Abstract
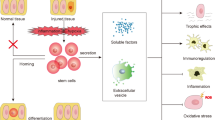
Injury to podocytes is an early event in diabetic nephropathy leading to proteinuria with possible progression to end-stage renal failure. The podocytes are unique and highly specialized cells that cover the outer layer of kidney ultra-filtration barrier and play an important role in glomerular function. In the past few decades, adult stem cells, such as mesenchymal stem cells (MSCs) with a regenerative and differentiative capacity have been extensively used in cell-based therapies. In addition to their capability for regeneration and differentiation, MSCs contributes to their milieu by paracrine action of a series of growth factors via antiapoptotic, mitogenic and other cytokine actions that actively participate in treatment of podocyte damage through prevention of podocyte effacement, detachment and apoptosis. It is hoped that novel stem cell-based therapies will be developed in the future to prevent podocyte injury, thereby reducing the burden of kidney disease.


Similar content being viewed by others
Abbreviations
- AD-MSCs:
-
Adipose-derived mesenchymal stem cells
- Ang II:
-
Angiotensin II
- ASCs:
-
Adult stem cells
- bFGF:
-
Basic fibroblast growth factor
- BM-MSCs:
-
Bone marrow mesenchymal stem cells
- BMP-7:
-
Bone morphogenetic protein-7
- DN:
-
Diabetic nephropathy
- EGF:
-
Epidermal growth factor
- ERK:
-
Extracellular signal-regulated kinase
- ESCs:
-
Embryonic stem cells
- FGF2:
-
Fibroblast growth factor 2
- FM-MSCs:
-
Fetal membranes mesenchymal stem cells
- GBM:
-
Glomerular basement membrane
- GDNF:
-
Glial cell-line derived neurotrophic factor
- HGF:
-
Hepatocyte growth factor
- IGF-I:
-
Insulin-like Growth Factor-I
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- iPSCs:
-
Induced pluripotent stem cells
- JNK:c:
-
Jun amino-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MSCs:
-
Mesenchymal stem cells
- PKC-α:
-
Protein kinase C-alpha
- ROS:
-
Reactive oxygen species
- SCs:
-
Stem cells
- TGF-β R′:
-
Transforming growth factor beta receptor
- TGF-β:
-
Transforming growth factor beta
- TNFα:
-
Tumor necrosis factor alpha
- UC-MSCs:
-
Umbilical cord mesenchymal stem cells
- VEGF:
-
Vascular endothelial growth factor
References
Sarje SK, Ghiware NB, Kawade RM, Gunjkar VN, Vadvalkar SM. Association of chronic complications of type 2 diabetes with the biochemical and physical estimations in subjects attending single visit screening for complications. Int J Res Pharm Chem. 2013;3:842–5.
Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015;11:508–24.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2008;14:631–40.
Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J kidney Dis Off J Natl Kidney Found. 2000;36:646–61.
Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–34.
Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia. 2008;51:1347–55.
Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–5.
Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401.
Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–9.
Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999;48:377–82.
Thirone AC, Scarlett JA, Gasparetti AL, Araujo EP, Lima MH, Carvalho CR, et al. Modulation of growth hormone signal transduction in kidneys of streptozotocin-induced diabetic animals: effect of a growth hormone receptor antagonist. Diabetes. 2002;51:2270–81.
Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, et al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–201.
Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:F830–9.
Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol JASN. 2004;15:1475–87.
Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep. 2007;9:403–8.
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–64.
Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, et al. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006;70:882–91.
Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–102.
Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Investig. 2008;118:1645–56.
Gross ML, Dikow R, Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int Suppl. 2005;94:S50–3.
Saleem MA. Biology of the human podocyte. Nephron Exp Nephrol. 2003;95:e87–92.
Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Choi HY, Chang JH, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–27.
Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol CJASN. 2007;2:529–42.
Chuang PY, He JC. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 2009;111:9–15.
Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47.
Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–14.
Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol. 2014;5:151.
Mundel P. Podocyte biology and response to injury. J Am Soc Nephrol JASN. 2002;13:3005–15.
Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–8.
Salmon AH, Neal CR, Harper SJ. New aspects of glomerular filtration barrier structure and function: five layers (at least) not three. Curr Opin Nephrol Hypertens. 2009;18:197–205.
Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–35.
Tossidou I, Starker G, Kruger J, Meier M, Leitges M, Haller H, et al. PKC-alpha modulates TGF-beta signaling and impairs podocyte survival. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2009;24:627–34.
Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60:1779–88.
Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51:1580–7.
Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–4.
Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Investig. 1997;99:342–8.
Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–60.
Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–72.
White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–9.
Lee HS. Pathogenic role of TGF-β in diabetic nephropathy. J Diabtes Metab. 2013;S9:1–7.
Dessapt C, Baradez MO, Hayward A, Dei Cas A, Thomas SM, Viberti G, et al. Mechanical forces and TGFbeta1 reduce podocyte adhesion through alpha3beta1 integrin downregulation. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2009;24:2645–55.
Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–33.
Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, et al. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–27.
Stieger N, Worthmann K, Schiffer M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metabol Res Rev. 2011;27:207–15.
