Abstract
Background
This meta-analysis aimed to assess the effects of exercise interventions on body composition and quality of life in overweight/obese breast cancer survivors.
Methods
Eligible randomized controlled trials (RCTs) were searched from the Cochrane Library, PubMed, and Embase databases and assessed using the Cochrane Collaboration’s assessing risk tool. The effect size was pooled as weighted mean difference (WMD) for body composition variables (i.e., body mass index [BMI], body fat, body weight, fat mass, lean mass, bone mineral density) and quality of life (i.e., physical health and mental health), and the confidence interval (CI) was set as 95%. Since heterogeneity existed, subgroup analysis was conducted to detect the source of heterogeneity.
Results
Eight articles from six RCTs containing 548 overweight/obese breast cancer survivors (BMI ≥ 25 kg/m2) were included and analyzed. Compared to routine care, exercise intervention significantly decreased the body mass index [WMD (95% CI) = -1.37 (-2.50, -0.23) kg/m2] and body fat [WMD (95% CI) = -3.80 (-6.59, -1.01) %] of overweight/obese breast cancer survivors. Exercise intervention showed a tendency to increase physical health [WMD (95% CI) = 2.65 (-10.19, 15.48)] and mental health [WMD (95%CI) = 1.38 (-4.18, 6.95)], but no statistical significance was observed. A subgroup analysis showed the duration of intervention was a source of heterogeneity on body composition. In the 16-week subgroup, exercise intervention decreased fat mass and BMI while increased lean mass and bone mineral density. The 52-week exercise intervention was effective in increasing lean mass. A significant exercise intervention effect on reducing body fat was only detected in the 12-week subgroup.
Conclusion
Exercise intervention significantly decreased the body mass index and body fat of overweight/obese breast cancer survivors. The benefits of exercise interventions for overweight/obese breast cancer survivors need more evidence from high-quality RCTs with large sample sizes.
Similar content being viewed by others
Background
Breast cancer is the most common malignancy in women [1], which is the fifth leading cause of cancer-related deaths with approximately 6.9% of them being reported worldwide in 2020 [2]. With advances in early screening and breast cancer treatments, breast cancer mortality has markedly declined during the last couple of decades, which has resulted in an increasing number of breast cancer survivors [3]. There was a 39% decrease in breast cancer mortality in the United States from 1989 to 2015, translating to approximately 322,600 patients saved from breast cancer-related deaths [4]. Despite the increasing survival rate, many breast cancer survivors frequently encounter chronic complications or long-term treatment sequelae that significantly impact their quality of life. These complications may include pain, limited upper limb function, fatigue, lipid disorders, obesity, premature menopause, and lymphedema [5,6,7]. It is crucial of secondary prevention or intervention strategies to maintain the overall health of the breast cancer survivors.
Exercise has traditionally not been advised in patients with cancer [8]. However, in recent years, studies on exercise have revealed the potential benefits for cancer survivors [9, 10]. Exercise is one type of physical activity that is planned, structured, and repetitive with an objective to improve or maintain physical fitness (such as cardiorespiratory endurance, muscular endurance, muscular strength, body composition, and flexibility) [11]. The American College of Sports Medicine roundtable proposes that exercise is safe for cancer survivors and advocates that they avoid inactivity, which helps to improve physical functioning, anxiety and depressive symptoms, cancer-related fatigue, and quality of life [8, 12]. Numerous studies have provided evidence for the benefits of exercise for breast cancer survivors, including improved shoulder range of motion and muscular strength, reduced anxiety and cancer-related fatigue, and enhanced self-esteem and overall well-being [13,14,15,16]. For instance, structured exercise programs have been shown to enhance shoulder range of motion in postoperative breast cancer patients [13]. Aerobic exercise has also been found to be effective in reducing cancer-related fatigue (CRF) among breast cancer survivors [14]. Overall, these findings provide compelling evidence for the benefits of incorporating exercise interventions in the care of breast cancer survivors.
However, it remains unclear the effects of exercise on body composition and quality of life among overweight or obese breast cancer survivors. Approximately 50% of breast cancer survivors worldwide are overweight or obese, and they usually gain more body weight following hormonal or adjuvant therapy [15]. According to the weight status classification established by World Health Organization and the National Institutes of Health individuals with a body mass index (BMI) of 30 kg/m2 or above are classified as obese and those with a BMI ranging from 25.0 to 29.9 kg/m2 are considered overweight [16, 17]. Breast cancer survivors are more likely to be obese, which is generally related to worse health-related quality of life [18]. In addition, overweight/obese breast cancer survivors have been documented to have a high risk of recurrence, all-cause mortality, and long-term morbidities (e.g., cardiovascular disease, diabetes) [19,20,21]. Studies have indicated that exercise and weight loss interventions (incorporating diet, exercise and psychosocial support) may improve the quality of life and reduce BMI, body weight and waist circumference of breast cancer survivors [22, 23]. While some other studies reported limited effect of exercise on body composition, such as body weight [24, 25], BMI [26, 27], fat, and lean mass [25, 28], or health-related quality of life [29, 30] among obese/overweight breast cancer survivors.
