Abstract
Background
Podocytes have become a crucial target for interventions in proteinuric kidney diseases. Many studies have reported that overexpression of transient receptor potential cation channel protein 6 (TRPC6) in podocyte injury upregulates intracellular Ca2+ influx and stimulates Ca2+-dependent protease calpain-1 signaling. The traditional Chinese drug, tetrandrine, a nonselective Ca2+ channel blocker, has long been used to treat chronic kidney disease. This research aimed to explore the possible mechanisms underlying the anti-proteinuric properties of tetrandrine.
Methods
We investigated the involvement of tetrandrine in Ca2+ dependent calpain-1 signaling in mouse podocytes and adriamycin-induced nephropathy rats. Cyclosporine A (CsA) and U73122 were used as positive controls. Cell viability, cytotoxicity, Ca2+ concentration, calpain activity, and mRNA and protein expression levels of calpain-1 signaling pathways were examined. The clinical and pathological changes were measured.
Results
Tetrandrine decreased intracellular Ca2+ influx in cultured TRPC6-overexpressing podocytes. In both in vitro and in vivo studies, the administration of tetrandrine downregulated calpain activity and the expression of calpain-1 and restored the expression of downstream Talin-1 and nephrin. Compared to CsA, tetrandrine treatment exhibited superior inhibitory effects on calpain activity and calpain-1 expression.
Conclusions
Tetrandrine has therapeutic potential in podocyte damage by blocking Ca2+-dependent activation of the calpain-1 signaling pathway. Tetrandrine reduced proteinuria, improved renal function, and alleviate renal pathological damage.
Similar content being viewed by others
Background
Podocytes are a prerequisite for maintaining the selective permeability of the glomerular filtration barrier and glomerular structural integrity. Podocyte injury is typically associated with proteinuric renal diseases, including focal segmental glomerulosclerosis (FSGS), membranous nephropathy, minimal change disease, and diabetic kidney disease [1,2,3,4]. Protection and restoration of podocytes from injurious factors constitute a major challenge in the successful treatment of kidney disease.
Podocytes are highly specialized, terminally differentiated cells located outside of the glomerular basement membrane and characterized by foot processes. Dysfunction of calcium signaling regulation in podocytes leads to podocyte actin cytoskeletal disorganization, foot process effacement, disruption of the slit diaphragm, and proteinuria. Transient receptor potential cation channel protein 6 (TRPC6), a novel slit diaphragm-associated protein in podocytes, has been identified as a nonspecific Ca2+-conducting ion channel [5,6,7,8]. Glomerular TRPC6 expression is increased in many hereditary and acquired proteinuric diseases. Previous studies have reported that overexpressing of TRPC6 in podocytes triggers a superior increase of intracellular Ca2+ influx, which is believed to be responsible for podocyte cell injury. Increased calcium load within podocytes activates downstream signaling pathways, including calpain-1, Talin-1, nephrin and calcineurin, to modulate the activity or expression of target genes [9,10,11,12].
Fangji Huangqi Tang is a classic traditional Chinese medicine (TCM) for the treatment of nephrotic syndrome [13]. It consists of six herbs: Radix Stephania Tetrandra (the roots of Stephania tetrandra), Radix Astragali (the roots of Astragalus membranaceus), Rhizoma Atractylodis Macrocephalae (the roots of Atractylodes macrocephala), Radix Glycyrrhizae (the roots of Glycyrrhiza uralensis), Rhizoma Zingiberis (the roots of Zingiber officinale) and Fructus Ziziphi Jujubae (Fructus of Ziziphus jujuba) [1]. Tetrandrine, one of the main active components of Radix Stephania Tetrandra, a non-selective Ca2+ channel blocker, has been tested in clinical trials and has been indicated to alleviate proteinuria and improve renal function [14, 15]. A previous study demonstrated the blocking effects of tetrandrine on Ca2+-dependent RhoA signaling in podocytes [16], but the underlying mechanisms was not elucidated. Herein, TRPC6-overexpressing models were developed in vitro and in vivo. Subsequent to administration of tetrandrine, changes in TRPC6-dependent Ca2+ signaling were evaluated, including downstream targets, calpain-1, Talin-1, nephrin and calcineurin. Cyclosporine A (CsA), an inhibitor of calcineurin, widely used in the treatment of proteinuria diseases) [17] and U73122, an inhibitor of TRPC6 channel opening [18] were used as positive controls.
