Abstract
Background
Cervical cancer is strongly associated with human papillomavirus (HPV) infection. In this retrospective study, we analyzed the data of postmenopausal women who were tested for HPV in Nanjing First Hospital from 2019 to 2021.
Methods
We retrospectively analyzed the data of 14,608 postmenopausal women aged 45–90 years, who underwent HPV examination in Nanjing First Hospital between January 2019 and December 2021. All participants were tested for 23 HPV genotypes. We subsequently analyzed the infection rate and evaluated the distribution of HPV using the chi-square test.
Results
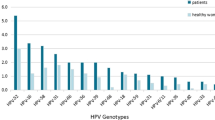
Our results showed that the HPV infection rate in postmenopausal women in Nanjing, China was 22.36%. In terms of age group, the infection rate was 19.54%, 24.30%, 26.58%, and 14.99% in those aged ≤ 50, 51–60, 61–70, and ≥ 71 years, respectively. The most common HPV subtypes were HPV52 (22.1 3%), HPV58 (15.86%), HPV53 (14.17%), HPV16 (12.61%), and HPV81 (11.66%), in that order. The single-HPV infection rate was 14.23%, and the multiple-genotype infection rate was 8.14% (1189/14,608).
Conclusions
This study showed that in Nanjing, China, the different age groups of post-menopausal women could have different rates of HPV infection, and the most common types were HPV52, HPV58, HPV53, HPV16 and HPV81. These findings highlighted the importance of understanding the epidemiology of HPV infection in specific populations, such as postmenopausal women in Nanjing, China. The results could provide valuable information for healthcare professionals and policymakers to develop targeted prevention and screening strategies for reducing the burden of HPV-related diseases in this population.
Similar content being viewed by others
Background
Human papillomavirus (HPV) is a spherical DNA virus, which can cause sexually transmitted diseases. Infection with HPV may occur through many methods, including sexual transmission, close contact, indirect contact, iatrogenic infection, and mother-to-child transmission. Studies have identified more than 200 types of HPV, which can be classified as either high- or low-risk, according to their carcinogenic ability [1]. High-risk factors for HPV infection include a large number of sexual partners; early cohabitation age; young primiparity age; suppressed and altered immune status; and hormone influence [2]. In the high-risk HPV group, HPV 16 and HPV 18 are the most common and have the highest cancer-causing ability. Studies have shown that 50–70% and 7–20% of cases were caused by HPV16 and HPV 18, respectively [3, 4].
HPV infection is very common worldwide, and most women will be infected with HPV in their lifetime [5]. Although most infections in female patients are asymptomatic and transient, persistent infection occurs in some patients, which may cause low-grade or high-grade cervical intraepithelial neoplasia and cervical cancer [6].
Cervical cancer is the second most common cancer in women worldwide, and almost 100% of women are infected with HPV at some point in their lifetime [7]. The risk factors of cervical cancer include multiple births, smoking, immunosuppression, malnutrition, and HPV infection. Among them, the most critical risk factors for cervical cancer are persistent HPV infection and lack of effective screening [8]. Furthermore, HPV is not only a carcinogen of cervical cancer, but also increases the risk of some vulva, vagina, penis, head and neck cancers [9].
The distribution of HPV genotypes shows regional differences [10, 11]. In the Asian population, HPV 18, 52, and 58 are more common. A study in Hunan, China showed that the HPV infection rate was approximately 10.16%, among which HPV16 was the most common sub-type, with an infection rate of 2.19% [12].
Another study on Uyghur women showed that the overall prevalence rate of HPV was 8.83%, for which the prevalence rate of high-risk HPV was 7.25%, and that of low-risk HPV was 1.58%. In order of infection rate, the five most common HPV subtypes are HPV16, HPV 51, HPV 31, HPV39, HPV 58 [13]. According to one survey, the estimated prevalence of HPV infection in women worldwide is 10.5% but varies widely across populations and geographic regions [14].
