Abstract
Background
Cancer remains a global health concern and constitutes an important barrier to increasing life expectancy. Malignant cells rapidly develop drug resistance leading to many clinical therapeutic failures. The importance of medicinal plants as an alternative to classical drug discovery to fight cancer is well known. Brucea antidysenterica is an African medicinal plant traditionally used to treat cancer, dysentery, malaria, diarrhea, stomach aches, helminthic infections, fever, and asthma. The present work was designed to identify the cytotoxic constituents of Brucea antidysenterica on a broad range of cancer cell lines and to demonstrate the mode of induction of apoptosis of the most active samples.
Methods
Seven phytochemicals were isolated from the leaves (BAL) and stem (BAS) extract of Brucea antidysenterica by column chromatography and structurally elucidated using spectroscopic techniques. The antiproliferative effects of the crude extracts and compounds against 9 human cancer cell lines were evaluated by the resazurin reduction assay (RRA). The activity in cell lines was assessed by the Caspase-Glo assay. The cell cycle distribution, apoptosis via propidium iodide (PI) staining, mitochondrial membrane potential (MMP) through 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) staining, and the reactive oxygen species (ROS) via 2´,7´-dichlorodihydrofluoresceine diacetate (H2DCFH-DA) staining, were investigated by flow cytometry.
Results
Phytochemical studies of the botanicals (BAL and BAS) led to the isolation of seven compounds. BAL and its constituents 3, (3-(3-Methyl-1-oxo-2-butenyl))1H indole (1) and hydnocarpin (2), as well as the reference compound, doxorubicin, had antiproliferative activity against 9 cancer cell lines. The IC50 values varied from 17.42 µg/mL (against CCRF-CEM leukemia cells) to 38.70 µg/mL (against HCT116 p53−/− colon adenocarcinoma cells) for BAL, from 19.11 µM (against CCRF-CEM cells) to 47.50 µM (against MDA-MB-231-BCRP adenocarcinoma cells) for compound 1, and from 4.07 µM (against MDA-MB-231-pcDNA cells) to 11.44 µM (against HCT116 p53+/+ cells) for compound 2. Interestingly, hypersensitivity of resistant cancer cells to compound 2 was also observed. BAL and hydnocarpin induced apoptosis in CCRF-CEM cells mediated by caspase activation, the alteration of MMP, and increased ROS levels.
Conclusion
BAL and its constituents, mostly compound 2, are potential antiproliferative products from Brucea antidysenterica. Other studies will be necessary in the perspective of the discovery of new antiproliferative agents to fight against resistance to anticancer drugs.
Similar content being viewed by others
Background
Cancer remains an incredible human killer and is recognized globally as an important barrier to increasing life expectancy. An incredible number of deaths is linked to tumors in many tissues such as the breast (685 thousand), stomach (769 thousand), liver (830 thousand), colon and rectum (916 thousand), and lung (1,8 million) [1]. It becomes even more dreadful as cancer cells rapidly develop drug-resistant phenotypes, leading to many clinical therapeutic failures and consequently an increased economic burden for patients and societies. Many factors are responsible for the development of recalcitrant tumors. These mostly include the development of resistance to chemically unrelated cytotoxic molecules resulting in the increased energy-dependent proteins that expelled compounds from cells; these also include the insensitivity to apoptosis induced by the cytotoxic molecule as well as the drug-detoxifying mechanisms [2]. Fighting cancer drug resistance appears as a challenging issue in chemotherapy, one of the major modes of treatment [3, 4].
The exploitation of medicinal plants traditionally used under rigorously well-conducted scientific investigations in vitro, in vivo, or in silico, has contributed significantly to the improvement of human health, with many pharmaceuticals deriving from botanicals [5,6,7].
The search for anticancer drugs capable of counteracting recalcitrant cancer from African medicinal plants has produced interesting results during the past two decades [8,9,10,11,12,13,14,15]. Some African plant extracts that have previously induced hypersensitivity of resistant cancer cells including Ambrosia maritma [8], Imperata cylindrica [16], Cola pachycarpa, Curcuma longa [14]. Similarly, some phytochemicals from African medicinal plants possessing corresponding effects include salvimulticanol and salvimulticaoic acid [17], maculine B [18], 5,7-dihydroxy-4'-methoxy-6,8-diprenylisoflavone [19], 8,8-bis-(dihydroconiferyl)-diferulate, aridanin, kihadanin B, progenin III, soyauxinium chloride [20,21,22,23,24,25].
Throughout Africa, one of the limits of the discovery of antiproliferative drugs remains the absence of clinical studies. Pending the implementation and intensification of clinical studies across the continent, scientists should increase as much as possible the library of bioactive molecules that can later undergo clinical studies. This is the rationale to perform this work aimed at evaluating the cytotoxic potential of botanicals and phytoconstituents of Brucea antidysenterica J. F. Mill. (Simaroubaceae) towards refractory cancer cells. The cellular mode of action of leaves methanol extract of Brucea antidysenterica (BAL) and its constituent, hydnocarpin (2) is also investigated. The plant is an erect tree native to Angola, Cameroon, Congo, Ethiopia, Nigeria, Tanzania, Upper Guinea, and Zimbabwe [26]. Brucea antidysenterica is traditionally used to treat cancer, dysentery, malaria, diarrhea, stomach aches, helminthic infections, fever, and asthma [27,28,29]. Its root and bark extracts earlier displayed the cytotoxic effects against PC-3 (prostate), A-549 (lung), and MCF-7 (breast) cancer cell lines [30]. However, the present study reports for the first time the antiproliferative activities of this plant on various models of drug-resistant cancer cells. Earlier phytochemical investigations of the root and bark of Brucea antidysenterica led to the identification of several secondary metabolites belonging to alkaloids, coumarins [30], triterpene quassinoids [29], sterols, fatty acids, and pregnane glycosides [31], and quassinoid glycosides [32] in this plant.
Methods
Chemicals
The phytoconstituents investigated herein were 3, (3-(3-methyl-1-oxo-2-butenyl))1H indole (1), hydnocarpin (2), (20R)-O-(3)-α-L-arabinopyranosyl-pregn-5-en-3β,20-diol (3), (20R)-O-(3)-β-D-glucopyranosyl(1 → 2)-α-L-arabinopyranosyl-pregn -5-en-3β,20-diol (4), canthin-6-one (5), cleomiscosin C (6), bruceolline F or 3-(2’,3’-dihydroxy-3’-méthylbutyl)-1N-β-glucopyranosylindole (7) (Fig. 1; Table 1). These compounds were purified from the methanol extracts of the leaves (BAL; 1–4) and stem (BAS; 1, 5–7) of Brucea antidysenterica J. F. Mill. (Simaroubaceae) as described below. Doxorubicin (purity > 98.0%) was as a drug control while geneticin (purity > 98%) was used to maintain the feature of the transfected cells. They were both obtained from Sigma-Aldrich (Taufkirchen Germany). The 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) used for the analysis of mitochondrial membrane potential was purchased from Biomol (Hamburg, Germany). The 2´,7´-dichlorodihydrofluorescein diacetate (H2DCFH-DA) used for the evaluation the levels of reactive oxygen species (ROS) as well as the reference molecule for ROS experiment, the hydrogen peroxide (H2O2), the reference mitochondrial gradient dissipation compound, valinomycin, and dimethyl sulfoxide (DMSO) were also purchased from Sigma-Aldrich.
