Abstract
Background
Ajuga bracteosa has been extensively used traditionally for the treatment of a variety of diseases. The aim of the study was to scientifically validate the wide-scale exploitation of A. bracteosa in folk medicine various in vitro and in vivo assays. Moreover, these activities were related to the intrinsic biologically active phytoecdysteroids of A. bracteosa.
Methods
Aerial and root parts of A. bracteosa were first extracted separately with chloroform (AbCA and AbCR) and the residue was again extracted with methanol (AbMA and AbMR). Total flavonoid and phenolic contents were assayed as quercetin (QE) and gallic acid equivalents (GAE), respectively. These extracts were analyzed for in vitro antioxidant assessment including DPPH and H2O2 (% inhibition of free radicals), and reducing power and phosphomolybdenum methods (ascorbic acid equivalents AAE mg/g DW). Further, these extracts were assayed in vivo in separate groups of Sprague–Dawley rats for carrageenan induced rat paw edema inhibition, hotplate antinociception, forced swim antidepression and anticoagulation. Dose of each crude extract and standard drug given to rats was 200 mg/Kg- and 10 mg/10 mL/Kg body weight respectively. Plant extracts and standard drugs were administered orally, 60 min prior to the conduction of assays. Moreover, biologically active phytoecdysteroids were screened in A. bracteosa with the help of RP-HPLC.
Results
AbMA represented highest values of flavonoids (QE 1.98 % DW) and phenolic contents (GAE 5.94 % DW), significantly scavenged DPPH radicles (IC50 36.9) and reduced ferric ions with 718.4 mg ascorbic acid equivalent/g (AAE). Highest total antioxidant capacity was expressed by AbMR (927 mg AAE) with an IC50 value 19.1 μg/mL. The extracts which were found potent anti-oxidants, were also good at in vivo activities. AbMA significantly reduced edema in all the three hours of treatment (67.9, 70.3 and 74.3 %). AbMA also showed maximum nociceptor suppression in analgesic assay by delaying the time to start licking of paws in rats (57.7 ± 4.9 s). In addition, maximum anti-coagulation was also exhibited by AbMA (89.3 s), while all extracts were found strong antidepressants (≤15.66 s immobility time). Screening of biologically active phytoecdysteroids revealed the presence of 20-hydroxyecdysone (20-HE), makisterone (MKA), cyasterone (CYP) and ajujalactone (AJL). Total phytoecdysteroid content found in A. bracteosa was 1232.5 μg/g DW and 20-HE was most abundant (1232.5 μg/g DW) as compared to other phytoecdysteroids.
Conclusion
Based on the tested in vitro and in vivo activities, AbMA was found to be a promising bioactive extract. These activities can be attributed to the intrinsic polyphenols and phytoecdysteroids contents of A. bracteosa.
Similar content being viewed by others
Background
Stress stimuli (biotic and abiotic) and intrinsic oxygen metabolism generate reactive oxygen species (ROS) as by-products. ROS production inactivates enzymes and damages vital cellular organelles and membranes, consequently causing cancers, aging, chronic inflammation, and plays a role in HIV infection, diabetes etc. [1–3]. ROS also modulate the principal neurotransmitters involved in the neurobiology of depression. Oxidative stress is implicated in depression and anxiety [4–6] and foremost depression is linked to lower levels of several endogenic antioxidant arrays [7]. This indicates that oxidative stress processes might also play a relevant role in depression. ROS are also known to provoke inflammation and associated pain caused by tissue injury [8].
Antioxidants protect biological systems from deleterious effects of ROS by free radicals scavenging mechanism. Antioxidants can mitigate ROS effect by reacting with free radicals, blocking the enzyme generating free radicles and provoking the expression of antioxidant enzymes [9]. Antioxidants include carotenoids, anthocyanidins, catalase, glutathione peroxidase ferritin, superoxide dismutase, ceruloplasmin, catechins, vitamin C, tocopherols vitamin E, glutathione and flavonoids etc. [10, 11]. Synthetic antioxidants like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) show toxicity to living systems [12]. Moreover, myocardial infarctions caused by intravascular blood clots are major cause of deaths worldwide. Although heparin has been used to treat acute thrombotic disorders as a primary drug, yet it poses some complications in its clinical usage [13, 14]. To cope with these problems, there is an increasing demand of alternative antioxidants and antithrombotic agent (anticoagulants). Medicinal plants have historically been used as primary source of antioxidants [15] and anticoagulants [16].
Ajuga bracteosa Wall. ex. Benth. (Family Labiateae) is a perennial plant and is distributed widely in Kashmir and sub-Himalayan tract. It is recommended in Ayurveda to treat numerous ailments [17–19]. Medicinal properties of A. bracteosa, such as astringent, anthelmintic, anti-inflammatory and anti-microbial led its use into folk medicine [17]. In a recently reported quantitative ethnobotanic survey of Khyber Pakhtunkhwa (KPK) province of Pakistan, 92 medicinal plants species were listed. Among them, A. bracteosa represented highest usage frequency (6) especially as blood purifier, carminative, anti-cough, anti-asthma, anti-jaundice and as a cooling agent [20]. Decoction of its bark is used to cure jaundice and sore throat [21]. Leaves extract of A. bracteosa is used as a remedy for acne, pimples, stomach disorders, ear and throats infections and headache. Moreover the extract of leaves of A. bracteosa is regarded as blood purifier and cooling agent e.g. effective in diuretic functions [22]. Moreover, it is found to be pharmacologically active against cancer, hypoglycemia, protozoal diseases, spasmodic activity, gastric ulcer and liver fibrosis [23, 24].