After menopause, immunity declines and the vaginal microecology changes, which increases the risk of HPV infection. Furthermore, in post-menopausal women, infections are more likely to develop into persistent ones, which could lead to cervical cancer. Simultaneously, as the population continues to age, the number of menopausal women increases and the number of patients with cervical cancer among older women has increased accordingly [15].
In this study, we assessed the distribution characteristics of HPV in postmenopausal women who underwent screening at Nanjing First Hospital from January 2019 to December 2021, to provide a theoretical reference for the cervical cancer screening strategy in postmenopausal women in China.
Methods
Target population
In this study, we retrospectively analyzed the data of 14,608 postmenopausal women aged 45–90 years, who underwent HPV examination in Nanjing First Hospital from January 2019 to December 2021. The exclusion criteria were total hysterectomy; systemic infection or autoimmune diseases; surgery for uterine diseases within 3 days; or other cancer types. Ethics approval was obtained from the Ethics Committee of Nanjing First Hospital, Nanjing Medical University (approval number: KY20210604-05). The need for obtaining informed consent was waived by the Institutional Review Board of Nanjing First Hospital, Nanjing Medical University due to the retrospective nature of the study.
HPV genotyping
HPV deoxyribonucleic acid (DNA) typing was performed using an HPV genotyping kit (Rapid HPV Genotyping MacroArray; Hybribio Ltd., Hong Kong). The assay was based on DNA amplification with HPV L1 consensus primers using polymerase chain reaction and the flow-through hybridization technique. It could recognize 23 HPV genotypes, including 17 high-risk HPV (HR-HPV) sub-types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk HPV (LR-HPV) sub-types (HPV 6, 11, 42, 43, 44, and 81). All protocols were performed according to the supplier’s manual, as previously described [16]. The sensitivity and specificity of the Hybribio HPV test kit, high risk-HPV and HPV 16/18 for cervical precancerous lesions screening were 95.1% (95% confidence interval [CI]: 88.1–98.1%) and 87.6% (95%CI: 86.9–88.2%) and 65.9% (95%CI: 55.1–75.2%) and 97.8% (95%CI: 97.5–98.1%), respectively [17].
Statistical analysis
The Chi-square test was used to compare the difference of HPV infection rate among different groups. A p-value of < 0.05 was considered statistically significant. The software SPSS version 25.0 was used for all statistical data analyses (SPSS, IBM, NY, USA).
Results
HPV prevalence
Table 1 presents the distribution of HPV infection in all the defined age groups. Of the 14,608 women enrolled, 3,267 tested positive for HPV. In terms of age group, the infection rate was 19.54% (1118), 24.30% (1467), 26.58% (585), and 14.99% (97) in those aged ≤ 50, 51–60, 61–70 and ≥ 71 years, respectively. These results revealed that the HPV infection rate in the 61–70-year-old population was higher than that in other populations. Statistical analysis revealed significant differences between the different age groups (P = 0.000, Table 1), indicating that people aged 61–70 were more susceptible to HPV infection.
Genotype distribution in the HPV positive population
As presented in Table 2, the most common sub-types of HPV infection were HPV52 (22.13%), HPV58 (15.86%), HPV53 (14.17%), HPV16 (12.61%), and HPV81 (11.66%). Statistical analysis revealed significant differences between age groups in terms of HPV52 (P = 0.013), HPV58 (P = 0.004), and HPV53 (P = 0.002). The rates of HPV16 (P = 0.166) and HPV81 (P = 0.805) infection did not show differences among the different age groups.
Prevalence of single- and multiple-HPV infection
Among the 14,608 women, 3267 tested positive for HPV, of whom 2,078 had a single sub-type of HPV, and 1,189 had multiple sub-type infections (Table 3). The single-HPV infection rate was 14.23% (2078/14,608). Furthermore, we found a significant difference between the different age groups (P = 0.000, Table 3). The multiple sub-type of infection rate was 8.14% (1189/14,608), with significant differences between different age groups (P = 0.000, Table 3).