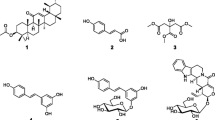
Chemical structure of compounds isolated from the leaves and stem of Brucea antidysenterica. 3, (3-(3-Methyl-1-oxo-2-butenyl))1H indole (1), hydnocarpin (2), (20R)-O-(3)-α-L-arabinopyranosyl-pregn-5-en-3β,20-diol (3), (20R)-O-(3)-β-D-glucopyranosyl(1 → 2)-α-L-arabinopyranosyl-pregn -5-en-3β,20-diol (4), canthin-6-one (5), cleomiscosin C (6), bruceolline F (7)
Plant material
The stem and leaves of Brucea antidysenterica were collected on May 2016 and September 2017 in Bazou in the West region of Cameroon (5° 03′ 60.00" N / 10° 27′ 59.99" E). No authorization to collect the plant sample was needed. The appropriate authorisation has been obtained for the collection of the plant and its use has been carried out in accordance with the relevant guidelines. The plant material (leave, bark, roots, whole plant picture) was authenticated by Mr Nana Victor at the Cameroon National Herbarium in Yaoundé (Voucher specimen number: 54605/HNC).
Extraction and isolation from leaves
The amounts of 2.2 kg of the air-dried and powdered leaves of Brucea antidysenterica were macerated in methanol (MeOH) for 72 h. After evaporation under reduced pressure, the obtained crude or (methanol) extract (60.5 g) was dissolved in hexane/ethyl acetate (AcOEt) 0.5% then 1%. The extract was evaporated to dryness and the residue to give 36 g was submitted to the column chromatography (CC) over silica gel (40–63 μm, 6 × 50 cm) using n-hexane-AcOEt and AcOEt-MeOH gradients as eluents. In total, 321 fractions of 100 ml each were obtained as follows: [(1–15), n-hexane-AcOEt 5%], [(16–32), n-hexane-AcOEt 7.5%], [(33–49), n-hexane-AcOEt 10%], [(50–66), n-hexane-AcOEt 12.5%], [(67–83), n-hexane-AcOEt 15%], [(84–100), n-hexane-AcOEt 17.5%], [(101–117), n-hexane-AcOEt 20%], [(118–134), n-hexane-AcOEt 25%], [(135–151), n-hexane-AcOEt 30%], [(152–168), n-hexane-AcOEt 40%], [(169–185), n-hexane-AcOEt 50%], [(186–202), n-hexane-AcOEt 60%], [(203–219), n-hexane-AcOEt 70%], [(220–236), n-hexane-AcOEt 80%], [(237–253), AcOEt 100%], [(254–270), AcOEt-MeOH 2.5%], [(271–287), AcOEt-MeOH 5%], [(288–304), AcOEt-MeOH 7.5%], [(305–321), AcOEt-MeOH 10%]. Analytic thin layer chromatography (TLC) was used to pooled them into 19 sub-fractions (F1′-F19′) as follows: fractions 5–11 (F1’); 12–29 (F2’); 30–45 (F3’); 46–60 (F4’); 61–81 (F5’); 82–97 (F6’); 98–116 (F7’); 117–133 (F8’); 134–150 (F9’); 151–164 (F10’); 165–185 (F11’); 186–202 (F12’); 203–220 (F13’); 221–234 (F14’); 235–250 (F15’); 251–269 (F16’); 270–282 (F17’); 283–305 (F18’); 306–321 (F19’). Theses fractions afforded compounds 1 (23.2 mg; yellow powder; from subfraction F5’), 2 (25.9 mg; yellow powder; from subfraction F10’), 3 (13.2 mg; beige powder; from subfraction F17’) and 4 (6.11 mg; white powder; from subfraction F14’) after precipitation and filtration.
Extraction and isolation from the stem
The amounts of 2.3 kg of the air-dried, finely powdered stem of Brucea antidysenterica were extracted with MeOH for 72 h. The solution was evaporated in vacuum to yield a brown residue of 24.10 g. The crude extract (22.10 g) was adsorbed on silica (22.10 g) and chromatographed over a silica gel CC (40–63 μm, 4.5 × 50 cm) with n-hexane-ethly acetate (AcOEt) and CHCl3-MeOH gradients as eluents. Fractions of 100 ml each were collected as follows: [(1–15), n-hexane 100%], [(16–32), n-hexane-AcOEt 2.5%], [(1–15), n-hexane-AcOEt 5%], [(16–32), n-hexane-AcOEt 7.5%], [(33–49), n-hexane-AcOEt 10%], [(50–66), n-hexane-AcOEt 12.5%], [(67–83), n-hexane-AcOEt 15%], [(84–100), n-hexane-AcOEt 17.5%], [(101–117), n-hexane-AcOEt 20%], [(118–134), n-hexane-AcOEt 25%], [(135–151), n-hexane-AcOEt 30%], [(152–168), n-hexane-AcOEt 40%], [(169–185), n-hexane-AcOEt 50%], [(186–202), n-hexane-AcOEt 60%], [(203–219), n-hexane-AcOEt 70%], [(220–236), n-hexane-AcOEt 80%], [(237–253), AcOEt 100%], [(254–270), AcOEt-MeOH 2.5%], [(271–287), AcOEt-MeOH 5%]. The analytic thin layer chromatography (TLC) was used to pooled them into 19 sub-fractions (F1-F19) as follows: fractions 7–15 (F1); 18–29 (F2); 32–48 (F3); 57–64 (F4); 66–81 (F5); 83–99 (F6); 101–107 (F7); 110–129 (F8); 133–147 (F9); 152–162 (F10); 166–184 (F11); 187–200 (F12); 203–219 (F13); 225–230 (F14); 234–248 (F15); 250–269 (F16); 273–289 (F17); 291–303 (F18); 307–319 (F19). Compound 1 (25 mg) was isolated by filtration of the precipitates of the sub-from F2. Compounds 5 (13.9 mg) was isolated as beige powder by filtration of the precipitates of the sub-from F4. From F14, compound 6 (10.5 mg) was obtained respectively as yellow powder. From F18, compound 7 (26 mg) was isolated as a white powder.
