazilin: an updated literature-based review on its promising therapeutic approaches and toxicological studies. South Afr J Bot. 2023.
Patel AA, Amanullah M, Elsaid FG, Soliman T, Eissa M et al. In-vitro evaluation of anti-cancer and genotoxic potential of medicinal herb Saussurea lappa extract in human cancer cell lines. European Journal of Molecular & Clinical Medicine. 2020.
Kinchington PR, Romanowski EG, Jerold Gordon Y. Prospects for adenovirus antivirals. J Antimicrob Chemother. 2005. https://doi.org/10.1093/jac/dki057.
Emam MH, Nageh H, Ali F, Taha M, ElShehaby HA, et al. Inhibition of SARS-CoV-2 spike protein entry using biologically modified polyacrylonitrile nanofibers: in vitro study towards specific antiviral masks. RSC Adv. 2022. https://doi.org/10.1039/d2ra01321e.
El-Nashar HAS, Mostafa NM, Eldahshan O, A,Singab. A N B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Natural product research. 2022. https://doi.org/10.1080/14786419.2020.1864365.
Abdelghffar EA, El-Nashar HA, Al-Mohammadi AG, Eldahshan OA. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021. https://doi.org/10.1039/d1fo01905h.
Bhuia MS, Chowdhury R, Sonia FA, Kamli H, Shaikh A, et al. Anticancer potential of the plant-derived saponin Gracillin: a Comprehensive Review of mechanistic approaches. Chem Biodivers. 2023. https://doi.org/10.1002/cbdv.202300847.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. https://doi.org/10.1016/0022-1759(83)90303-4.
Xue Y, Zhang T, Zhang B, Gong F, Huang Y, et al. Cytotoxicity and apoptosis induced by silver nanoparticles in human liver HepG2 cells in different dispersion media. J Appl Toxicology: JAT. 2016. https://doi.org/10.1002/jat.3199.
Loutfy SA, Shalaby RH, Hamed AR, Mohamed MB, Barakat A et al. Evaluation of cytotoxic effect of metallic nanoparticles in an in vitro liver cancer model. Chem Pharm Res. 2015.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008. https://doi.org/10.1038/nprot.2008.73.
Paek AR, Lee CH, You HJ. A role of zinc-finger protein 143 for cancer cell migration and invasion through ZEB1 and E-cadherin in colon cancer cells. Mol Carcinog. 2014. https://doi.org/10.1002/mc.22083.
Loutfy SA, Abdel-Salam AI, Moatasim Y, Gomaa MR, Abdel Fattah NF, et al. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil-CNPs) against SARS-CoV-2 (in silico and in vitro study). RSC Adv. 2022. https://doi.org/10.1039/d2ra00905f.
Elmahdy EM, Ahmed NI, Shaheen MNF, Mohamed EB, Loutfy SA. Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J Water Health. 2019. https://doi.org/10.2166/wh.2019.303.
Iwashina T, Smirnov SV, Damdinsuren O, Kondo K. Saussurea species from the Altai Mountains and adjacent area, and their flavonoid diversity. Bull Natl Mus Nat Sci. 2010.
Alaagib RM, O,Ayoub SMH. On the chemical composition and antibacterial activity of Saussurea lappa (Asteraceae). The Pharma Innovation. 2015.
Dawa Z, Zhou Y, Bai Y, Gesang S, Liang J, et al. Analysis of Saussurea species from tibet using HPLC-DAD-ESI-MS n. Acta Chromatographica. 2010. https://doi.org/10.1556/achrom.22.2010.1.11.
Dawa Z, Zhou Y, Bai Y, Gesang S, Bai B, et al. Development of an HPLC-DAD–ESI-MSn method for quantitative analysis of Saussurea tridactyla. J Pharm Biomed Anal. 2008. https://doi.org/10.1016/j.jpba.2008.08.016.
Ferreres F, Llorach R, Gil-Izquierdo A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J mass Spectrometry: JMS. 2004. https://doi.org/10.1002/jms.586.
Liao Z-X, Zhang B-B, Ding L-S, Zhou Y. Development of an UPLC-QTOF-MS method for qualitative and quantitative analysis of Saussurea eopygmaea. Acta Chromatographica. 2013. https://doi.org/10.1556/achrom.25.2013.1.11.