Various extracts of A. bracteosa has been evaluated by some groups worldwide to assess its pharmacological applications. Ethanolic extract of A. bracteosa (70 %: EEAB), when employed over mouse ear edema, resulted in a significant and dose-dependent anti-inflammatory activity at the dose of 0.5 and 1.0 mg/ear [18]. EEAB also resulted in a significant and dose dependent inhibitory effect against acute and chronic arthritic models of albino rats comparable to aspirin [17]. In mice models, CCl4 induced hepatic fibrosis was treated with chloroform extract of A. bracteosa which shielded the liver from injury by reducing the activity of plasma aminotransferase and inhibiting the hepatic mRNA expression of CD14 and TNF-α [24]. Various extracts of A. bracteosa was assessed in Swiss albino mice models for their analgesic potentials in acetic acid-induced writhing test and tail immersion test. Chloroform and aqueous extracts showed significant and dose-dependent analgesic effects at a 200 and 400 mg/Kg, i.p doses [23]. Some extracts of A. chamaecistu were analyzed for formalin induced antinociceptive activity in mice. Hexane fraction at 200 mg/Kg body weight of mice (BWM) caused significant analgesic effects on the chronic phase (15–60 min after formalin injection). However, to obtain the same analgesic effects by aqueous and diethyl ether extracts, a dose of 400 mg/Kg BWM was required [25]. Besides these extracts, methanolic extract of A. bracteosa induced a strong in vivo anti-nociceptive effect in treated animals at a dose of 500 and 750 mg/Kg BWM in three different analgesic assays [26].
These activities can be attributed to the intrinsic natural products of A. bracteosa like phytoecdysteroids, withanolides, clerodanes etc. Phytoecdysteroids are attributed to impart the antioxidant character in A. bracteosa. They also regarded to prevent body from infections and depression [27]. Ecdysteroids, major constituents of A. bracteosa has been exploited commercially and an ecdysteroid containing adaptogenic preparation (Leventon) was found to increase in anticoagulation potential in athletes [28]. Therefore, the assessment of a variety of extracts of A. bracteosa for in vitro antioxidant activities and in vivo anti-inflammatory, analgesic, antidepressant and anticoagulant activities on Sprague–Dawley rats were performed.
Methods
Equipment, chemicals and reagents
The solvents were purchased from Sigma Aldrich, GmbH Buchs Switzerland. Whatman filter paper 1 was used to filter the extracts which were concentrated in BUCHI Rotavapor R-200 rotary evaporator. Dried crude extracts were dissolved with the help of Elmasonic Sonicator (E30-H Germany). Salts, standards, chemicals and reagents were purchased from Sigma Aldrich USA, Merck Germany, Panreac Quimica SA Barcelona Spain and BDH Reagents & Chemicals England. Change in absorbance was recorded by UV–VIS Spectrophotometer (Agilent technologies). For hot plate analgesia, Eddy’s hot plate (IKA, Germany) was used. Paw volume was measured by Plethysmometer (Ugo Basile 7140).
Collection and identification of plant
Ajuga bracteosa Wall. ex Benth. was collected from University Campus of Quaid-i-Azam University (QAU) (Fig. 1). The plant was identified by taxonomist in Plant Sciences department wide a voucher specimen (HMP-460) which was deposited in “Herbarium of medicinal Plants of Pakistan” at QAU Islamabad, Pakistan.
Preparation of extracts
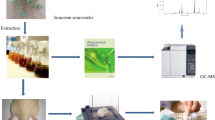
Fresh plant material was rinsed with distilled water and aerial part was separated from root. Both parts were air dried under shade. Extract was prepared by soaking ground plant powder (both parts separately) in chloroform. After 24 h, chloroform extract was filtered with white cotton cloth. The residue was extracted two more times in the same way. Mixture was further filtered and concentrated in rotary evaporator at 40 °C. The subsequent residue was extracted with methanol in the same way as described before. Semi-liquid extracts were dried in fume hood and stored at −20 °C for further usage. The overall scheme of extraction process is shown in Fig. 2.
The names of extracts were abbreviated as; the first two italicized letters represent plant (Ab, Ajuga bracteosa), the third letter represents the solvent (M, Methanol; C, Chloroform) and the last letter represent the plant part (A, Aerial portion; R, Root). For example AbMA represents the methanolic extract of aerial parts of A. bracteosa.
Determination of total flavonoid content
Aluminum chloride colorimetric method [29] was used to assay total flavonoid content with some modifications. Briefly, an aliquot of 0.5 mL of various extracts (1 mg/mL) were added to 1.5 mL of methanol, followed by the addition of aluminum chloride (0.1 mL, 10 %), potassium acetate (1 M, 0.1 mL) and distilled water (2.8 mL). This reaction blend was incubated for 30 min at room temperature and its absorbance was recorded with spectrophotometer at 415 nm. The calibration curve (0 μg/mL to 8 μg/mL) was plotted by using quercetin as a standard. The total flavonoids were expressed as mg quercetin equivalent (QE/g) dry weight.
Determination of total phenolic content
Folin-Ciocalteu reagent method was used to measure total phenolic content [29]. Briefly, an aliquot of various extracts (0.1 mL, 4 mg/mL) was mixed with Folin-Ciocalteu reagent (0.75 mL, 10-fold diluted with dH2O). The resultant mixture was left at room temperature and exactly after 5 min sodium carbonate (0.75 mL of 6 %) was added to it. It was followed by a 90 min of reaction time and finally its absorbance was recorded at 725 nm. The standard calibration (0 μg/mL to 25 μg/mL) curve was plotted by using Gallic acid as standard and total phenolic content was expressed as mg Gallic acid equivalent (GAE/g) per gram dry weight of extract.
In vitro antioxidant assays
Extracts of A. bracteosa were analyzed for their antioxidant potential at 1000, 500, 250, 125, 62.5, 31.25, 15.63 and 7.81 μg/mL concentrations (prepared in DMSO). In all the assessment methods, ascorbic acid was used as positive control. For a more reliable conclusion, extracts were assayed by four different antioxidant determination methods.