Prevalence of high- and low-risk HPV infection
Among the 3267 women who tested positive for HPV, 2717 women were infected by at least one sub-type of HR-HPV, and 550 had multiple infections (Table 4). Overall, the HR-HPV infection rate was 18.60% (2717/14,608) and the LR-HPV infection rate was 3.76% (2717/14,608). Statistical analysis showed that the HR-HPV infection rate was higher than the LR-HPV infection rate in all age groups, and individuals aged 61–70 were significantly more likely to be infected with HR-HPV (P = 0.000, Table 4).
Prevalence of single- and Multiple-HPV infection of different sub-types
As shown in Table 5, among the women infected with HPV16, 51.21% had single-HPV infection and 48.79% had multiple-HPV infection. The single-HPV infection rates of HPV 52 (48.41%), HPV58 (44.98%), HPV53 (42.76%), HPV81 (44.62%), HPV18 (35.21%) were lower than those of multiple-HPV infection. Statistical analysis showed that the single-HPV infection rate of HPV16 was significantly higher than the other HPV genotypes (P = 0.009, Table 5).
Discussion
Cervical cancer is one of the most common gynecological malignancies. It is one of the leading causes of cancer death in women worldwide. The World Health Organization has set a global goal of eliminating cervical cancer, defined as achieving an incidence of less than 4 cases per 100,000 women per year. The ‘90-70-90’ goal has also been set, defined as follows: 90% of girls should be fully vaccinated against human papillomavirus (HPV) vaccine by age 15, 70% of women should undergo screening with high performance tests by the age of 35 years, and again by the age of 45 years, and 90% of women with cervical disease should receive appropriate treatment [18].
Almost all cervical cancers are caused by persistent HPV infections. Numerous studies have shown that the most common HPV sub-types were HPV16, HPV18, HPV52 and HPV58. Older women, particularly pre- and post-menopausal women, exhibit decreases in immune capability, which results in a weakened ability to clear previous and new HPV infections, which is also reflected in the high HPV infection rate.
In a previous study examining women in Wuhan, the total HPV infection rate was found to be 13.10%, with most women being infected with a high-risk sub-type. There was no significant difference in the prevalence of HPV infection between women before and after menopause. However, the prevalence of high-risk sub-types of HPV was higher in those older than 65 years [15]. In this study, among the total 14,608 women evaluated, 3,267 tested positive for HPV: 22.36% of postmenopausal women had HPV infection. The HPV infection rate in this study was higher than that reported in previous studies [12], which may have been related to the postmenopausal population being more susceptible to HPV infection. In addition, since cervical screening is performed more frequently, an increasing number of individuals in the community could undergo screening. When the results of cervical screening are abnormal, patients are transferred to a higher level of care for further treatment. Nanjing First Hospital is a tertiary referral hospital, which may treat more people with abnormal cervical screenings, leading to a higher infection rate in this research.
Our analysis further showed that the most common HPV sub-types in postmenopausal women were HPV52, HPV58, HPV53, and HPV16. Among them, HPV52 accounted for 22.13% of all infections. HPV16, HPV52, and HPV58 were more likely to occur in people ≥ 71 years old, while HPV53 was more likely to occur in those aged 61–70 years. This inconsistency may have been caused by regional differences and different pathogenicities of different HPV types.
Multiple HPV infections had a higher correlation with high-grade cervical squamous intraepithelial lesions, and their duration was longer than that of single HPV infection [19]. This may have been due to the change in immune status and increase in viral load [10]. In our study, the single HPV infection rate was 14.23%, which is greater than the multiple HPV infection rate of 8.14%. Single HPV infections were more common in people aged from 51 to 60, while multiple HPV infections were more common in people aged from 61 to 70. Studies have demonstrated that multiple HPV infections could increase the incidence of cytological abnormality compared with single HPV infection [20]. Multiple HPV infections could lead to prolonged persistent infection. In addition, patients with multiple high-risk viral loads had a 4 to 6-fold higher risk in cervical precancerous cytology than those with a single high-risk viral load [21]. As seen from Table 5, the HR-HPV infection rate (18.60%) was higher than those of only LR-HPV infection rate (3.76%). The HR-HPV infection rate was higher than the LR-HPV infection rate in every age group. Furthermore, people aged from 61 to 70 were more susceptible to HR-HPV. Among the entire cohort, the HPV infection rate (26.58%, 585/2201) and HR-HPV infection rate (24.03%, 529/2201) were the highest in women aged 61–70 years, followed by 51–60 years old people (24.30%, 1467/6038; 20.07%, 1212/6038). The HR-HPV infection rate was higher than the rate in Beijing found in a previous study, which may be because the examined population in Beijing comprised all people who underwent free screening [22].