Studied cell lines and culture settings
In this work, 9 cancer cell lines with various drug resistance patterns as well as AML12 hepatocytes as normal control cell line were used. The origins of the leukemia cell lines, CCRF-CEM (drug-sensitive) and CEM/ADR5000 (its subline overexpressing multidrug-resistant (MDR) P-glycoprotein (P-gp)) have been reported by several authors [33,34,35]; Similarly, the source of the HepG2 hepatocarcinoma [36], glioblastoma U87MG cells and its sub-line U87MG.ΔEGFR cells transfected with epidermal growth factor receptor (EGFR) [37], HCT116 p53+/+ colon adenocarcinoma cells and its knock-out clone HCT116 p53−/− cells [38], and MDA-MB-231-pcDNA3 breast adenocarcinoma cells and breast cancer resistance protein (BCRP)-transfected, and its multidrug-resistant MDA -MB-231-BCRP clone 23 cells [39]. The AML12 hepatocytes were used for the comparison of the activities of the samples with HepG2 [40]. CCRF-CEM and CEM/ADR5000 cells were kindly provided by Dr. J. Beck (Department of Pediatrics, University of Greifswald, Greifswald, Germany); MDA-MB-231-pcDNA3 and MDA-MB-231-BCRP cells were obtained from Dr. Douglas D. Ross (University of Maryland Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD); HCT116 p53+/+ and HCT116 p53−/− cells were a generous gift from Dr. B. Vogelstein and H. Hermeking (Howard Hughes Medical Institute, Baltimore, MD); U87MG and U87MG.ΔEGFR cells were kindly provided by Dr. W. K. Cavenee (Ludwig Institute for Cancer Research, San Diego, CA); HepG2 and AML12 cells were obtained from ATCC (USA). The RPMI-1640 medium was used for the culture of CCRF-CEM and CEM/ADR5000 cells whilst DMEM medium was used for carcinoma cells. The culture media were supplemented (complete medium) with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Eggenstein, Germany). The CEM/ADR5000 cell line was maintained in a complete medium containing 5000 ng/ml of doxorubicin to maintain its resistant phenotype. To maintain the resistance phenotype of the resistant carcinoma cell lines such as MDA-MB-231/BCRP and HCT116 p53−/−, and U87MG.ΔEGFR cells, the complete DMEM medium containing geneticin at 800 ng/ml and 400 µg/ml, respectively were used [41, 42].
Evaluation of the cytotoxicity using resazurin reduction assay (RRA)
The antiproliferative activity of botanicals (BAL and BAS), phytoconstituents (1–7) from Brucea antidysenterica, and doxorubicin was monitored by RRA [43] under the previously reported work conditions [44,45,46]. Cells were cultured in a standard condition consisted of a humidified 5% CO2 atmosphere and an incubation temperature of 37 °C. The tested concentration ranges were 0.63—80 µg/mL for the botanicals and 0.78—100 µM for phytochemicals. The incubation time of the treated cells was 72 h, and the fluorescence was measured with Infinite M2000 Pro™ plate reader (Tecan, Crailsheim, Germany). The wavelengths used were 544 nm for the excitation and 590 nm for the emission. The IC50 values of samples were referred to as the concentrations required to inhibit 50% of the cell proliferation and determined using calibration curve as reported previously [47]. The degree of resistance (D.R.) was set as the ratio of the IC50 values of samples in the resistant cell line vs that of corresponding sensitive cell line. The selectivity index (S.I.) was the ratio of the IC50 value of AML12 hepatocytes vs. that of HepG2 cells [13, 48].
Evaluation of the cell cycle distribution by flow cytometry
The modification of the cell cycle distribution of CCRF-CEM induced by the botanicals BAL, the flavonolignan 2, the control drug, doxorubicin, or the solvent control DMSO was determined by flow cytometry under the previously reported work conditions [49]. BAL and compound 2 were applied to CCRF-CEM cells (1 ml; 1 × 106 cells) at their ¼ IC50, ½ IC50, IC50, and 2 × IC50 values. The whole was then incubated for 24 h as earlier indicated followed by the analysis of the cell cycle distribution with a BD Accury C6 Flow Cytometer (BD Biosciences, Heidelberg, Germany) [49, 50]. Assays were performed three times in triplicates.
Evaluation of apoptosis by flow cytometry
The annexin V/propidium iodide (PI) staining combined to the flow cytometry was used to detect apoptotic cells in CCRF-CEM cells by BAL, the flavonolignan 2, doxorubicin, or the solvent (DMSO) [20, 25, 46]. BAL and compound 2, as well as doxorubicin were applied to CCRF-CEM cells (1 ml; 1 × 106 cells) at their ¼ IC50, ½ IC50, IC50, and 2 × IC50 values followed by a 24 h incubation as indicated earlier. Afterward, apoptosis detection was undertaken with a fluorescein isothiocynate (FITC)-conjugated annexin V/PI assay kit (eBioscience™ Annexin V; Invitogen, San Diego, USA). The measurement was done using BD Accury C6 Flow Cytometer (BD Biosciences) under experimental conditions previously reported [20, 25]. The assays were done thrice independently with three repetitions each.
Evaluation of caspases activity by Caspase-Glo assay
The activity of caspases in CCRF-CEM cells treated with BAL and the flavonolignan 2 was determined by Caspase-Glo assay [42]. BAL and compound 2 were applied to CCRF-CEM cells (100 µl; 1.5 × 104 cells for caspase 3/7 assay or 3 × 104 cells for caspase 8 and caspase 9 assays) at their ½ IC50, IC50, and 2 × IC50. The whole was then incubated for 6 h (standard culture conditions) and the activity of caspases was detected by spectrophotometry as previously described [42].
Quantification of mitochondrial membrane potential (MMP) alteration and reactive oxygen species (ROS) production by Flow cytometry
The modification of the MMP as well as the ROS levels after the application of BAL, and flavonolignan 2 at ¼ IC50, ½ IC50, IC50, and 2 × IC50 to CCRF-CEM cells (1 × 106) was performed by flow cytometry [45]. DMSO was used as solvent control while valinomycin served as a positive control. The cells were incubated for 24 h and further stained for 30 min with JC-1 [45] and measured (1 × 104 cells) in an LSR-Fortessa FACS analyzer (Becton–Dickinson) [45]. For ROS determination, CCEF-CEM cells were similarly treated BAL, the flavonolignan 2, hydrogen peroxide (H2O2; positive control), DMSO (solvent control) as in the MMP analysis, then incubated for 24 h. They were further stained with H2DCFH-DA and measured (1 × 104 cells) in an LSR-Fortessa FACS analyzer [40, 51, 52].
Statistical analysis
The Graph Pad Prism 5 software was used for the statistical analysis. Data from independent assays are presented as mean value ± standard deviation. The difference between the mean values of test samples and the control, One-way Analysis Variance (ANOVA) and post hoc Tukey’s test was used to establish the significance. The significant differences were regarded as p-value < 0.05.