Methods
Cell culture and plasmid construction
Conditionally immortalized mouse podocyte cell line (MPC5) cells, obtained from Professor Peter Mundel (Mount Sinai School of Medicine, USA), were maintained at 33 °C for proliferation, and then shifted to 37 °C for differentiation. The TRPC6 expression plasmid was constructed by cloning the full length of the coding DNA sequence of the TRPC6 gene into the EcoRI/BamHI sites of the pCDH-GFP-PURO vector, and packaged into lentivirus. When differentiated and grown to approximately 80% confluence, podocytes were seeded at a cell density of 4 × 104 per cm2 and incubated in combined with TRPC6-overexpressing lentivirus. The TRPC6 stable cell line, verified using TRPC6 protein and mRNA expression, was used for further experiments. Thus, MPC5 podocytes were divided into three groups, normal control (NC) group, blank lentivirus (Lv-NC) group, and TRPC6-overexpressing (OverTRP) group.
In vitro cell drug treatment and cytotoxicity assay
Cell viability was accessed using the Cell Counting kit-8 (CCK-8) assay, according to the manufacturer’s instructions. A total of 5000 MPC5 podocytes per well were grown in triplicate in 96-well plates. Cells were treated with a series of CsA (0–2 μmol/L; Sigma-Aldrich, St. Louis, MO, USA) and tetrandrine (0–40 μmol/L; Sigma-Aldrich) at 37 °C for 48 h. CCK-8 was then added into the plates (10 μL/well), which were then incubated in the dark for 2 h. Absorbance at 450 nm was measured using a microplate reader (BioTek Instruments, USA). Cytotoxicity was assayed by quantifying, lactate dehydrogenase (LDH) release using the Cytotoxicity LDH Assay Kit-WST (Dojindo Laboratories, Japan). As a positive control, cells were treated with 10 μmol/L U73122 (Calbiochem, USA) at 37 °C for 10 min.
RNA extraction and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was prepared from cultured cells and kidney tissues using RNAiso Plus reagent (Takara, Japan) and reverse-transcribed into cDNA PrimeScript™ RT Master Mix kit (Takara) according to the manufactures’s protocol. qRT-PCR was performed using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, Germany). The primer sequences of target and reference genes are listed in Supplementary Table S1. The data were collected using the ABI Prism 7900HT FAST Real-Time PCR system. Relative quantification of target gene expression was normalized using the comparative 2-ΔΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control.
Western blotting
Total protein samples were extracted from cultured cells and kidney tissues. Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific). Samples were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. The membrane was then blocked overnight at 4 °C with primary antibodies, namely anti-TRPC6 (Santa Cruz, USA), anti-calpain-1 (Abcam, UK), anti-Talin-1 (Abcam), anti-nephrin (Santa Cruz) and anti- calcineurin (Abcam). After washing, the membrane was incubated with goat anti-rabbit IgG (H + L; Jackson ImmunoResearch Laboratories, USA). The membrane was also processed to detect GAPDH (Abcam) for equal loading. The bands recognized by the primary antibody were visualized, and the optical densities of the protein bands were analyzed using TanonImage software (Tanon, China).
Determination of cytosolic Ca2+ in cultured podocytes
MPC5 podocytes were seeded and treated with tetrandrine, CsA and U73122 for 48 h, 48 h, and 10 min, respectively. Podocytes were then incubated with RPMI-1640 supplemented with 10 μM Fluo-3 AM (Dojindo Laboratories) for 30 min in the dark. Thereafter, they were immediately washed with phosphate-buffered saline, in which they were maintained and excited at 488 nm. The relative concentration of cytosolic Ca2+ ion was evaluated by measuring fluorescence intensity using a Zeiss laser confocal imaging system (Zeiss, USA).
Measurement of calpain activity
Calpain activity was determined using the Calpain Activity Assay Kit from Abcam Inc., according to the manufacturer’s protocol. The supernatant (150 uL) of podocytes or kidney tissues was taken in the appropriate buffer solution (145 mmol/L Nacl, 100 mmol/L Tris-HCL, pH 7.3) and 0.15 mL N-Succinyl-Leu-Tyr-AMC (500 umol/L). After a 1-h incubation at 37 °C in the dark, the samples were read using a fluorometer equipped with a 360 nm excitation filter and a 440 nm emission filter.