Additionally, some postmenopausal women may wish to preserve their fertility after being diagnosed with cervical or other gynecologic malignancies because of personal, cultural, religious, or family considerations [23]. Although it is known that postmenopausal people are more likely to be infected with HPV, there have been very few studies evaluating HPV infection specifically in postmenopausal women. Persistent infection with high-risk HPV could increase the risk of cervical cancer, vagina cancer, and vulvar cancer in postmenopausal women. These cancers typically occur after menopause and are difficult to detect due to the atrophy of cervical tissues. Furthermore HPV persistence is one of the most important factors predicting the risk of CIN2 + recurrence. The risk of CIN2 + recurrence increased with the increase of HPV persistence for up to 1 year. The persistence of HPV after the first year does not appear as a risk factor [24]. Therefor further recommendations have to take into account this feature. Attempts are needed to better categorize patients with HPV infection, thus providing useful information for prognostications and tailoring the most appropriate surveillance [25].
The study found variations in HPV infection rates among different age groups of postmenopausal women. This information is valuable as it helps identify specific cohorts that may require more vigilant screening and monitoring for cervical cancer. For example, the higher infection rates observed in the 61–70 age group might suggest the need for increased attention and targeted interventions in this age range.This information can guide developing more effective and targeted screening strategies, such as recommending more frequent screening or incorporating HPV testing into routine screening protocols for this specific population.
Cervical cancer incidence declines with further promotion of cervical screening. Therefore, it is necessary to promote cervical screening among postmenopausal people and provide timely treatment.
Conclusions
This study sought to provide valuable insights into the epidemiology of HPV infection in this specific population. Our findings showed that the HPV infection rate differed between the different age groups, and the most common types were HPV52, HPV58, HPV53, HPV16 and HPV81. These results indicated that more attention should be paid to cervical cancer screening to achieve the goal of eliminating cervical cancer.
The study was conducted at a single hospital, which may have introduced a potential source of bias due to the specific characteristics of the patient population in that particular healthcare facility. Therefore, the findings may not fully represent the HPV prevalence and distribution characteristics in postmenopausal women in the broader population. However, by providing data on HPV prevalence and distribution in Nanjing, the study can contribute to informing public health authorities and policymakers in the region about the current state of HPV infection. The study contributes to the body of scientific literature on HPV epidemiology, particularly by focusing on postmenopausal women in a specific geographical region. Our findings can provide information for the benefit of healthcare practices, screening guidelines, and interventions for reducing the burden of cervical cancer in this population. This information can help in developing targeted prevention and screening strategies to reduce the incidence of HPV-related diseases among postmenopausal women.
Data availability
The datasets used and/or analysed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- HPV:
-
Human papillomavirus
- CIN:
-
Cervical intraepithelial neoplasia
- HR-HPV:
-
High-risk human papillomavirus
- LR-HPV:
-
Low-risk human papillomavirus
References
Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol. 2016;27:e21.
Schneider A. Pathogenesis of genital HPV infection. Genitourin Med. 1993;69:165–73.
Muñoz N, Castellsagué X, Berrington de González A, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suupl 3):31–10.
Koutsky L. The epidemiology behind the HPV vaccine discovery. Ann Epidemiol. 2009;19:239–44.
Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8.
Ghittoni R, Accardi R, Chiocca S, Tommasino M. Role of human papillomaviruses in carcinogenesis. Ecancer. 2015;9:526.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9.
Reid J. Women’s knowledge of pap smears, risk factors for cervical cancer, and cervical cancer. J Obstet Gynecol Neonatal Nurs. 2020;30:299–305.
Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):311–25.
Oyervides-Muñoz MA, Pérez-Maya AA, Sánchez-Domínguez CN, Berlanga-Garza A, Antonio-Macedo M, Valdéz-Chapa LD, et al. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses. 2020;12:380.
Li M, Du X, Lu M, Zhang W, Sun Z, Li L, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91:473–81.
Tang SY, Liao YQ, Hu Y, Shen HY, Wan YP, Wu YM. HPV prevalence and genotype distribution among women from Hengyang District of Hunan Province, China. Front Public Health. 2021;9:710209.
Sui S, Jiao Z, Niyazi M, Sulaiya S, Lu P, Qiao YL. Genotype distribution and behavioral risk factor analysis of human papillomavirus infection in Uyghur women. Asian Pac J Cancer Prev. 2013;14:5861–5.
Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJF, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–8.
Shen Y, Xia J, Li H, Xu Y, Xu S. Human papillomavirus infection rate, distribution characteristics, and risk of age in pre- and postmenopausal women. BMC Women’s Health. 2021;21:80.
Low HC, Silver MI, Brown BJ, Leng CY, Blas MM, Gravitt PE, et al. Comparison of Hybribio GenoArray and Roche human papillomavirus (HPV) linear array for HPV genotyping in anal swab samples. J Clin Microbiol. 2015;53:550–6.
Zhang SK, Luo XP, Li ZF, Su Z, Xia JC, Hu GY, et al. Performance of human papillomavirus typing test in cervical precancer lesions and cervical cancer screening. Zhonghua Zhong Liu Za Zhi. 2020;42:252–6.
Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603.
Kim M, Park NJ, Jeong JY, Park JY. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13:1342.
Jia H, Ding L, Han Y, Lyu Y, Hao M, Tian Z, et al. Genotype-specific distribution and change of high-risk human papillomavirus infection and the association with cervical progression risk in women with normal pathology and abnormal cytology in a population-based cohort study in China. J Cancer. 2021;12:4379–88.
Zhu B, Liu Y, Zuo T, Cui X, Li M, Zhang J, et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in China: the pooled analysis of 1.7 million women. Cancer Med. 2019;8:5373–85.
Shen J, Gao LL, Zhang Y, Han LL, Wang JD. Prevalence of high-risk HPV and its distribution in cervical precancerous lesions among 35–64 years old women who received cervical cancer screening in Beijing. Zhonghua Yu Fang Yi Xue Za Zhi. 2018;52:493–7.
Gullo G, Cucinella G, Chiantera V, Dellino M, Cascardi E, Török P, et al. Fertility-sparing strategies for early-stage endometrial Cancer: stepping towards Precision Medicine based on the Molecular Fingerprint. Int J Mol Sci. 2023;24:811.
Bogani G, Sopracordevole F, Ciavattini A, Vizza E, Vercellini P, Giannini A, et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023;32(6):525–32.
Bogani G, Sopracordevole F, Ciavattini A, Vizza E, Vercellini P. Ghezzi F. HPV persistence after. Cervical surgical excision of high-grade cervical lesions.Cancer Cytopathol. 2023 Sep 25.
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
XY: Data Collection, Manuscript writing; CZ: Data collection, Data analysis; XW: Data collection, Data analysis; JF: Data collection; JX: Supervision, Manuscript review; YL: Project development, Data Collection, Manuscript editing. All authors contributed to, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. The study was conducted in accordance with the principles of the Declaration of Helsinki, and ethics approval was obtained from the Ethics Committee of Nanjing First Hospital, Nanjing Medical University (approval number: KY20210604-05).
Consent to participate
The need for obtaining informed consent was waived by the Institutional Review Board of Nanjing First Hospital, Nanjing Medical University due to the retrospective nature of the study.
Consent for publication
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yin, X., Zhang, C., Wu, X. et al. HPV prevalence and distribution characteristics in postmenopausal women from Nanjing, China. BMC Women's Health 24, 68 (2024). https://doi.org/10.1186/s12905-024-02904-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-02904-8