Results
Phytochemistry
Seven phytochemicals were purified from the methanol extracts of the leaves (1–4) and stem (1, 5–7) of Brucea antidysenterica. Their chemical structures (Fig. 1) were determined from their NMR spectra and a comparison with data from the literature. Their general characteristics phytochemicals 1–7 are given in Table 1. They included three alkaloids: 3, (3-(3-Methyl-1-oxo-2-butenyl))1H indole (1), canthin-6-one (5) and bruceolline F (7), a flavonolignan, hydnocarpin (2), two sterol glycosides: (20R)-O-(3)-α-L-arabinopyranosyl-pregn-5-en-3β,20-diol (3) and (20R)-O-(3)-β-D-glucopyranosyl(1 → 2)-α-L-arabinopyranosyl-pregn -5-en-3β,20-diol (4), and a coumarinolignans, cleomiscosin C (6). The NMR spectra of the isolated phytoconstituents are available as supporting information.
Cytotoxicity
The cytotoxic effects BAL, BAS, their isolated secondary metabolites as well as doxorubicin were determined by RRA towards the studied cell lines. The results are shown in Tables 2 and 3. The IC50 values of BAL (Table 2), phytochemiclas 1 and 2, and doxorubicin (Table 3) were obtained against the nine cancer cell lines investigated. The other tested samples (BAS, compounds 3–7) had selective cytotoxic effects. The obtained IC50 values varied from 17.42 µg/ml (against CCRF-CEM leukemia cells) to 38.70 µg/mL (against HCT116 p53−/− colon adenocarcinoma cells) for BAL (Table 2); from 19.11 µM (against CCRF-CEM cells) to 47.50 µM (against MDA-MB-231-BCRP breast adenocarcinoma cells) for compound 1, from 4.07 µM (against MDA-MB-231-pcDNA cells) to 11.44 µM (against HCT116 p53+/+ cells) for compound 2, and from 0.02 µM (against CCRF-CEM cells) to 122.96 µM (against CEM/ADR5000 cells) for doxorubicin. The three most active samples (BAL, compounds 1 and 2) also displayed lower cytotoxicity (S.I. > 2.12) against AML12 cells vs HepG2 cells (Tables 2 and 3). Hypersensitivity (collateral sensitivity) of MDA-MB-231-BCRP cells vs. MDA-MB-231-pcDNA cells (D.R. of 0.51), and U87.MGΔEGFR glioblastoma cells vs. U87.MG cells (D.R. of 0.69) to BAL was observed (Table 2). This was also the case for CEM/ADR5000 cells compared to CCRF-CEM cells (D.R. of 0.72), HCT116 p53−/− cells compared to HCT116 p53+/+ cells (D.R. of 0.59), and U87.MGΔEGFR cells compared to U87.MG cells (D.R. of 0.58) vis-a-vis compound 2 (Table 3). Normal sensitivity of CEM/ADR5000 cells compared to CCRF-CEM cells (D.R. of 1.08) and HCT116 p53−/− cells compared to HCT116 p53+/+ cells (D.R. of 1.04) to BAL was also noted. The tested cancer cells showed cross-resistance to compound 1 (Table 2).
Cell cycle distribution and apoptosis
The PI staining was applied to study the distribution of the various cycle phases in CCRF-CEM cells treated the test samples. The results summarized in Fig. 2 show that BAL, flavonolignan 2, and doxorubicin induced significant changes (p < 0.05) of various phases of the cycle of CCRF-CEM cells in a dose-dependent manner. The cell cycle was arrested in S and G2/M phases as the result of treatment with BAL and doxorubicin, while that with compound 2 induced G0/G1 cycle arrest. The amounts of cells in sub-G0/G1 phase also change upon treatment with the test samples, with values significantly increasing (p < 0.05) from 2.67 ± 0.2% (1/4 × IC50) to 22.00 ± 1.4% (2 × IC50) with BAL, from 3.41 ± 0.2% (1/4 × IC50) to 38.40 ± 2.6% (2 × IC50) with flavonolignan 2, and from 3.28 ± 1.1% (1/4 × IC50) to 12.05 ± 0.9% (2 × IC50) with doxorubicin. Apoptosis can be suggested by the increase of the cells in sub-G0/G1 phase and can be evidenced by annexin V/PI staining. For instance, the data depicted in Fig. 3 indicate that CCRF-CEM cells treated with BAL moderately underwent early apoptosis with 6.5 ± 0.4% annexin V ( +)/PI (-) cells (2 × IC50) vs. 2.6 ± 0.1% for non-treated control (Q2-UR; Fig. 3) and late apoptosis with 9.5 ± 0.76% annexin V ( +)/PI ( +) cells (2 × IC50) vs. 6.1% for non-treated control (Q2-UR; Fig. 3). Flavonolignan 2 also induced 9.4 ± 0.8% and 18.5 ± 1.3% early and late apoptotic cells at 2 × IC50, respectively.
Cycle distribution of CCRF-CEM cells treated with the crude extract (BAL), hydnocarpin (2) from Brucea antidysenterica, and doxorubicin for 24 h. IC50 values were 17.42 µg/mL for BAL, 4.28 µM for phytochemical 2 and 0.02 µM for doxorubicin. Control cells were treated with DMSO to a final concentration of 0.1%. (**): values are significantly different to that of untreated control (P < 0.05)
Apoptosis in CCRF-CEM cells treated with the crude extract (BAL), hydnocarpin (2) from Brucea antidysenterica, and doxorubicin for 24 h, as determined by the annexin V/PI test. Cells were measured by flow cytometric after annexin V-PI double staining. IC50 values were 17.42 µg/mL for BAL, 4.28 µM for phytochemical 2 and 0.02 µM for doxorubicin. Necrotic cells lose membrane integrity, allowing PI entry. Q2-LL: viable cells exhibit annexin V (-)/PI (-); Q2-LR: early apoptotic cells exhibit annexin ( +)/PI (-); Q2-UR and Q2-UL: late apoptotic cells or necrotic cells exhibit annexin V ( +)/PI ( +) or annexin V (-)/PI ( +)
Caspase activation
The results of the caspase activity resulting in the application of BAL and flavonolignan 2 to CCRF-CEM cells are summarized in Fig. 4. At 2 × IC50, BAL increased the activity of caspases 3/7, 8, and 9 in CCRF-CEM cells by 1.90-fold, 2.12-fold, and 2.67-fold, respectively, whilst that with phytochemical 2 caused increased by 190-fold, 2.12-fold, and 2.67-fold. This is an indication that both the botanical (BAL) and its isolate (compound 2) activated the caspases enzymes.