Adriamycin-induced nephropathy (ADRN) rat model
Based on Fermi approximation of sample size [19] and proteinuria difference between the control and model groups in our preliminary experiments, the total number of rats required in our study was determined. A total of 56 healthy male Sprague–Dawley rats, weighing approximately 200 g and aged approximately 8 weeks (Zhejiang Institute of TCM), were randomly divided into seven groups (n = 8 rats/group), namely the ADR group, ADR+ tetrandrine low-dose group (4 mg/kg/d), ADR+ tetrandrine middle-dose group (8 mg/kg/d), ADR+ tetrandrine high-dose group (16 mg/kg/d), ADR+ CsA treated group (30 mg/kg/d), ADR+ middle dose of tetrandrine (8 mg/kg/d) in combination with CsA (30 mg/kg/d) group, and NC group. Rats in the model group and drug treatment groups were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg), and two doses of ADR (4 mg/kg) injected into the caudal vein to develop the FSGS rat model. After 12 weeks, urine samples of the rats were collected for 24 h using metabolic cages, followed by collection of blood and harvest of the kidneys. Clinical efficacy was observed, and the changes in 24-h urine protein, plasma albumin, serum creatinine (Scr), and blood urine nitrogen (BUN) levels were measured using commercially available diagnostic kits with an automated chemistry analyzer (HITACHI 7180).
All experimental procedures involving the use of animals were approved by the Ethics Committee on Animal Experiments at Zhejiang Institute of TCM (Tianmushan Road No.132) and were strictly performed according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Histologic staining, electron microscopy and analysis
Rat kidneys were fixed in paraformaldehyde, dehydrated using a graded series of ethanol, and embedded in paraffin. Sections (5-μm-thick) were stained with hematoxylin and eosin (H&E) and Masson’s trichrome and examined under an optical microscope. Kidneys were perfused and fixed with glutaraldehyde and then cut into 1-mm3 tissue blocks for epoxy embedding. Electron microscopy was performed using standard procedures. Five ultra-micrographs were randomly obtained from each specimen. Podocyte foot processes fusion rate (PFR) was calculated using the following formula: PFR (%) = ∑LFP / ∑LBM × 100%, where ∑LFP is the total length of the fused foot process and ∑LBM is the total length of the peripheral capillary basement membrane (BM).
Statistical analysis
Data are expressed as mean ± standard error of measurement and analyzed using SPSS 20.0 (IBM Statistics SPSS, US) and GraphPad Prism 6 for Windows (GraphPad Software Inc., USA). Normal distribution was assessed by qq plots and S-W test. The data showed a normal distribution. When two groups of data were compared, the independent samples t-test was applied. Differences among three or more independent groups were compared using one-way analysis of variance followed by Tukey’s post hoc test. Statistical significance was set at p < 0.05.
Results
Selection of optimal concentrations and cytotoxicity of tetrandrine, CsA and U73122
The CCK-8 assay was used to detect cell viability. As shown in the Fig. 1a and b, the maximum concentration of 10 μmol/L of tetrandrine and 0.1 μmol/L of CsA did not affect the viability of MPC5 podocytes. The LDH release assay further confirmed that 10 μmol/L tetrandrine and 0.1 μmol/L CsA for 48 h showed no toxicity, whereas 10 μmol/L U73122 for 10 min showed espected toxicity (Fig. 1c). Therefore, the subsequent cell experiments utilized tetrandrine and CsA at 10 μmol/L and 0.1 μmol/L, respectively.
Optimization of drug concentrations. MPC5 podocyte viability under treatment with different concentrations of tetrandrine (a) and CsA (b) detected by CCK8 assay. c The LDH cytotoxicity assay in MPC5 podocytes treated with tetrandrine, CsA and U73122. * P<0.05. NC: normal control. Lv-NC: blank lentivirus. OverTRP: TRPC6-overexpressing
Tetrandrine decreased intracellular Ca2+ influx in the cultured MPC5 cells overexpressing TRPC6
Using plasmid transfection, a cell model of TRPC6 overexpression in MPC5 podocytes was successfully established. qRT-PCR and western blot analysis demonstrated that the expression levels of TRPC6 in the OverTRP group increased significantly compared to that in the NC and Lv-NC groups (p < 0.05; Fig. 2a and b). And Full-length gel and blot of TRPC6 was included in the Supplementary Fig. S1.