2, 2- Diphenyl-1-picryl-hydrazyl radical (DPPH) assay
Already reported method was followed for DPPH assay with some modifications [30]. Briefly, 950 μL of methanolic solution of DPPH (3.2 mg/100 mL) was mixed with 50 μL of each extract in eppendorf tubes, mixed and kept in dark at 37 °C for 1 h. Change in absorbance was recorded at 517 nm with spectrophotometer. IC50 value was calculated by table curve method and percentage inhibition was measured by following equation:
Hydrogen peroxide (H2O2) scavenging assay
Hydrogen peroxide radicle scavenging activity of the extracts was measured according to an established protocol with some modifications [31]. Briefly, a solution of H2O2 (2 mM) was prepared in phosphate buffer (50 mM, pH 7.4) and 0.3 mL of fresh buffer was mixed with 0.1 mL of various plant extracts separately. It was followed by the addition of H2O2 solution (0.6 mL) and brief vortexing. The reaction mixture was left for 10 min and absorbance was recorded at 230 nm. The H2O2 radicle scavenging ability was calculated by the following formula:
Reducing power
The reducing potential of the extracts was assayed as described before [32]. Plant extracts (0.2 mL of each concentration separately) was added to phosphate buffer (0.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe (CN)6] (1 %), mixed and incubated at 50 °C for 20 min. Further trichloroacetic acid (0.5 mL, 10 %) was added to it and the mixture was centrifuged for 10 min at 3000 rpm. The supernatant (0.5 mL) was mixed with distilled water (0.5 mL) and ferric chloride [0.1 mL of 0.1 % (w/v) FeCl3]. After 10 min, change in absorbance was recorded at 700 nm.
Total antioxidant activity
Total antioxidant activity was assessed by phosphomolybdenum assay [33]. Briefly, an aliquot of crude extract (0.1 mL) was combined with reagent solution [1 mL, 0.6 M sulphuric acid (H2SO4), 28 mM sodium phosphate (Na3PO4) and 4 mM ammonium molybdate (NH4)6Mo7O24.4H2O]. It was incubated at 95 °C for 90 min and cooled to room temperature. Change in absorbance was measured at 765 nm and antioxidant effect was determined by following equation.
In vivo assays
Test animals, their grouping and dosage
For all in vivo assays, Sprague–Dawley rats (6 weeks; 185–205 g) provided by the NIH Islamabad of either sex were selected. The study protocol for laboratory animal use and care was legitimated by the Institutional Animal Ethics Committee (Quaid-i-Azam University Islamabad). They were maintained and bred in the Animal House at environmental conditions: 12 h light and dark cycles, 25 ± 1 °C temperature and 50 % relative humidity. The rats were kept in stainless steel standard cages under hygienic conditions and fed with an autoclaved feed and water ad libitum. Rats were acclimatized for 2 weeks in this environment before the conduction of experiments. Distilled water was used for the oral administration of standard drug and plant extracts in all in vivo assays. Before the conduction of each assay, rats were divided into six groups, weighed and marked with numbers and each group received different treatment administered orally (through feeding tube) 60 min prior to the conduction of assays. First group received only distilled water (blank or negative control) while second group received standard drugs (positive control) at 10 mg/10 mL/ Kg rat body weight concentration. Group 3, 4, 5 and 6 received AbMR, AbMA, AbCR and AbCA respectively at 200 mg/Kg body weight of rats.
Anti-inflammatory assay
Crude extracts of A. bracteosa were evaluated for their anti-inflammatory activity by carrageenan induced edema assay [34, 35]. For edema induction, 100 μL of 1 % λ-carrageenan, prepared in 0.9 % NaCl was injected sub-plantar into the left hind paw of each rat. The volume of the rat paw was determined before and after injection followed by one hour regular reading from 0 to 4th hour with the help of UGO Basile Plethysmometer (7140) calibrated with electrolyte solution (0.05 % NaCl and 0.3 % surfactant). Data was recorded in triplicate for every interval. Dichlofenac potassium and saline were used as positive and negative controls respectively. The increase in paw volume was determined by minusing the 0 h reading from respective interval reading while % edema inhibition was calculated by following equation.
Where; NC = Mean value of edema in negative control
CE = Mean value of edema in respective extract
Analgesic assay (Hot-plate method)
For the conduction of hot plate analgesia, a modified form of already optimized method [36] was followed. Standard drug, dichlofenac potassium was used as positive control. The animals were placed on Eddy’s hot plate kept at a temperature of 55 °C. Reaction time was documented when licking or jumping response against heat stimuli appeared in rats [37]. Moreover a number of lickings in proceeding 30 s after first lick were also recorded.
Antidepressant assay
Forced swim method [38] was used to screen the extracts for their potential as antidepressants. Fluoxetine HCl was used as positive control. The rats were acclimatized in a water filled glass tank (46 cm height, 21 cm diameter and 30 cm depth) maintained at 37 °C. The level of water was adjusted in such a way that rats could support themselves by retaining their tails at bottom. Rats were allowed to move freely in water tank for six minutes to complete their swimming practice. During the conduction of assay, rats were placed in water tank one by one and then after 2 min immobility time calculated with the help of stop watch. Immobility time refers to that time when rats halt all movement except those necessary for survival or to balance their body [39]. It can be calculated by the following amended formula [40].
Anticoagulant assay
Capillary tube experiment was performed to determine the blood clotting activity of the crude extracts [41]. One hour after the oral dosage, the blood was collected in capillary tube by pricking the tails of rats with sterile needles. The blood filled tubes were sealed and placed in water bath at 37 °C. Then after every 10 s small part of tube was broken until the thread formation of fibrin protein started. The clotting time was determined by taking the blood appearance on rat tail as starting point and thread formation as end point.
Extraction of phytoecdysteroids and RP-HPLC analysis
Six ecdysteroids screened in the study were; 20-ydroxyecdysone (20-HE), Ajugalactone (AJL), Sengosterone (SG), Cyasterone (CYP), Polypodine (PoB) and Makisterone A (MKA) (Fig. 3). For the extraction and analytical HPLC of ecdysteroids, already optimized protocol was followed [42].