Activity of caspases in CCRF-CEM cells treated with the crude extract (BAL), hydnocarpin (2) from Brucea antidysenterica for 6 h, IC50 values were 17.42 µg/mL for BAL, 4.28 µM for phytochemical 2. Caspase activity is expressed as percentage (%) compared to untreated cells. Results display mean values ± SD of three assays
Effects of test samples on the mitochondrial membrane and ROS production
After treatment of CCRF-CEM cells with BAL, flavonolignan 2, and valinomycin, the JC-1 staining was applied to analyze the integrity of MMP. The results illustrated in Fig. 5 indicate that the application of BAL and flavonolignan 2 to CCRF-CEM cells considerably altered the MMP. The amounts of 59.0% and 10.4% intact MMP cells resulted from BAL and flavonolignan 2 applications, respectively, at 2 × IC50 vs. 92.6% for non-treated control (Q1; Fig. 5). BAL at 2 × IC50 also induced 30.4% cells with MMP loss (Q2; Fig. 5) and 10.6% cells with disrupted MMP (Q3 and Q4; Fig. 5) in CCRF-CEM cells whereas the phytochemical 2 caused 70.5% cells with MMP loss and 19.1% cells with disrupted MMP. The amounts of 38.8% healthy cells were noted after treatment with valinomycin (10 µM) while 44.5% cells and 16.8% had MMP loosed or disrupted, respectively.
Action of the crude extract (BAL), hydnocarpin (2) from Brucea antidysenterica or valinomycin after 24 h on the mitochondrial membrane potential (MMP) of CCRF-CEM cells. IC50 values were 17.42 µg/mL for BAL, 4.28 µM for phytochemical 2. Q1: cells with undamaged MMP, Q2: Cells with MMP loss, Q3-Q4: cell with disrupted MMP
Regarding ROS production, the application of BAL and flavonolignan 2 to CCRF-CEM cells induced a significant dose-dependent raise (Fig. 6). In effect, the ROS levels in treated cells increased from 0.7% (1/4 × IC50) to 17.80% (2 × IC50) and from 6.00% (1/4 × IC50) to 49.00% (2 × IC50) with BAL and phytoconstituent 12, respectively. H2O2 (positive control) caused significant raise in the ROS levels to 94.30% at 50 µM vs. 0.6% in non-treated CCRF.CEM cells.
Action of the crude extract (BAL), hydnocarpin (2) from Brucea antidysenterica or hydrogen peroxide (H2O2) for 24 h on the production of reactive oxygen species in CCRF-CEM cells. IC50 values were 17.42 µg/mL for BAL, 4.28 µM for phytochemical 2. Shown are mean values ± SD of three independent experiments. Control cells were treated with DMSO to a final concentration of 0.1%. (**): values are significantly different to that of untretaed control (P < 0.05)
Discussion
The importance of medicinal plants in the search of drugs is well established today [5,6,7]. Some illustrative anticancer molecules include vinblastine, vincristine, paclitaxel, or camptothecin [53]. Given the great diversity of plant constituents, botanicals constitute an enormous reservoir for the discovery of antiproliferative molecules to tackle the drug resistance of cancer cells. Substances intended to fight against refractory cancers must be more (i.e., collateral sensitivity or hypersensitivity) or as active (normal sensitivity) on resistant than sensitive cells [48, 54]. Consequently, various resistant cell models were used in the present study to validate the role of the tested botanicals and phytochemicals to combat cancer drug resistance. Their drug-sensitive congeners were also used to determine their collateral sensitivity (hypersensitivity), i.e., more sensitivity of the resistant counterpart to the applied sample. They resistant model of leukemia cell line include CEM/ADR5000 cells. This cell line overexpressed P-gp (ABCB1/MDR1) which is the adenosine triphosphate-binding-cassette transporter (ABC). The resistant carcinoma cell lines included were MDA-MB231/BCRP breast cancer adenocarcinoma cells, HCT116 p53−/− −/− colon adenocarcinoma cells, and U87.MGΔEGFR glioblastoma multiforme cells. HCT116 p53−/− cells had p53 knockout and represent as a model of many suppressor genes [20, 25]; MDA-MB231/BCRP cells bear the breast cancer resistance protein (BCRP) [55]. U87.MGΔEGFR cells harbor a mutation-activated EGFR gene (ΔEGFR) [56, 57]. In this work, the hypersensitivity of MDA-MB-231-BCRP cells vs. MDA-MB-231-pcDNA cells and U87.MGΔEGFR cells vs. U87.MG cells to the botanical (BAL) were obtained. Hypersensitivity of CEM/ADR5000 cells vs. CCRF-CEM cells, HCT116 p53−/− cells vs HCT116 p53+/+ cells, and U87.MGΔEGFR cells vs. U87.MG cells to flavonolignan 2 was also achieved. These are indications that the two most active samples (BAL and compound 2) could help to combat the cancer drug resistance. IC50 values lower than 20 μg/ml for plant extracts or 10 μM for phytochemicals were set as thresholds for good naturally occurring cytotoxic agents [58]. The crude methanol extract from the leaves (BAL) and stem (BAS) of Brucea antidysenterica (BAL) had IC50 ≤ 20 μg/ml on 3/9 and 1/9 cancer cell lines, respectively (Table 2). In addition, the IC50 of BAL was noted towards the nine cancer cell lines investigated, clearly showing that it should more likely be considered instead of BAS. Meanwhile, flavonolignan 2 had IC50 ≤ 10 μM on 8/9 studied cancer cell lines (Table 3). No other compounds displayed IC50 values lower than 10 μM suggesting that phytochemical 2 is the most active cytotoxic constituent of BAL.
The cytotoxicity of the crude extract from Brucea antidysenterica towards various models of drug-resistant cancer cells is reported here for the first time. Nonetheless, other cytotoxic molecules have been isolated from this taxon. The cytotoxicity of the crude extract on sensitive cancer cell lines was also reported. The root and bark extract had cytotoxic effects on A-549 lung carcinoma cells, MCF-7 breast cancer cells, and PC-3 prostate cancer cells (IC50 values varying from 65.1 to 80.5 μg/ml) [30]. This was also the case with the isolated compounds bruceacanthinones A (IC50 values = 195.5 μM on PC-3) and B (IC50 values = 150.3 to 160.5 μM), canthin-6-one (IC50 values = 170.3 to 177.3 μM), 1-methoxycanthin-6-one (IC50 values = 125.8 to 155.1 μM), 2-methoxycanthin-6-one (IC50 values = 121.5 to 130.9 μM), 2-hydroxy-1,11-dimethoxycanthin-6-one (IC50 values = 138.1 to 151.5 μM) and cleomiscosin A (IC50 values = 130.1 to 130.8 μM) [30]. Makong et al. found that cleomiscosin C was not active against the three reported cancer cell line. The low cytotoxicity of canthin-6-one and otherwise that of cleomiscosin C corroborates the results of the present study. The good cytotoxicity of hydnocarpin was reported by Bueno Pérez and his team toward 697 pre-B acute lymphoblastic leukemia cell line with the IC50 value of 8.7 μM [59]. The good cytotoxicity of hydnocarpin was also reported against murine L-1210 (IC50 values = 3.65 μg/ml), and Tmolt3 (IC50 values = 32.94 μg/ml) leukemia leukemia cells, KB nasopharynx cancer cells (IC50 values = 1.15 μg/ml), S-480 colon adenocarcinoma (IC50 values = 2.00 μg/ml), TE-418 osteosarcoma cells (IC50 values = 2.14 μg/ml), and HeLa-S3 uterine cells (IC50 values = 2.02 μg/ml), MB-9812 lung cancer cells (IC50 values = 8.18 μg/ml) [60]. Lee et al. have also reported the IC50 value of 20.3 μM for hydnocarpin toward SW480 colon cancer cells [61].