Construction of TRPC6 overexpressing cell model, and detection of Ca2+ influx among groups. The mRNA and protein levels of TRPC6 were determined by qRT-PCR (a) and western blotting (b), respectively. The intracellular Ca2+ influx was determined using Fluo-3 AM under confocal microscope (c) and semiquantitative analysis (d). Different letters represent statistically different values (P < 0.05), whereas the same letters indicate no significant difference
Fluorescence imaging (Fig. 2c) and semi-quantitative analysis (Fig. 2d) indicated that overexpression of TRPC6 enhanced intracellular Ca2+ influx, whereas there were no obvious differences between the NC and Lv-NC groups. Then OverTRP group cells were then treated with the experimental drugs. The drug pretreatment groups (tetrandrine, CsA and U73122) had inhibitory effects on intracellular Ca2+ influx (P < 0.05), while there was no statistically significant difference among the three groups of drugs.
Effects of tetrandrine on activity and the expression of calpain-1, calcineurin, Talin- 1 and nephrin in MPC5 cells
It has been shown that intracellular Ca2+ activates calpain, a Ca2+-dependent cysteine protease, and calcineurin, a Ca2+/calmodulin-dependent protein phosphatase. In line with previous observations, the activity and expression levels of calpain and calcineurin in the OverTRP group were all higher than those in the NC and Lv-NC groups (p < 0.05; Fig. 3a-e, h and i). Drup intervention in cultured TRPC6-overexpressing podocytes established that tetrandrine inhibited calpain activity and calpain-1 expression. The calpain activity and calpain-1 mRNA levels were significantly reduced in the tetrandrine-exposed group than in the CsA intervention group (p < 0.05; Fig. 3a and d). Western blotting results demonstrated the considerable decrease in calpain-1 protein levels because of tetrandrine (p < 0.05; Fig. 3c and h). In addition, calcineurin activity was significantly reduced (p < 0.05; Fig. 3b) after tetrandrine exposure, but the mRNA and protein expression levels of calcineurin remain unchanged (Fig. 3e and i).
Tetrandrine decreased Ca2+-dependent calpain-1 signaling in cultured podocytes. a Calpain and b calcineurin activity were measured. c Typical bands showing the protein levels of TRPC6, calpain-1, calcineurin, Talin-1 and nephrin, normalized to GAPDH. Detection of mRNA d-g and protein levels h-k of calpain-1, calcineurin, Talin-1 and nephrin, respectively. CsA and U73122 were used as positive controls. Different letters represent statistically different values (P < 0.05), whereas the same letters indicate no significant difference
Treatment with CsA did not block calpain activation (Fig. 3a), and the effect was not as robus as that of tetrandrine in calpain-1 mRNA expression (p < 0.05; Fig. 3d). In addition, the inhibiting effect on the activity and expression of calcineurin was more obvious in the CsA-treated group than in the tetrandrine-treated group (p < 0.05; Fig. 3b, e and i).
The podocyte cytoskeleton-associated anchoring protein Talin-1 and the slit diaphragm protein nephrin are two specific targets of calpain-1. When treated with tetrandrine, there was an increasing trend in mRNA and protein expression of Talin-1 (p > 0.05) and nephrin (p < 0.05), compared with that in the OverTRP group, but this finding was not statistically significant among the three treatment groups (p > 0.05; Fig. 3f, g, j, and k).
Full-length gels and blots were included in the Supplementary Fig. S1.
In vivo experiments using a rat model of ADRN
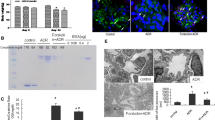
To characterize the response to tetrandrine-mediated inhibition of TRPC6/calpain-1 signaling in vivo, a model of ADRN was developed in rats. The mRNA and protein expression profiles of TRPC6 were significantly increased in the ADRN group compared to those in the normal-chow fed rats (NC group; p < 0.05; Fig. 4a and b). Consistent with the above studies in cell-based models, the activity and expression levels of calpain and calcineurin increased remarkably in ADRN group (p < 0.05; Fig. 4c-g, j and k) than in the NC group.