Statistical analysis
Crude extracts were assessed in vitro at eight different concentrations were triplicated. The same extracts were assessed in vivo at one concentration and triplicated with three rats in each replicate except analgesic assay which was hexaplicated. Statistical analysis consisted of descriptive statistics using SPSS Statistical Package (version 16.0) and represented as means ± standard deviation.
Results and discussion
Total flavonoid and phenolic content
Considerable differences were observed among the flavonoids and phenolic contents of various extracts (Fig. 4). AbMA represented highest values of flavonoids (mg QE/g DW 1.98 ± 0.06) followed by AbMR (mg QE/g DW 1.51 ± 0.14) with a significant difference. Results clearly demonstrate that high flavonoid contents are found in methanolic extracts and aerial portions of A. bracteosa. Like flavonoids, the highest phenolics content was also found in AbMA (mg GAE/g DW 5.94 ± 1.98), followed by AbCR (mg GAE/g DW 4.76 ± 0.11). In the present study, the levels of flavonoids and phenolic compounds were found to be higher in aerial portion of plant than in roots as reported earlier [43]. Moreover, the solvents of different polarity affect polyphenol content and antioxidant activity [44]. In folk medicines, aqueous extract of aerial parts of A. bracteosa is used generally for the treatment of a variety of ailments. We infer polar solvent (methanol) are rich in polyphenols, which might be a reason for the potency of folk use of aqueous extracts.
Antioxidant assays
Antioxidant behavior of plant extracts can be attributed to their ability to prevent chain initiation, block nonstop hydrogen abstraction, bind to transition metal ion catalysts, peroxides decomposition, reductive competency and radical scavenging [45, 46]. Antioxidant activity measured by one method cannot show the true antioxidant potential of any substance, because one method of quantification relay on only one mechanism [47]. Commonly used antioxidant assays are; DPPH assay, H2O2 quenching assay, reducing power, total antioxidant activity etc. [48]. In the present study we conducted four antioxidant assays, in order to evaluate a broad range of antioxidant activity of A. bracteosa extracts.
DPPH assay
Extracts which can donate hydrogen atom to DPPH radical are considered good antioxidants. DPPH free radical scavenging was found highest for AbMA (Fig. 5a). AbMA, at 31.25 μg/ mL, 62.5 μg/ mL and 125 μg/ mL represented 40.7 ± 3.3, 70.74 ± 3.6 % and 75.52 ± 4.8 % free radical scavenging activity respectively, which is the higher among all the tested extracts. AbMA represented promising DPPH free radical scavenging activity and highest flavonoids and phenolic contents. As flavonoids and phenols are cogent free radical scavengers [49], there seems a strong positive correlation between DPPH free radical scavenging activity and flavonoids and phenolic compounds of methanolic extracts of aerial parts.
Hydrogen peroxide scavenging (H2O2) activity
There is a decrease in absorbance of H2O2 upon its oxidation. AbCA represented significantly high H2O2 free radical scavenging activity at all the tested concentrations (Fig. 5b). It represented 51.29 ± 2.2 % and 92.08 ± 4 % scavenging of H2O2 radicals at 7.81 and 1000 μg/mL concentration respectively. H2O2 is a free radical that rapidly decomposes into oxygen and water. It can also produce hydroxyl radicals that can initiate lipid peroxidation (LPO) and cause DNA damage in the body. To neutralize it, phenolic compounds donate electron to H2O2 to generate water [50]. It can be suggested that phenolic content found in the extract offered electron to H2O2.
Reducing power assay
Antioxidants alter oxidation state of iron by donating electron and reducing ferric ion to ferrous ion (Fe + 3 to Fe + 2) [51]. Highest reducing power was exhibited by AbMA with 718.4 ± 36 mg ascorbic acid equivalent/g measured at 1000 μg/mL concentration. It was followed by AbCR 529.1 ± 35.9 mg ascorbic acid equivalent/g measured at 1000 μg/mL concentration. AbCA and AbMA represented in the same way with least reducing powers. This assay revealed AbMA as having potent reducing power and antioxidant activity at 15.62 to 1000 μg/mL concentration as compared to the other extracts (Fig. 6a).
Phosphomolybdenum method
In phosphomolybdenum assay, Mo (VI) is reduced to Mo (V) by the analyte extracts and subsequent formation of a green phosphate Mo (V) complex [48]. Total antioxidant activity determination gave highest value for AbMR i.e. 506.14 ± 16.68 and 927 ± 50.62 mg ascorbic acid equivalent/g (AAE) at 7.81 and 1000 μg/mL concentration. It was followed by AbMA and AbCR with an AAE of 866 ± 47.94 and 838 ± 46.40 mg/g respectively at 1000 μg/mL concentration. This method represented AbMR as valuable antioxidant extract (Fig. 6b). The logic behind this behavior may be explained by the structure of the antioxidants that can donate electrons/hydrogen to free radicals [52].
Comparison of antioxidant assays
When IC50 values of the extracts were compared to evaluate antioxidant capabilities, IC50 value of AbMA in DPPH radical scavenging (36.9 μg/mL) and reducing power assay (1.5 μg/mL) were found promising. AbMR also represented significant IC50 value in phosphomolybdenium radical reduction (19.1 μg/mL). Chloroform extract of aerial parts only exhibited significant activity in hydrogen peroxide assay. The results of antioxidant assays show the scavenging of free radicals or reduction of chemicals in concentration dependent manner. Our results clearly depicted that methanolic extracts of aerial part of A. bracteosa possess better antioxidant potential than its chloroform extracts and that of root portion (Table 1). The literature also suggests the polarity of the solvent as an important parameter to extract the antioxidant compounds from a plant [53]. Although the principal compounds of A. bracteosa harboring antioxidant activity are not known, polyphenols got attention due to their antioxidant potential especially free radical scavenging [54]. Phenolic compounds are competent source for free radical scavenging [55]. Polyphenolic compounds are extensively present in plant derived food products, and they confer additional antioxidant attributes. Higher phenolic contents of the medicinal plants take priority over other attributes to treat different diseases [56]. It is considered that a positive correlation exists between phenolic contents and free radical scavenging [44]. The plants containing abundant flavonoids are considered to be a probable source of natural antioxidants [54]. Besides polyphenolic compounds, phytoeceysteroids are also regarded as strong antioxidants and majority of them are reported and isolated from A. bracteosa (discussed in detail in section 3.3.5).