Apoptosis is recognized as one of the modes of cancer cell death induced by plant extracts and their consituents [9]. It was shown in this work that BAL and flavonolignan 2 induced apoptosis in CCRF-CEM cells. Apoptosis was also observed with hydnocarpin (2) derivatives in the lung and melanoma cancer cells via caspase activation [62]. The activation of caspases as observed in this study corroborates these previous findings. When it's stimulated, mitochondria release the pro-apoptotic proteins that activate caspases leading to apoptosis [63]. The crude extract, BAL, and hydnocarpin modified the MMP in CCRF-CEM cells and increases the levels of ROS, indicating their mode of induction of apoptosis.
Conclusion
This work has demonstrated the antiproliferative potential of the crude extracts and the phytoconstituents of Brucea antidysenterica towards multifactorial drug-resistant cancer cell lines. The main cytotoxic constituents of the plant identified included hydnocarpin and 3, (3-(3-Methyl-1-oxo-2-butenyl))1H indole. Hydnocarpin was the most active compound and induced, together with the leave extract, the apoptotic cell death in leukemia CCRF-CEM cells. The leave extract of Brucea antidysenterica and hydnocarpin have good antiproliferative activities. Further in-depth studies including the investigation of more molecular targets, western blots, and in vivo anticancer studies are to be performed in way of discovering new anticancer drugs from these samples.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- 1 :
-
3, (3-(3-Methyl-1-oxo-2-butenyl))1H indole
- 2 :
-
Hydnocarpin
- 3 :
-
(20R)-O-(3)-α-L-arabinopyranosyl-pregn-5-en-3β,20-diol
- 4 :
-
(20R)-O-(3)-β-D-glucopyranosyl(1 → 2)-α-L-arabinopyranosyl-pregn -5-en-3β,20-diol
- 5 :
-
Canthin-6-one
- 6 :
-
Cleomiscosin C
- 7 :
-
Bruceolline F
- ABC:
-
ATP-binding cassette
- BAL:
-
Methanol extract of the leaves of Brucea antidysenterica
- BAS:
-
Methanol extract of the stem of Brucea antidysenterica
- BCRP:
-
Breast cancer resistance protein
- CC:
-
Column chromatography;
- DMSO:
-
Dimethylsulfoxide
- D.R.:
-
Degree of Resistance
- EGFR:
-
Epidermal growth factor receptor
- AcOEt:
-
Ehyl acetate
- FITC:
-
Flourescein isothiocynate
- H2O2 :
-
Hydrogen peroxide
- H2DCFH-DA:
-
2´,7´-Dichlorodihydrofluoresceine diacetate
- IC50 :
-
50% Inhibitory concentration
- JC-1:
-
The 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- MDR:
-
Multidrug resistant
- MeOH:
-
Methanol
- P-gp:
-
P-glycoprotein
- PI:
-
Propidium iodide;
- RRA:
-
Resazurin reduction assay
- ROS:
-
Reactive oxygen species
- S.I.:
-
Selectivity index
- TLC:
-
Thin layer chromatography
References
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F: Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer 2020, Available 17 May 2022: https://gco.iarc.fr/today.
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27.
Zeino M, Saeed ME, Kadioglu O, Efferth T. The ability of molecular docking to unravel the controversy and challenges related to P-glycoprotein-a well-known, yet poorly understood drug transporter. Invest New Drugs. 2014;32(4):618–25.
Volm M, Efferth T. Prediction of cancer drug resistance and implications for personalized medicine. Front Oncol. 2015;5:282.
Wang T, Jiang X, Ruan Y, Zhuang J, Yin Y. Based on network pharmacology and in vitro experiments to prove the effective inhibition of myocardial fibrosis by Buyang Huanwu decoction. Bioengineered. 2022;13(5):13767–83.
Wang T, Zhou Y, Wang K, Jiang X, Wang J, Chen J. Prediction and validation of potential molecular targets for the combination of Astragalus membranaceus and Angelica sinensis in the treatment of atherosclerosis based on network pharmacology. Medicine (Baltimore). 2022;101(26): e29762.
Wang T, Jiang X, Ruan Y, Li L, Chu L. The mechanism of action of the combination of Astragalus membranaceus and Ligusticum chuanxiong in the treatment of ischemic stroke based on network pharmacology and molecular docking. Medicine. 2022;101(28): e29593.
Saeed ME, Abdelgadir H, Sugimoto Y, Khalid HE, Efferth T. Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug-resistant cancer cells. J Ethnopharmacol. 2015;174:644–58.
Kuete V, Efferth T. African flora has the potential to fight multidrug resistance of cancer. Biomed Res Int. 2015;2015: 914813.
Kuete V, Djeussi DE, Mbaveng AT, Zeino M, Efferth T. Cytotoxicity of 15 Cameroonian medicinal plants against drug sensitive and multi-drug resistant cancer cells. J Ethnopharmacol. 2016;186:196–204.
Saeed MEM, Meyer M, Hussein A, Efferth T. Cytotoxicity of South-African medicinal plants towards sensitive and multidrug-resistant cancer cells. J Ethnopharmacol. 2016;186:209–23.
Alves-Silva JM, Romane A, Efferth T, Salgueiro L. North African medicinal plants traditionally used in cancer therapy. Front Pharmacol. 2017;8:383.
Mbaveng AT, Kuete V, Efferth T. Potential of Central, Eastern and Western Africa medicinal plants for cancer therapy: spotlight on resistant cells and molecular targets. Front Pharmacol. 2017;8:343.
Mbaveng AT, Manekeng HT, Nguenang GS, Dzotam JK, Kuete V, Efferth T. Cytotoxicity of 18 Cameroonian medicinal plants against drug sensitive and multi-factorial drug resistant cancer cells. J Ethnopharmacol. 2018;222:21–33.