Tetrandrine-mediated inhibition of Ca2+/calpain-1 signaling in adriamycin-induced nephropathy rats. TRPC6 overexpressing rat model was established (a, b). c Calpain and (d) calcineurin activity was measured. e Typical bands showing the protein levels of TRPC6, calpain-1, calcineurin, Talin-1 and nephrin, normalized to GAPDH. The mRNA (f-i) and protein expressions (j-m) of calpain-1, Talin-1, calcineurin and nephrin, respectively. CsA was used as the positive control. Different letters represent statistically different values (P < 0.05), whereas the same letters indicate no significant difference
To determine the effect of tetrandrine, the rats were treated at various concentrations as indicated. A significant decrease in the activity of calpain and calcineurin was observed after tetrandrine exposure, compared with that in the ADR group (p < 0.05; Fig. 4c and d). The mRNA and protein levels of calpain-1 declined significantly in the tetrandrine middle- and high-dose groups (p < 0.05), whereas their levels were maintained in the low-dose group (p > 0.05), compared with those in the ADR group (Fig. 4f and j). The mRNA expression level of calcineurin in the high dose of tetrandrine group was down compared to the ADR group (Fig. 4g).
Treatment with CsA did not block the calpain activation induced by TRPC6 overespression (Fig. 4c), and the effect was not as robus as that of tetrandrine on calpain-1 protein expression (p < 0.05; Fig. 4j). In addition, the inhibiting effect on the activity and expression of calcineurin was more obvious in the CsA-treated group than in the tetrandrine-treated group (p < 0.05; Fig. 4d, g and k).
Furthermore, the expression of Talin-1 and nephrin were evaluated (Fig. 4h, i, l, and m). Both Talin-1 and nephrin levels were reduced in ADRN rats compared to that in the NC group (p < 0.05). Upon treatment with tetrandrine, the mRNA levels and protein expression of Talin-1 and nephrin were visibly increased. The high-dose group of tetrandrine recovered the expression of the two structural components, similar to the ADR + CsA group (p > 0.05).
Full-length gels and blots were included in the Supplementary Fig. S2.
Tetrandrine suppressed ADRN progression and rescued histopathology
The effect of tetrandrine on kidney function in Sprague-Dawley rats treated with ADRN was subsequently evaluated (Fig. 5a-d). There were no initial significant differences in 24-h proteinuria, Scr and BUN levels among the groups. Twelve weeks later, the levels of 24 h-proteinuria, Scr and BUN in the ADRN group were statistically increased, whereas that of plasma albumin was markedly reduced, compared to those in the NC group (P < 0.05). After treatment with tetrandrine, the levels of proteinuria, Scr and BUN were markedly decreased, whereas the level of albumin was significantly improved compared to those in the ADR group (P < 0.05). Among the three dose-response subgroups of tetrandrine, a significant difference were observed: the middle dose demonstrated considerably better efficacy than that other two doses (P < 0.05). There was no significant difference in the levels of serum alanine aminotransferase, before and after treatment among groups (P > 0.05; data not shown).
Protective effects of drugs on renal function in adriamycin-induced nephropathy rats. 24 h-proteinuria (a), plasma albumin (b), serum creatinine (c), and blood urea nitrogen (d) were measured and compared. Different letters represent statistically different values (P < 0.05), whereas the same letters indicate no significant difference
Changes in histomorphology are shown in Fig. 6. In ADRN rats, H&E and Masson staining showed FSGS, tubulointerstitial fibrosis, and renal interstitial inflammatory cell infiltration. In addition, electron microscopy imaging of glomeruli revealed highly effaced, flattened, and fused podocyte foot processes with 80–100% podocyte PFR. Rats in the tetrandrine sub-groups (middle-dose and high-dose groups) presented with fewer lesions than the ADR group with various extents, and the improvement in the high-dose group excelled that of the middle-dose group. In the low-, middle-, and high- dose of tetrandrine-treated groups, the podocte PFRs were 70 –80%, 50 –60%, and 30 –40%, respectively. These data suggest that tetrandrine protected against structural damage of renal glomeruli induced by ADR administration. There were no significant differences in pathological changes under light microscope among the groups (high-dose tetrandrine, CsA, and tetrandrine in combination with CsA). Additionally, the podocte PFR in the CsA-treated ADRN group was the same as that in the high-dose of tetrandrine-treated groups.