Sometimes plants with high phenolic and flavonoid content exhibit low antioxidant activity and vice versa. We find AbMA in DPPH and reducing power assay while AbMR in phosphomolybdenum assay as potent extract to scavenge free radicals. These extracts also possess highest amounts of flavonoids and phenolic contents. But when we compare these extracts to AbCA in H2O2 assay, it represented a drastically low IC50 value (1.5 μg/mL) and low flavonoids and phenolic contents. This indicates that the antioxidant potential of pant extract analyte does not always dependent on total amount of polyphenolic compounds only [57].
In vivo assays
Anti-inflammatory assay
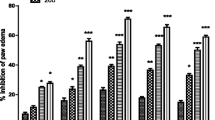
Paw edema is an inflammation induced by carrageenan and a decrease in rat paw volume is an indication of anti-inflammatory effects. Test samples represented maximum carrageenan induced rat paw edema inhibition after 3rd hour of treatment (Fig. 7a). Among all the extracts, AbMA performed much better in edema inhibition at all the three hours after treatment at 200 mg/Kg dose (67.9 ± 2.6 %, 70.3 ± 0.9 % and 74.3 ± 4.3 %). Edema inhibition at 3rd hour of treatment is comparable to AbCR (74.4 ± 1.8 %). We suggest that phytoecdysteroids present in A. bracteosa are responsible for this activity. These results are supported by a previous study [58] where authors argued that anti-inflammatory activity of Pfaffia iresinoides and Polypodium decumanum are due to the presence of phytoecdysteroids in these plants. Phytoecdysteroids are reported hepatoprotective being able to regenerate the damaged liver [59]. Phytoecdysteroid improves the cure of wounds or burns [60] and several commercial cosmetics. They inhibit psoriasis activity and are used in cosmetics [61].
Analgesic assay
Paw licking in rats is an indication of pain caused by burning from hotplate. Hot plate analgesia assay revealed that AbMA represented maximum analgesic value in delaying the mean time of start of licking (57.7 ± 4.9 s) by suppressing the nociceptors activity in paws (Fig. 7b). AbMR also exhibited a delay in start of licking time (53.7 ± 7.2 s). Good analgesic agents/extracts cause suppression of nociceptors and represent minimum number of lickings. AbMR displayed least number of lickings in 30 s i.e. 12 ± 1.2 s. Depending on the results, both AbMA and AbMR thus were found as beneficial analgesic candidates. Phytoecdysteroids are attributed for the analgesic activity of A. bracteosa. Our speculation can be supported by another study [27] where Pfaffia iresinoides and Helleborus species are regarded to contain active analgesic principle i.e. phytoecdysteroids for which they are capable to relieve pain.
Antidepressant assay
Immobility time of a rat in force swim test is an indication of stress and anxiety. The rats were fairly active after oral dose of the extracts. AbCR represented itself as a good antidepressant (2 ± 0.35 s) candidate followed by AbMR (7.3 ± 2.08 s), AbCA (11.66 ± 2.51) and AbMA (15.66 ± 1.52) (Fig. 7c). All potential antidepressant agents increase the mobility time of the rats in forced swim antidepressant assay [62] This strong antidepressant activity of A. bracteosa can be linked to the presence of phytoecdysteroids in it. Our findings are supported by previous studies where phytoecdysteroids are reported to be responsible antidepressants in Leuzea carthamoides and Sida carpinifolia plant species [58]. Lack of endogenous antioxidants results in oxidative stress in depressive disorders [7] and antidepressant potential of phytoecdysteroids can be further explained due to their antioxidant properties.
Anticoagulant assay (Clotting time determination)
Extracts of medicinal plants play their haemostatic role being anti-infective, wound healer, and antineoplastic. Fibrin sealants start coagulation cascade when delivered to the bleeding sites and thrombin converts fibrinogen into fibrin to solidify the whole mixture [63]. AbMA represented significantly better inhibitory effects on coagulation activity (Fig. 7d). It delayed coagulation from 32.66 ± 3.51 s (negative control) to 89.3 ± 4.04 s followed by AbMA (68.33 ± 5.03 s). Chloroform extracts remained least effective in anticoagulation property. Herbal resources offer a safe anticoagulant treatment. Dichrocephala intergrifolia is used as an effective anticoagulant agent in Cameroon where 95.3 % interviewed patients reported its safety. A Turkish herbal extract (Ankaferd Blood Stopper) is recently approved for the management of external hemorrhage and dental surgery bleeding to reduce coagulation time effectively [64]. Anticoagulation activity of A. bracteosa can be attributed to phytoecdysteroid content. This speculation is supported by a previous report in which the researcher [28] administered an adaptogenic preparation containing ecdysteroid (Leveton) to the athletes for 20 days. Besides an increase in working potential of athletes, a significant decline in the blood coagulation was found.
Comparison of in vivo assays
Anti-inflammatory agents are usually considered to possess analgesic activities too. In the present study, methanolic extract of aerial portion (AbMA) was found to be a potent extract possessing anti-inflammatory as well as analgesic activities. Moreover, the same extract delayed the coagulation time of rat blood. Besides this, all the extracts exhibited enormous anti-depressant activities. In vivo assays showed their activity in concentration dependent manner. Antidepressants are being used as analgesics for various pain related disorders and their analgesic activity is well recognized [65]. Antidepressants may also benefit the patients having depression and inflammatory pains. Fluoxetine, a standard antidepressant drug is found to significantly decrease inflammation in carrageenan induced rat paw oedema [65]. The anti-inflammatory activity of A. bracteosa can be attributed to the presence of phytoecdysteroids and withanolides. Dinan and Lafont reported many studies which describe the wide-scale pharmacological applications of phytoecdysteroids on mammals [27, 58, 66–69]. Evaluation of ethanolic extract (70 %) of A. bracteosa is reported expressing a promising anti-inflammatory activity probably mediated through inhibition of cholinesterase enzymes I and II (COX-1 and COX-2). Further 6-deoxyharpagide (isolate of the same extract) exhibited significant COX-2 inhibition [18]. Moreover, withanolides isolated from A. bracteosa Bractin A, B and Bractic acid displayed inhibitory potential against enzyme lipoxygenase (LOX), while four of diterpenoids were found to inhibit COX enzymes in a concentration-dependent manner [70]. Based on in vivo studies mentioned here, methanolic extract of aerial portion of A. bracteosa hence proved to as an elixir.