Madhuri K, Elango K, Ponnusankar S. Saussurea lappa (Kuth root): review of its traditional uses, phytochemistry and pharmacology. Orient Pharm Experimental Med. 2012. https://doi.org/10.1007/s13596-011-0043-1.
Antunes-Ricardo M, Moreno-García BE, Gutiérrez-Uribe JA, Aráiz-Hernández D, Alvarez MM et al. Induction of apoptosis in colon cancer cells treated with isorhamnetin glycosides from Opuntia ficus-indica pads. Plant foods for human nutrition (Dordrecht, Netherlands). 2014. https://doi.org/10.1007/s11130-014-0438-5.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004. https://doi.org/10.1016/j.cell.2004.12.012.
Xu X, Peng W, Liu C, Li S, Lei J, et al. Flavone-based natural product agents as new lysine-specific demethylase 1 inhibitors exhibiting cytotoxicity against breast cancer cells in vitro. Bioorg Med Chem. 2019. https://doi.org/10.1016/j.bmc.2018.12.013.
Fukuda K, Akao S, Ohno Y, Yamashita K, Fujiwara H. Inhibition by costunolide of phorbol ester-induced transcriptional activation of inducible nitric oxide synthase gene in a human monocyte cell line THP-1. Cancer Lett. 2001. https://doi.org/10.1016/s0304-3835(00)00704-7.
Choi SH, Im E, Kang HK, Lee JH, Kwak HS, et al. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005. https://doi.org/10.1016/j.canlet.2005.01.011.
Suganya K, Poornima A, Sumathi S, Chigurupati S, Alyamani NM, et al. Rutin induces endoplasmic reticulum stress-associated apoptosis in human triple-negative breast carcinoma MDA-MB-231 cells-In vitro and in silico docking studies. Arab J Chem. 2022. https://doi.org/10.1016/j.arabjc.2022.104021.
Goodarzi S, Tabatabaei MJ, Mohammad Jafari R, Shemirani F, Tavakoli S, et al. Cuminum cyminum fruits as source of luteolin- 7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat Prod Res. 2020. https://doi.org/10.1080/14786419.2018.1519824.
Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003. https://doi.org/10.1034/j.1600-065x.2003.00048.x.
van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006. https://doi.org/10.1038/sj.cr.7310028.
Choi JH, Bogenberger JM, Tibes R. Targeting apoptosis in Acute myeloid leukemia: current status and future directions of BCL-2 inhibition with Venetoclax and Beyond. Target Oncol. 2020. https://doi.org/10.1007/s11523-020-00711-3.
Zheng X, Guo R, Liu Q, Wakae K, Watanabe N et al. Identification of natural compounds extracted from crude drugs as novel inhibitors of hepatitis C virus. Biochemical and Biophysical Research Communications. 2021. https://doi.org/10.1016/j.bbrc.2021.06.022.
Ravi L. Procyanidin B2 of Cassia fistula a potent inhibitor of COVID19 protease: a molecular dynamic simulation analysis. Asian J Pharm (AJP). 2020.
Shahat A, Ismail S, Hammouda F, Azzam S, Lemiere G, et al. Anti-HIV activity of flavonoids and proanthocyanidins from Crataegus sinaica. Phytomedicine. 1998. https://doi.org/10.1016/S0944-7113(98)80010-X.
Zhang W, Qiao H, Lv Y, Wang J, Chen X, et al. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0110429.
Chiang LC, Ng LT, Cheng PW, Chiang W, Lin CC. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clinical and experimental pharmacology & physiology. 2005. https://doi.org/10.1111/j.1440-1681.2005.04270.x.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Heba A. S. El-Nashar: Data analysis, writing original draft, & editing, Omayma A. Eldahshan: Conceptualization, data analysis, reviewing and editing the manuscript, Nasra F Abdel Fattah has performed all biological and virological experiments, Samah A Loutfy has supervised and edited biological and virological investigations, Ibrahim M Abdel-Salam Conceptualization, data analysis, reviewing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Nashar, H.A., Eldahshan, O.A., Fattah, N.F.A. et al. HPLC-ESI/MS-MS characterization of compounds in Dolomiaea costus extract and evaluation of cytotoxic and antiviral properties: molecular mechanisms underlying apoptosis-inducing effect on breast cancer. BMC Complement Med Ther 23, 354 (2023). https://doi.org/10.1186/s12906-023-04164-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-04164-9