Discussion
Podocytes have become increasingly crucial as a target for interventions in FSGS and related nephrotic diseases [20, 21]. Herein, based on in vitro and in vivo data, it was demonstrated that the podocyte protection and antiproteinuric effect of tetrandrine, a non-selective Ca2+ channel blocker, was mediated via inhibition of intracellular Ca2+ influx and Ca2+-dependent calpain-1 signaling cascade, including Talin-1, nephrin and calcineurin. Tetrandrine was more effective than in hindering calpain-1 inhibition.
According to previous findings, podocytic TRPC6 is the channel that mainly contributes to changes in Ca2+ influx under normal and pathological conditions [7, 22]. It is widely known that calpain activity is dependent on calcium levels and is associated with kidney injury, particularly renal anti-glomerular BM injury [10, 23]. Among the 15 calpain isoforms identified, calpain-1 has recently been shown to link TRPC6 activity to podocyte injury [24]. Calpain-1 is reported to cleave the podocyte cytoskeleton–associated anchoring protein Talin-1 and the slit diaphragm protein, nephrin, which disturbs podocyte integrity and induce severe proteinuria [8,9,10,11,12, 16]. Calcineurin is a Ca2+-dependent phosphatase that has previously been demonstrated to play a role in podocytes and kidney function. Accumulating evidence increasingly indicates that calpain can cleave and activate calcineurin in a proteolytic manner [25, 26]. This study created TRPC6 overexpression models by virus infection in cultured podocytes and adriamycin injection in SD rats. In accordance, we showed that TRPC6-medicated Ca2+ influx activated the calpain activity and the calpain family member calpain-1 expression. Increased calpain-1 subsequently cleaved the downstream Talin-1 and nephrin.
Tetrandrine is an alkaloid extracted from a traditional China medicine plant and is a non-selective Ca2+ channel blocker. Herein, it was reaffirmed that tetrandrine inhibited TRPC6 overexpression and reduced the influx of Ca2+ in both cells and rats overexpressing TRPC6, as documented previously [16]. More importantly, this study focus on the intervening effect of tetrandrine on the calcium-dependent downstream signaling pathway. It was observed that unlike CsA, tetrandrine reduced calcium-activated neutral protease (calpain) activity. Furthermore, tetrandrine decreased the expression of the calcium-activated protease family, calpain-1, and restored the downregulated expression of Talin-1 and nephrin. In comparison with CsA, tetrandrine administration exhibited superior effects on calpain inactivation and downregulated expression of calpain-1. These results showed that tetrandrine has therapeutic potential in podocyte damage by blocking TRPC6-dependent activation of the calpain-1 signaling pathway.
Phospholipase C (PLC) has been identified to be a TRPC6-binding partner [27]. U73122 inhibits PLC, so that less diacylglycerol is generated and the TRPC6 channel opening is reduced [18, 24]. U73122 was, thus, used in parallel as a positive control, and it did show a noticeable effect in reducing intracellular Ca2+ influx, inhibiting calpain activity and calpain-1 expression in podocytes overexpressing TRPC6. However, in vitro cytotoxicity assays showed apparent podocyte toxicity.
In this study, after 12 weeks of ADR injection, serious proteinuria, hypoalbuminemia, and elevated BUN and Scr levels were evident in ADR--treated rats. Light microscopy revealed FSGS, tubulointerstitial fibrosis, and renal interstitial inflammatory cell infiltration in the ADRN rats. Electron microscopy demonstrated widespread effacements of foot processes. The in vivo data further demonstrated that tetrandrine therapy can reduce proteinuria, improve renal function, and attenuate the progression of glomerulosclerosis. After tetrandrine treatment, pathological changes under light microscope and electron microscope, 24-h proteinuria, plasma albumin, Scr and BUN in ADRN rats were significantly improved compared to those in the NC group.