In this study we for the first time report the hotplate analgesic assay of A. bracteosa. In previous studies, acetic acid-induced writhing test and tail immersion test of chloroform and water extracts (200 and 400 mg/Kg, i.p.) showed significant and dose-dependent analgesic effects probably mediated through opioid receptors [23]. We found all the tested extracts especially AbMR and AbMA exhibiting highly significant analgesic effects at 200 mg/Kg per os concentration. It is suggested that the mechanism of this extract may be linked partly to inhibition of LOX and/or COX in peripheral tissues decreasing prostaglandin E2 synthesis and interfering with the mechanism of transduction in primary afferent nociceptor [23]. Antidepressants are also known to possess intrinsic antinociceptive activity. Antidepressants by inhibiting the uptake of monoamines lead to increased amount of noradrenaline and serotonin in the synaptic cleft causing reinforcement of descending pain inhibitory pathways [71].
Phytoecdysteroids profiling
Among the six tested phytoecdysteroids, four were successfully detected in A. bracteosa: 20-hydroxyecdysone (20-HE), makisterone (MKA), cyasterone (CYP) and ajujalactone (AJL). Among naturally occurring phytoecdysteroids, 20-HE is most studied and most abundant. (1232.5 μg/g DW) (Fig. 8a-c) and total phytoecdysteroid content detected was 3098 μg/g DW. It is remarkable that certain medicinal plants used in traditional Chinese medicine have high ecdysteroid content. Studies conducted on polar extract of Serratula coronata resulted in a significant inhibition of lipid peroxidation (LPO) and phytoecdysteroids and flavonoids present in this extract were suspected for this activity. The fraction rich in ecdysteroids also displayed a significantly higher LPO inhibition in the enzyme-dependent system (10-fold) and in enzyme independent system (two-fold) than α-tocopherol acid succinate [72]. This idea is further supported by the repeated doses of phytoecdysteroids which remarkably regenerated erythrocytes and raised hemoglobin levels in rats [73]. 20-HE was tested on mitochondrial fraction and found oxidizing lipid free radicals in concentration-dependent fashion [74]. It is found that 20-HE successfully terminated the oxidation of lipid free radicals and it showed a double antiradical effect than vitamin D3 (cholecalciferol) and hydroquinone at 8 μM concentration [75, 76]. In another study, 20-HE at a concentration ranging from 10−6 to 10−3 M also exhibited antiradical and antioxidant properties comparable to that of the known inhibitors of lipid peroxidation: diethyl para-phenylenediamine and ethylenediaminetetraacetate [77].
Conclusion
We for the first time report a broad range of in vitro and in vivo activities of methanolic and chloroform extract of different parts of A. bracteosa. Methanolic extract of aerial parts of plant represented promising in vitro antioxidant and in vivo anti-inflammatory, analgesic, antidepressant and anticoagulant properties and can be suggested as a potent elixir. These activities can be linked to intrinsic active compounds like phytoecdysteroids which are found in highest amounts in methanolic extracts.
Abbreviations
- 20-HE:
-
20-hydroxyecdysone
- AAE:
-
Ascorbic acid equivalents
- AbCA:
-
A. bracteosa chloroform extract
- AbCR:
-
A. bracteosa chloroform root extract
- AbMA:
-
A. bracteosa methanolic extract
- AbMR:
-
A. bracteosa methanolic root extract
- AJL:
-
Ajujalactone
- BHA:
-
Butylated hydroxyanisole
- BHT:
-
Butylated hydroxytoluene
- BWM:
-
Body weight of mice
- CYP:
-
Cyasterone
- DMSO:
-
Dimethyl sulfoxide
- DPPH:
-
2 2- Diphenyl-1-picryl-hydrazyl
- DW:
-
Dry eight
- EEAB:
-
Ethanolic extract of A. bracteosa
- GAE:
-
Gallic acid equivalents
- KPK:
-
Khyber Pakhtunkhwa
- MKA:
-
Makisterone
- QE:
-
Quercetin
- ROS:
-
Reactive oxygen species
- RP-HPLC:
-
Revrs phase high perormane liui homtograpy
References
Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95.
Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171(6):3010–8.
Karuppanapandian T, Moon JC, Kim C, Manoharan K, Kim W. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci. 2011;5(6):709–25.
Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12(2):140–7.
Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2(2):63–7.
Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–75.
Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants: fact or fiction? CNS Drugs. 2012;26(6):477–90.
Winrow V, Winyard P, Morris C, Blake D. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49(3):506–22.
Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14(4):840–60.
Benzie IF. Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):113–26.
Chaudière J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37(9):949–62.
Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res. 2002;519(1):103–19.
Moll S, Roberts HR. Overview of anticoagulant drugs for the future. In: Semin Hematol: 2002. Philadelphia: Elsevier; 2002. p. 145–157.
Lapikova E, Drozd N, Tolstenkov A, Makarov V, Zvyagintseva T, Shevchenko N, Bakunina I, Besednova N, Kuznetsova T. Inhibition of thrombin and factor Xa by Fucus evanescens fucoidan and its modified analogs. Bull Exp Biol Med. 2008;146(3):328–33.
Ortega‐Ramirez LA, Rodriguez‐Garcia I, Leyva JM, Cruz‐Valenzuela MR, Silva‐Espinoza BA, Gonzalez‐Aguilar GA, Siddiqui MW, Ayala‐Zavala JF. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: a hypothesis. J Food Sci. 2014;79(2):R129–37.