El-Seedi HR, Yosri N, Khalifa SAM, Guo Z, Musharraf SG, Xiao J, Saeed A, Du M, Khatib A, Abdel-Daim MM, et al. Exploring natural products-based cancer therapeutics derived from egyptian flora. J Ethnopharmacol. 2021;269: 113626.
Kuete V, Sandjo LP, Wiench B, Efferth T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol. 2013;149:245–53.
Hegazy MF, Hamed AR, El-Halawany AM, Hussien TA, Abdelfatah S, Ohta S, Pare PW, Abdel-Sattar E, Efferth T. Cytotoxicity of abietane diterpenoids from Salvia multicaulis towards multidrug-resistant cancer cells. Fitoterapia. 2018;130:54–60.
Nganou BK, Mbaveng AT, Fobofou SAT, Fankam AG, Bitchagno GTM, Simo Mpetga JD, Wessjohann LA, Kuete V, Efferth T, Tane P. Furoquinolines and dihydrooxazole alkaloids with cytotoxic activity from the stem bark of Araliopsis soyauxii. Fitoterapia. 2019;133:193–9.
Adem FA, Mbaveng AT, Kuete V, Heydenreich M, Ndakala A, Irungu B, Yenesew A, Efferth T. Cytotoxicity of isoflavones and biflavonoids from Ormocarpum kirkii towards multi-factorial drug resistant cancer. Phytomedicine. 2019;58: 152853.
Mbaveng AT, Chi GF, Nguenang GS, Abdelfatah S, Tchangna Sop RV, Ngadjui BT, Kuete V, Efferth T. Cytotoxicity of a naturally occuring spirostanol saponin, progenin III, towards a broad range of cancer cell lines by induction of apoptosis, autophagy and necroptosis. Chem-Biol Interact. 2020;326: 109141.
Mbaveng AT, Damen F, Guefack MF, Tankeo SB, Abdelfatah S, Bitchagno GTM, Çelik İ, Kuete V, Efferth T. 8,8-bis-(Dihydroconiferyl)-diferulate displayed impressive cytotoxicity towards a panel of human and animal cancer cells. Phytomedicine. 2020;70: 153215.
Mbaveng AT, Chi GF, Bonsou IN, Ombito JO, Yeboah SO, Kuete V, Efferth T. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J Ethnopharmacol. 2020;267: 113632.
Mbaveng AT, Chi GF, Bonsou IN, Abdelfatah S, Tamfu AN, Yeboah EMO, Kuete V, Efferth T. N-acetylglycoside of oleanolic acid (aridanin) displays promising cytotoxicity towards human and animal cancer cells, inducing apoptotic, ferroptotic and necroptotic cell death. Phytomedicine. 2020;76: 153261.
Mbaveng AT, Noulala CGT, Samba ARM, Tankeo SB, Fotso GW, Happi EN, Ngadjui BT, Beng VP, Kuete V, Efferth T: Cytotoxicity of botanicals and isolated phytochemicals from Araliopsis soyauxii Engl. (Rutaceae) towards a panel of human cancer cells. J Ethnopharmacol 2020, 267:113535.
Mbaveng AT, Noulala CGT, Samba ARM, Tankeo SB, Abdelfatah S, Fotso GW, Happi EN, Ngadjui BT, Beng VP, Kuete V, Efferth T. The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem-Biol Interact. 2021;333: 109334.
Exell AW, Fernandes A, Wild H: Flora Zambesica. vol. 2. . London (UK): Crown Agents 1963.
Watt J, Breyer-Brandwyk M. The medicinal and poisonous plants of Southern and Easthern Africa. London: Livingstone; 1962.
Grace OM, Fowler DG: Brucea antidysenterica, medicinal plants/plantes medicinales. Mill, JF, Ed; PROTA: Wageningen, The Netherlands 2008.
Cuendet M, Pezzuto JM. Antitumor activity of bruceantin: an old drug with new promise. J Nat Prod. 2004;67(2):269–72.
Makong YS, Mouthé Happi G, Djouaka Bavoua JL, Wansi JD, Nahar L, Kamdem Waffo AF, Martin C, Sewald N, Sarker SD. Cytotoxic stilbenes and canthinone alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules. 2019;24(23):4412.
Makong YS, Fotso GW, Mouthe GH, Lenta B, Rennert R, Sewald N, Arnold N, Wansi JD, Ngadjui BT: Bruceadysentoside A, a new pregnane glycoside and others secondary metabolites with cytotoxic activity from Brucea antidysenterica J. F. Mill. (simaroubaceae). Nat Prod Res 2021, 35(12):2037–2043.
Toyota T, Fukamiya N, Okano M, Tagahara K, Chang JJ, Lee KH: Antitumor agents, 118. The isolation and characterization of bruceanic acid A, its methyl ester, and the new bruceanic acids B, C, and D, from Brucea antidysenterica. J Nat Prod 1990, 53(6):1526–1532.
Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64(2):382–94.
Gillet J, Efferth T, Steinbach D, Hamels J, de Longueville F, Bertholet V, Remacle J. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64(24):8987–93.
Kimmig A, Gekeler V, Neumann M, Frese G, Handgretinger R, Kardos G, Diddens H, Niethammer D. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990;50(21):6793–9.
Kuete V, Nkuete AHL, Mbaveng AT, Wiench B, Wabo HK, Tane P, Efferth T. Cytotoxicity and modes of action of 4′-hydroxy-2′,6′-dimethoxychalcone and other flavonoids toward drug-sensitive and multidrug-resistant cancer cell lines. Phytomedicine. 2014;21(12):1651–7.
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95(10):5724–9.
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282(5393):1497–501.
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95(26):15665–70.
Kuete V, Sandjo LP, Djeussi DE, Zeino M, Kwamou GM, Ngadjui B, Efferth T. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Invest New Drugs. 2014;32:1053–62.
Kuete V, Mbaveng AT, Sandjo LP, Zeino M, Efferth T. Cytotoxicity and mode of action of a naturally occurring naphthoquinone, 2-acetyl-7-methoxynaphtho[2,3-b]furan-4,9-quinone towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2017;33:62–8.
Mbaveng AT, Ndontsa BL, Kuete V, Nguekeu YMM, Celik I, Mbouangouere R, Tane P, Efferth T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine. 2018;43:78–85.
O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267(17):5421–6.
Adem FA, Kuete V, Mbaveng AT, Heydenreich M, Koch A, Ndakala A, Irungu B, Yenesew A, Efferth T. Cytotoxic flavonoids from two Lonchocarpus species. Nat Prod Res. 2019;33(18):2609–17.
Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-mesistant tumor cells. Evid Based Complement Alternat Med. 2013;2013: 285903.
Mbaveng AT, Bitchagno GTM, Kuete V, Tane P, Efferth T. Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy. Phytomedicine. 2019;60: 152832.
Kuete V, Ngameni B, Wiench B, Krusche B, Horwedel C, Ngadjui BT, Efferth T. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011;77:1984–9.