The calcineurin inhibitor, CsA, has long been used to treat proteinuric kidney diseases [28]. Calcineurin is clinically important as a direct target of the immunosuppressive drug CsA [17, 29]. CsA reduces the expression of calcineurin and diminished calcineurin-mediated nuclear factor of activated T-cells [30,31,32]. Recent evidence supports the stabilizing effect of CsA on the podocyte actin cytoskeleton, such as synaptopodin by blocking the calcineurin-mediated dephosphorylation of synaptopodin [17]. It has also been demonstrated that calpain can cleave and activate calcineurin in a proteolytic manner [25, 26]. Data from this study reaffirmed that CsA inhibited calcineurin activity and expression more strongly than tetrandrine. As previously reported, CsA does not appear to directly affect calpain activity.
A major limitation of the study was the failure to demonstrate a dose-dependent effect among the tetrandrine sub-groups (low-dose, middle-dose, and high-dose groups). One possible explanation could be that the dose range we used was too high for rats. In addition, the individual differences in the animal experiment may be considerably greater than the dose differences. The relatively small sample size may reduce the statistical power. Therefore, further studies including considerably smaller doses and appropriate sample size are needed to ascertain the exact dose range and effect of tetrandrine in ARDN rats.
In summary, in the regulation of calcium signaling pathways, tetrandrine exhibited an obvious inhibitory effect on calpain-1, whereas CsA directly influenced calcineurin inhibition. These findings suggest that combined tetrandrine and CsA treatment may affect multiple targets of the calcium signaling network to improve clinical outcomes. Confirmatory studies are necessary to validate these observations as well as to elucidate the mechanisms of synergistic effects.
Conclusion
In conclusion, this study provides evidence that tetrandrine protects podocytes by inhibiting Ca2+ influx and blocking calcium-dependent protease calpain-1 to cleave Talin-1 and nephrin, both in TRPC6-overexpressing podocytes and in a rat model of ADRN. Tetrandrine is reconfirmed to reduce proteinuria, improve renal function and alleviate renal pathological damage in animal experiments. Taken together, these results suggest that tetrandrine plays an important role in protecting against podocyte injury.
Availability of data and materials
The datasets used and/or analyzed during the current study are contained within the manuscript and the appendix, and available from the corresponding author on reasonable request.
Abbreviations
- FSGS:
-
Focal segmental glomurular sclerosis
- MCD:
-
Minimal change disease
- MN:
-
Membranous nephropathy
- DKD:
-
Diabetic kidney disease
- TRPC6:
-
Transient receptor potential cation channel protein 6
- Ca2+ :
-
Calcium
- TCM:
-
Classic traditional Chinese medicine
- Tet:
-
Tetrandrine
- CsA:
-
Cyclosporine A
- Scr:
-
Serum creatinine
- BUN:
-
Blood urine nitrogen
- PFR:
-
Processes fusion rate
- BM:
-
Basement membrane
- ADRN:
-
Adriamycin-induced nephropathy
- PLC:
-
Phospholipase C
References
Garg P. A review of podocyte biology. Am J Nephrol. 2018;47(Suppl 1):3–13.
Khalilpourfarshbafi M, Hajiaghaalipour F, Selvarajan KK, et al. Mesenchymal stem cell-based therapies against podocyte damage in diabetic nephropathy. Tissue Eng Regen Med. 2017;14:201–10.
Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89:1221–30.
Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307.
Riehle M, Büscher AK, Gohlke B-O, et al. TRPC6 G757D loss-of-function mutation associates with FSGS. J Am Soc Nephrol. 2016;27:2771–83.
Ilatovskaya DV, Blass G, Palygin O, et al. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J Am Soc Nephrol. 2018;29:1917–27.
Reiser J, Polu KR, Möller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–44.
Huang H, You Y, Lin X, et al. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem. 2017;41:163–72.
Verheijden KAT, Sonneveld R, Bakker-van Bebber M, et al. The calcium-dependent protease Calpain-1 links TRPC6 activity to podocyte injury. J Am Soc Nephrol. 2018;29:2099–109.
Peltier J, Bellocq A, Perez J, et al. Calpain activation and secretion promote glomerular injury in experimental glomerulonephritis: evidence from calpastatin-transgenic mice. J Am Soc Nephrol. 2006;17:3415–23.
Tian X, Kim JJ, Monkley SM, et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Investig. 2014;124:1098–113.