Chaves DSA, Costa SS, Almeida AP, Frattani F, Assafim M, Zingali RB. Secondary metabolites from vegetal origin: a potential source of antithrombotic drugs. Quim Nova. 2010;33(1):172–80.
Kaithwas G, Gautam R, Jachak SM, Saklani A. Antiarthritic effects of Ajuga bracteosa Wall ex Benth. in acute and chronic models of arthritis in albino rats. Asian Pac J Trop Biomed. 2012;2(3):185–8.
Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of Ajuga bracteosa Wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol. 2011;133(2):928–30.
Singh R, Patil S, Pal G, Ahmad M. Evaluation of in vivo and in vitro anti-inflammatory activity of Ajuga bracteosa Wall ex Benth. Asian Pac J Trop Dis. 2012;2:404–7.
Barkatullah, Ibrar M, Rauf A, Hadda TB, Mubarak MS, Patel S. Quantitative ethnobotanical survey of medicinal flora thriving in Malkand Pass Hills, Khyber PakhtunKhwa, Pakistan. J Ethnopharmacol. 2015;169:335–46.
Rastogi RP, Mehrotra B, Sinha S, Seth R. Compendium of Indian medicinal plants, vol. 3. New Delhi: Central Drug Research Institute and Publications & Information Directorate; 2001.
Barkatullah, Ibrar M, Hussain F. Ethnobotanical studies of plants of Charkotli hills, Batkhela district, Malakand, Pakistan. Front Biol Chin. 2009;4(4):539–48.
Pal A, Pawar R. A Study on Ajuga bracteosa Wall ex. Benth for analgesic activity. Int J Cur Bio Med Sci. 2011;1(2):12–4.
Hsieh WT, Liu YT, Lin WC. Anti-inflammatory properties of Ajuga bracteosa in vivo and in vitro study and their effects on mouse model of liver fibrosis. J Ethnopharmacol. 2011;135(1):116–25.
Khanavi M, Davoodipoor AM, Sadati SN, Ardekani MRS, Sharifzadeh M. Antinociceptive effect of some extracts from Ajuga chamaecistus Ging. spp. tomentella (Boiss.) Rech. f. aerial parts. DARU J Pharm Sci. 2014;22(1):1–6.
Khatri RS, Ahmad M, Pal G, Ashwlayan VD. Evaluation of antinociceptive activity of Ajuga bracteosa wall ex benth. Int J Green Pharm. 2013;7(1):73–6.
Báthori M, Pongrácz Z. Phytoecdysteroids-from isolation to their effects on humans. Curr Med Chem. 2005;12(2):153–72.
Azizov A. Effects of eleutherococcus, elton, leuzea, and leveton on the blood coagulation system during training in athletes. Eksp Klin Farmakol. 1996;60(5):58–60.
Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–82.
Obied HK, Allen MS, Bedgood DR, Prenzler PD, Robards K, Stockmann R. Bioactivity and analysis of biophenols recovered from olive mill waste. J Agric Food Chem. 2005;53(4):823–37.
Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–8.
Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–41.
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp Biol Med. 1962;111(3):544–7.
Adeyemi O, Okpo S, Ogunti O. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae). Fitoterapia. 2002;73(5):375–80.
Zulfiker A, Rahman MM, Hossain MK, Hamid K, Mazumder M, Rana MS. In vivo analgesic activity of ethanolic extracts of two medicinal plants-Scoparia dulcis L. and Ficus racemosa Linn. Biol Med. 2010;2(2):42–8.
Toma W, Gracioso JS, Hiruma-Lima C, Andrade F, Vilegas W, Brito AS. Evaluation of the analgesic and antiedematogenic activities of Quassia amara bark extract. J Ethnopharmacol. 2003;85(1):19–23.
Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2.
Castagné V, Porsolt RD, Moser P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur J Pharmacol. 2009;616(1):128–33.
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012;59:1–5.
Ismail H, Mirza B. Evaluation of analgesic, anti-inflammatory, anti-depressant and anti-coagulant properties of Lactuca sativa (CV. Grand Rapids) plant tissues and cell suspension in rats. BMC Complement Altern Med. 2015;15(1):1.
Kayani WK, Palazòn J, Cusidò RM, Mirza B. The effect of rol genes on phytoecdysteroid biosynthesis in Ajuga bracteosa differs between transgenic plants and hairy roots. RSC Adv. 2016;6(27):22700–8.
Ghasemzadeh A, Jaafar HZ, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules. 2010;15(6):4324–33.
Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006;99(4):835–41.
Oktay M, Gülçin İ, Küfrevioğlu Öİ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci Technol. 2003;36(2):263–71.
Diplock AT. Will the’good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radic Res. 1997;27(5):511–32.
Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant capacities. Food Anal Methods. 2009;2(1):41–60.
Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21(2):143–52.
Ahmed D, Fatima K, Saeed R. Analysis of phenolic and flavonoid contents, and the anti-oxidative potential and lipid peroxidation inhibitory activity of methanolic extract of Carissa opaca roots and its fractions in different solvents. Antioxidants. 2014;3(4):671–83.
Gülçin I, Küfrevioǧlu Öİ, Oktay M, Büyükokuroǧlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90(2):205–15.
Moein MR, Moein S, Ahmadizadeh S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules. 2008;13(11):2804–13.
Loo A, Jain K, Darah I. Antioxidant activity of compounds isolated from the pyroligneous acid. Rhizophora apiculata Food Chem. 2008;107(3):1151–60.
Settharaksa S, Jongjareonrak A, Hmadhlu P, Chansuwan W, Siripongvutikorn S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int Food Res J. 2012;19(4):1581–7.
Jan S, Khan MR, Rashid U, Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public health Res Perspect. 2013;4(5):246–54.
Qian ZJ, Jung WK, Byun HG, Kim SK. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour Technol. 2008;99(9):3365–71.