Efferth T, Saeed MEM, Kadioglu O, Seo EJ, Shirooie S, Mbaveng AT, Nabavi SM, Kuete V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol Adv. 2020;38: 107342.
Mbaveng AT, Fotso GW, Ngnintedo D, Kuete V, Ngadjui BT, Keumedjio F, Andrae-Marobela K, Efferth T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine. 2018;48:112–9.
Adem FA, Kuete V, Mbaveng AT, Heydenreich M, Ndakala A, Irungu B, Efferth T, Yenesew A. Cytotoxic benzylbenzofuran derivatives from Dorstenia kameruniana. Fitoterapia. 2018;128:26–30.
Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130(4):1910–7.
Cossarizza A, Ferraresi R, Troiano L, Roat E, Gibellini L, Bertoncelli L, Nasi M, Pinti M. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat Protoc. 2009;4(12):1790–7.
Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–75.
Efferth T, Kadioglu O, Saeed MEM, Seo EJ, Mbaveng AT, Kuete V. Medicinal plants and phytochemicals against multidrug-resistant tumor cells expressing ABCB1, ABCG2, or ABCB5: a synopsis of 2 decades. Phytochem Rev. 2021;20(1):7–53.
Cheng J, Demeulemeester J, Wedge DC, Vollan HKM, Pitt JJ, Russnes HG, Pandey BP, Nilsen G, Nord S, Bignell GR, et al. Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors. Nat Commun. 2017;8(1):1221.
Navolanic PM, Steelman LS, McCubrey JA. EGFR family signaling and its association with breast cancer development and resistance to chemotherapy (Review). Int J Oncol. 2003;22(2):237–52.
Yan GE, Efferth T. Broad-spectrum cross-resistance to anticancer drugs mediated by epidermal growth factor receptor. Anticancer Res. 2019;39(7):3585–93.
Boik J. Natural compounds in cancer therapy. Minnesota USA: Oregon Medical Press; 2001.
Bueno Pérez L, Pan L, Sass E, Gupta SV, Lehman A, Kinghorn AD, Lucas DM. Potentiating effect of the flavonolignan (-)-hydnocarpin in combination with vincristine in a sensitive and P-gp-expressing acute lymphoblastic leukemia cell line. Phytother Res. 2013;27(11):1735–8.
Sharma DK, Hall IH. Hypolipidemic, anti-inflammatory, and antineoplastic activity and cytotoxicity of flavonolignans isolated from Hydnocarpus wightiana seeds. J Nat Prod. 1991;54(5):1298–302.
Lee MA, Kim WK, Park HJ, Kang SS, Lee SK. Anti-proliferative activity of hydnocarpin, a natural lignan, is associated with the suppression of Wnt/β-catenin signaling pathway in colon cancer cells. Bioorg Med Chem Lett. 2013;23(20):5511–4.
Arya JS, Joseph MM, Sherin DR, Nair JB, Manojkumar TK, Maiti KK. Exploring mitochondria-mediated intrinsic apoptosis by new phytochemical entities: An explicit observation of cytochrome c dynamics on lung and melanoma cancer cells. J Med Chem. 2019;62(17):8311–29.
Dejean LM, Martinez-Caballero S, Kinnally KW. Is MAC the knife that cuts cytochrome c from mitochondria during apoptosis? Cell Death Differ. 2006;13(8):1387–95.
Kumar V, Bulumulla HNK, Wimalasiri WR, Reisch J. Coumarins and an indole alkaloid from Pamburus missionis. Phytochemistry. 1994;36(4):879–81.
Afifi MSA, Ahmed MM, Pezzuto JM, Kinghornt AD. Cytotoxic flavonolignans and flavones from Verbascum sinaiticum leaves. Phytochemistry. 1993;34(3):839–41.
Kamperdick C, Sung TV, Thuy TT, Tri MV, Adam G. (20R)-O-(3)-α-L-arabinopyranosyl-pregn-5-en-3β, 20-diol from Brucea javanica. Phytochemistry. 1995;38(3):699–701.
Liu JQ, Wang CF, Li XY, Chen JC, Li Y, Qiu MH. One new pregnane glycoside from the seeds of cultivated Brucea javanica. Arch Pharm Res. 2011;34(8):1297–300.
Fukamiya N, Okano M, Aratani T, Negoro K, McPhail AT, Ju-ichi M, Lee KH: Antitumor agents, 79. Cytotoxic antileukemic alkaloids from Brucea antidysenterica. J Nat Prod 1986, 49(3):428–434.
Koike K, Ohmoto T. Quassinoids from Quassia indica. Phytochemistry. 1994;35(2):459–63.
Anil BR, Sunil KC, Sandeep K. Structures of cleomiscosins, coumarinolignoids of Cleome viscosa seeds. Tetrahedron. 1985;41(1):209–14.
Ouyang KKY, Ohmoto T. Indole alkaloids from Brucea mollis var. Tonkinensis Phytochemistry. 1994;37(2):575–8.
Brahemi G, Kona FR, Fiasella A, Buac D, Soukupova J, Brancale A, Burger AM, Westwell AD. Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. J Med Chem. 2010;53(7):2757–65.
Acknowledgements
ATM, SBT and VK acknowledge the Alexander von Humboldt (AvH) Foundation for the research stays fellowships and trips in Mainz (Germany). Authors grateful to the Institute of Molecular Biology gGmbH (IMB) (Mainz, Germany) where the flow cytometry measurements of MMP were done.
Funding
This study was financially supported by the Alexander von Humboldt Foundation Through the research stays fellowships and trips in Mainz (Germany) to ATM, SBT, and VK.
Author information
Authors and Affiliations
Contributions
LMY, YSDM, ATM, SBT, and VK performed the Laboratory work. GWF, BLN, JDW, NS, and BTN elucidated the chemical structures. VPB, VK and TE designed the study. ATM and VK drafted the manuscript. TE supervised the work and provided the reagents and the equipment for the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research project was approved by the Faculty of Science of the University of Dschang. The plant was identified and authenticated by Mr. NANA Victor as Brucea antidysenterica J. F. Mill. (Simaroubaceae) in comparison with the specimen of the herbarium recorded under the voucher number 54605/HNC.
Consent for application
Not applicable.
Competing interests
VK is a Senior Editor of BMC Complementary Medicine and Therapy; ATM is an Associate Editor of BMC Complementary Medicine and Therapy. The remaining authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Youmbi, L.M., Makong, Y.S.D., Mbaveng, A.T. et al. Cytotoxicity of the methanol extracts and compounds of Brucea antidysenterica (Simaroubaceae) towards multifactorial drug-resistant human cancer cell lines. BMC Complement Med Ther 23, 48 (2023). https://doi.org/10.1186/s12906-023-03877-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-023-03877-1