Terada Y, Inoshita S, Nakashima O, Tamamori M, Ito H, Kuwahara M, et al. Cell cycle inhibitors (p27Kip1 and p21CIP1) cause hypertrophy in LLC-PK1 cells. Kidney Int. 1999;56:494–501.
Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–4.
Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2010;25:3874–84.
Ilic D, Polak JM. Stem cells in regenerative medicine: introduction. Br Med Bull. 2011;98:117–26.
Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respir Int Rev Thoracic Dis. 2013;85:3–10.
Das BC, Tyagi A. Chapter 23—Stem cells: a trek from laboratory to clinic to industry. In: Singh ASV, editor. Animal biotechnology. San Diego: Academic Press; 2014. p. 425–50.
Whitworth DJ, Banks TA. Stem cell therapies for treating osteoarthritis: prescient or premature? Vet J. 2014;202:416–24.
Romano G. Stem cell transplantation therapy: controversy over ethical issues and clinical relevance. Drug News Perzspect. 2004;17:637–45.
Obokata H, Vacanti CA. Chapter 31—Stem cells in tissue engineering. In: Vacanti RLL, editor. Principles of tissue engineering. 4th ed. Boston: Academic Press; 2014. p. 595–608.
Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–73.
Bernard-Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes. 2001;50:S30–5.
Forbes S, Poulsom R, Wright N. Hepatic and renal differentiation from blood-borne stem cells. Gene Ther. 2002;9:625–30.
Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49.
Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol JASN. 2006;17:3028–40.
Ito T. Stem cells of the adult kidney: where are you from? Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2003;18:641–4.
Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Investig. 2004;114:795–804.
Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–55.
Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–97.
Chen J, Park H, Addabbo F, Ni J, Pelger E, Li H, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–89.
Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–55.
Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201.
Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells (Dayton, Ohio). 2003;21:105–10.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol JASN. 2009;20:1053–67.
Burst V, Putsch F, Kubacki T, Volker LA, Bartram MP, Muller RU, et al. Survival and distribution of injected haematopoietic stem cells in acute kidney injury. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2013;28:1131–9.
Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Investig. 2005;115:1743–55.
Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42.
Gazit Z, Pelled G, Sheyn D, Kimelman N, Gazit D. Chapter 19—Mesenchymal stem cells. In: Lanza R, Atala A, editors. Essentials of stem cell biology. 3rd ed. Boston: Academic Press; 2014. p. 255–66.
Prochazkova M, Chavez MG, Prochazka J, Felfy H, Mushegyan V, Klein OD. Chapter 18—Embryonic versus adult stem cells. In: Ramalingam AVSS, editor. Stem cell biology and tissue engineering in dental sciences. Boston: Academic Press; 2015. p. 249–62.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60.
Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–70.
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30:85–92.
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43.
Lv S, Liu G, Wang J, Wang W, Cheng J, Sun A, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17:275–82.
Zhou H, Tian HM, Long Y, Zhang XX, Zhong L, Deng L, et al. Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J. 2009;122:2573–9.
Ezquer F, Ezquer M, Simon V, Pardo F, Yanez A, Carpio D, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1354–65.
Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract. 2014;104:143–54.
Inoki K, Haneda M, Maeda S, Koya D, Kikkawa R. TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999;55:1704–12.
Ali IHA, Brazil DP. Under the right conditions: protecting podocytes from diabetes-induced damage. Stem Cell Res Ther. 2013;4:119/111–2.
Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B, Fu B, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103.
Li L, Truong P, Igarashi P, Lin F. Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol. 2007;18:3067–77.
Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9:444–50.
Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–35.
Guo JK, Schedl A, Krause DS. Bone marrow transplantation can attenuate the progression of mesangial sclerosis. Stem Cells. 2006;24:406–15.
Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–55.
Meyer-Schwesinger C, Lange C, Brocker V, Agustian P, Lehmann U, Raabe A, et al. Bone marrow-derived progenitor cells do not contribute to podocyte turnover in the puromycin aminoglycoside and renal ablation models in rats. Am J Pathol. 2011;178:494–9.
Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465–73.
Qi W, Lu S, Liu G, Cheng J, Song Y, Ming T, et al. Human umbilical cord mesenchymal stem cells eo-culture ameliorates podocytic apoptosis: a possible role of HGF. Zhonghua Shenzangbing Zazhi. 2014;30:933–8.
Zhang L, Li K, Liu X, Li D, Luo C, Fu B, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22:3074–86.
Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–35.
Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. J Am Soc Blood Marrow Transpl. 2013;19:538–46.
Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metabol Syndr. 2014;6:34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Manizheh Khalilpourfarshbafi and Fatemeh Hajiaghaalipour had equal contribution in this work.
Rights and permissions
About this article
Cite this article
Khalilpourfarshbafi, M., Hajiaghaalipour, F., Selvarajan, K.K. et al. Mesenchymal Stem Cell-Based Therapies against Podocyte Damage in Diabetic Nephropathy. Tissue Eng Regen Med 14, 201–210 (2017). https://doi.org/10.1007/s13770-017-0026-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0026-5