Petti S, Scully C. Polyphenols, oral health and disease: A review. J Dent. 2009;37(6):413–23.
Javanmardi J, Stushnoff C, Locke E, Vivanco J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83(4):547–50.
Sláma K, Lafont R. Insect hormones-ecdysteroids: their presence and actions in vertebrates. Eur J Entomol. 1995;92:355–77.
Badal’yants K, Nabiev A, Khushbaktova Z, Syrov V. Mechanism of hepatoprotective action of ecdystene in acute heliotrine intoxication. In: Doklady Akademii Nauk Respubliki Uzbekistana. 1996;1996:46–8.
Meybeck A, Bonté F. Ecdysteroid-containing liposomes for wound healing and skin regeneration. Demande FR 2,637,182. In: Chemical Abstracts: 1990. 1990. p. 30138r.
Inaoka Y, Yamamoto M, Tsuji K. Psoriasis inhibitors containing ecdysteroid analogs. Jpn. Kokai Tokkyo Koho JP 09 02,955 [97 02,955]. In: Chemical Abstracts: 1997. 1997. p. 166507r.
Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282(2):967–76.
Scarano A, Murmura G, Di Cerbo A, Palmieri B, Pinchi V, Mavriqi L, Varvara G. Anti-hemorrhagic agents in oral and dental practice: an update. Int J Immunopathol Pharmacol. 2013;26:847–54.
Agbor AM, Naidoo S, Mbia AM. The role of traditional healers in tooth extractions in Lekie Division. Cameroon J Ethnobiol Ethnomed. 2011;7(1):1–8.
Chugh PK, Kalra BS, Kaushik N, Tekur U. Evaluation of anti-inflammatory activity, effect on blood pressure and gastric tolerability of antidepressants. Indian J Med Res. 2013;138(1):99–103.
Syrov V. Comparative experimental investigation of the anabolic activity of phytoecdysteroids and steranabols. Pharm Chem J. 2000;34(4):193–7.
Kholodova Y. Phytoecdysteroids: biological effects, application in agriculture and complementary medicine (as presented at the 14-th Ecdysone Workshop, July, 2000, Rapperswil, Switzerland). Ukr Biokhim Zh. 2000;73(3):21–9.
Bathori M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev Med Chem. 2002;2(3):285–93.
Lafont R, Dinan L. Practical uses for ecdysteroids in mammals including humans: and update. J Insect Sci. 2003;3(1):7.
Riaz N, Nawaz SA, Mukhtar N, Malik A, Afza N, Ali S, Ullah S, Muhammad P, Choudhary MI. Isolation and enzyme inhibition studies of the chemical constituents from Ajuga bracteosa. Chem Biodivers. 2007;4(1):72–83.
Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005;71(3):483–90.
Báthori M, Zupkó I, Hunyadi A, Gácsné-Baitz E, Dinya Z, Forgó P. Monitoring the antioxidant activity of extracts originated from various Serratula species and isolation of flavonoids from Serratula coronata. Fitoterapia. 2004;75(2):162–7.
Syrov V, Nasyrova S, Khushbaktova Z. The results of experimental study of phytoecdysteroids as erythropoiesis stimulators in laboratory animals. Eksp Klin Farmakol. 1996;60(3):41–4.
Kuz’menko A, Morozova R, Nikolenko I, Donchenko G. Antioxidant effect of 20-hydroxyecdysone in a model system. Ukr Biokhim Zh. 1998;71(3):35–8.
Kuzmenko A, Morozova R, Nikolenko I, Korniets G, Kholodova Y. Effects of vitamin D3 and ecdysterone on free-radical lipid peroxidation. Biochemistry Biokhimiia. 1997;62(6):609–12.
Kuz’Menko A, Morozova R, Nikolenko I, Donchenko G. Characteristics of antioxidant properties of 20-hydroxyecdysone in low density lipoproteins by kinetic parameters of chemiluminescence. Ukr Biokhim Zh. 1998;71(6):37–42.
Osinskaia L, Saad L, Kholodova I. Antiradical properties and antioxidant activity of ecdysterone. Ukr Biokhim Zh. 1991;64(1):114–7.
Acknowledgements
Six standard ecdysteroids were kindly provided by Prof. Josep Coll-Toledano, Department of Biological Chemistry and Molecular Modeling, Spanish National Research Council (SNRC), Barcelona, Spain. We thank the chromatography team at Parc Científic de Barcelona, Spain for RP-HPLC experiments. This project was supported by The Higher Education Commission of Pakistan.
Funding
WKK received a research fellowship from Higher Education Commission (HEC) Pakistan (PIN NO.106-1559-BM6-048).
Availability of data and materials
The plants were collected from the Quaid-i-Azam University campus Islamabad and identified by Prof. Dr. Rizwana Aleem Qureshi (taxonomist) in Plant Sciences, Department Quaid-i-Azam University (QAU). A voucher specimen (HMP-460) was deposited in the “Herbarium of Medicinal Plants of Pakistan” in QAU Islamabad, Pakistan.
Authors’ contributions
WKK conceived the study, performed in vitro experiments, carried out RP-HPLC of phytoecdysteroids and wrote the manuscript. TA helped drafting the manuscript, performed statistical analysis and helped HI in in vivo assays. HI carried out in vivo assays and wrote the relevant portion. ED helped to design of the study, processed the plant samples and helped in write-up of the manuscript. BM supervised the study and reviewed the manuscript. All the authors read and approved the manuscript.
Competing interests
Authors and the funding body (Higher Education Commission) declare no competing financial interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Rats were handled according to the Ethical Guidelines for Laboratory Animals of Quaid-i-Azam University Islamabad and the ARRIVE guidelines. The study protocol for laboratory animal use and care was approved by the Institutional Animal Ethics Committee (Quaid-i-Azam University Islamabad, Pakistan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kayani, W.K., Dilshad, E., Ahmed, T. et al. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complement Altern Med 16, 375 (2016). https://doi.org/10.1186/s12906-016-1363-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1363-y