Jiang L, Ding J, Tsai H, et al. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp Biol Med (Maywood). 2011;236:184–93.
Liu X, Zhou Q-G, Zhu X-C, et al. Screening for potential active components of Fangji Huangqi tang on the treatment of nephrotic syndrome by using integrated metabolomics based on “correlations between chemical and metabolic profiles”. Front Pharmacol. 2019;10:1261.
Liu KC, Lin YJ, Hsiao YT, et al. Tetrandrine induces apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells by endoplasmic reticulum stress and Ca2+/Calpain pathways. Anticancer Res. 2017;37:6107–18.
Wang YJ, He LQ, Sun W, et al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol. 2012;139:757–64.
Yu J, Zhu C, Yin J, et al. Tetrandrine suppresses transient receptor potential Cation Channel protein 6 overexpression- induced podocyte damage via blockage of RhoA/ROCK1 signaling. Drug Des Devel Ther. 2020;14:361–70.
Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine a. Nat Med. 2008;14:931–8.
Klein RR, Bourdon DM, Costales CL, et al. Direct activation of human phospholipase C by its well known inhibitor U73122. J Biol Chem. 2011;286:12407–16.
Reynolds PS. When power calculations won't do: Fermi approximation of animal numbers. Lab Anim (NY). 2019;48:249–53.
Ahn W, Bomback AS. Approach to diagnosis and Management of Primary Glomerular Diseases due to Podocytopathies in adults: Core curriculum 2020. Am J Kidney Dis. 2020;75:955–64.
Assady S, Wanner N, Skorecki KL, Huber TB. New insights into podocyte biology in glomerular health and disease. J Am Soc Nephrol. 2017;28:1707–15.
Ilatovskaya DV, Staruschenko A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol Renal Physiol. 2015;309:F393–7.
Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801.
Farmer LK, Rollason R, Whitcomb DJ, et al. TRPC6 binds to and activates Calpain, independent of its channel activity, and regulates podocyte cytoskeleton, cell adhesion, and motility. J Am Soc Nephrol. 2019;30:1910–24.
Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61:123–37.
Ding F, Li X, Li B, Guo J, Zhang Y, Ding J. Calpain-mediated cleavage of Calcineurin in Puromycin Aminonucleoside-induced podocyte injury. PLoS One. 2016;11:e0155504.
Kanda S, Harita Y, Shibagaki Y, et al. Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-γ1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell. 2011;22:1824–35.
Schönenberger E, Ehrich JH, Haller H, Schiffer M. The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant. 2011;26:18–24.
Aramburu J, Heitman J, Crabtree GR. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 2004;5:343–8.
el-S A, Doller A, Babelova A, et al. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin a and FK506. J Immunol. 2008;181:2831–45.
Schlöndorff J, Del Camino D, Carrasquillo R, et al. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009;296:C558–69.
Nijenhuis T, Sloan AJ, Hoenderop JG, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol. 2011;179:1719–32.
Acknowledgements
We thank all the individuals who participated in this study.
Funding
This work was supported by Hangzhou Agricultural and Social Development Research Project (2019120B136). The funding body had no role in the design of the study, collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Data interpretation, figure preparation and manuscript writing: Yin Ding. Histological and laboratory analysis and interpretation: Xuanli Tang. Study design, data analysis and interpretation: Yuhui Wang, Dongrong Yu and Caifeng Zhu. Study design, experiment performance, data analysis and figure preparation: Jin Yu. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal procedures were approved by the Animal experiment Ethics Committee of Zhejiang Institute of Traditional Chinese Medicine in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. And all studies involving animals are reported in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Consent for publication
Not applicable.
Competing interests
The authors declare that there exist no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1
. Sequences of the primers used in this study.
Additional file 2: Supplementary Figure S1
. Protein expressions determined by western blotting in cultured podocytes.
Additional file 3: Supplementary Figure S2
. Protein expressions determined by western blotting in adriamycin-induced nephropathy rats.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, Y., Tang, X., Wang, Y. et al. Tetrandrine alleviates podocyte injury via calcium-dependent calpain-1 signaling blockade. BMC Complement Med Ther 21, 296 (2021). https://doi.org/10.1186/s12906-021-03469-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03469-x