Previous research has shown that compared to normal-weight women, overweight or obese women had different energy expenditures in a day (higher portions in sedentary and light physical activity) [31]; when completing same intensity exercise, overweight or obese women utilized significantly more calories than normal-weight women [32]. These studies suggest overweight or obese women may experience different effects from exercise. Although exercise interventions had been proved to have a significant impact on improving health-related quality of life and reducing body weight and waist circumference in breast cancer survivors [33], no consensus has been made among overweight or obese breast cancer survivors . Therefore, we conducted a meta-analysis based on randomized controlled trials (RCTs) to assess the effects of exercise interventions on the body composition and quality of life of overweight/obese breast cancer survivors.
Materials and methods
Screening eligible studies
Studies that met all the following criteria were included: (1) involving breast cancer survivors who were overweight or obese; (2) reporting the differences in outcomes between the exercise and control groups (routine care); (3) involving one or more of the following outcomes, including body weight, BMI, fat mass, lean mass, bone mineral density, body fat, and 36-Item Medical Outcomes Survey-Short Form (SF-36); and (4) studies that were RCT. The exclusion criteria were (1) reviews, conference abstracts, comments, and other non-original articles; (2) involving other strategies in addition to exercise interventions with weight loss as the primary goal; and (3)involving participants who had regular daily exercise habits. If any of the exclusion criteria was met, the study will not be included in the meta-analysis. Additionally, if multiple studies utilized the same dataset, only the study with the most detailed data will be extracted and analyzed.
Retrieval strategies
To investigate the effect of exercise on body composition and quality of life in overweight/obese breast cancer survivors, studies were retrieved from PubMed, Cochrane Library, and Embase databases until June 9, 2022, without language restrictions. The retrieval terms were as follows: “overweight” OR “obese” OR “obesity” AND “exercise” OR “physical activity” OR “sports” OR “training” OR “exercising” AND “breast neoplasms” OR “breast cancer.” Additionally, references cited in the included studies and relevant reviews were retrieved.
Outcome variables
This study examined several outcome variables, including body composition indicators (BMI and body weight, fat mass, lean mass, and bone mineral density) and quality of life (physical and mental health summary score). Body composition indicators were assessed using internationally standardized metrics, while quality of life was assessed using validated scales. All outcome variables were treated as continuous data in the analysis (see details in Figs. 2 and 3).
Extracting data and quality assessment
The following information was extracted: name of the first author, year of publication, area in which the study was conducted, intensity of exercise of the involved participants, definition of overweight and obesity, exercise intervention program, demographic information of the involved participants (age, and race), and outcomes (BMI, body weight, fat mass, lean mass, bone mineral density, quality of life). Information extraction was completed by two independent investigators, and the third researcher would be consulted for resolution in case of any disagreements. The Cochrane Collaboration tool was used to assess the risk of bias in the included RCT studies, which encompasses six domains of bias, namely selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The included randomized controlled trials (RCTs) were categorized into low risk,or unclear risk in the aforementioned domains [34].
Statistical analysis
All statistical analyses were performed using RevMan 5.3 and Stata12.0. The weighted mean difference (WMD) and 95% confidence interval (CI) were used as effect sizes to pool the results. WMD is a statistical measure used to assess the difference in continuous variables between two groups, which calculates the average difference between the two groups, with each study’s contribution weighted by the sample size. A narrower 95% CI indicates a more precise estimate. Cochran’s Q test and the I2 test were used to assess heterogeneity of all outcome variables. A random-effects model was used for the meta-analysis when there was significant heterogeneity (P < 0.05, I2 > 50%); otherwise, a fixed-effects model was used (P ≥ 0.05, I2 ≤ 50%). The random-effects model allows for the possibility that studies are drawn from different populations, which means that differences across studies may be due to unidentified sources of variation. This model provides a more conservative estimate of treatment effects by considering both the within-study sampling error and the between-study variability [35]. A subgroup analysis was conducted in groups divided by the duration of the intervention. Sensitivity analysis was performed using a one-study-removed approach to assess the significant impact of individual included studies on the results of the meta-analysis [36]. Egger’s test [37] and funnel plot [38] was used to assess publication bias among the studies.
Results
Screening eligible studies
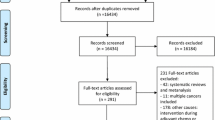
The workflow of the study retrieval and screening is shown in Fig. 1. First, we retrieved 1,516 studies from three databases and excluded 551 repetitive studies. Among the remaining 965 studies, 948 that did not meet the inclusion criteria were removed after reviewing the title and abstract. Nine studies were excluded after reviewing their full text. Finally, we identified eight eligible studies [23,24,25,26,27,28,29,30] for this meta-analysis.
Detailed information for the included studies
A total of 8 articles were assessed and found to meet the inclusion criteria. Among them, two articles [23, 29] from the same RCT by Brown et al. (NCT01515124) and two articles [24, 30] from the same RCT by Dieli-Conwright et al.(NCT01140282) met the inclusion criteria. A total of 548 participants were included, ranging from 28 to 177 participants in different studies. All participants were breast cancer survivors with stages I-III tumors and with BMI equal or greater than 25 kg/m2. The study by Hooshmand et al. [25] was conducted in Iran, and the other studies were all conducted in the United States. Heterogeneity was observed among the studies on the intensity of exercise and exercise intervention programs of the participants (Table 1). The publication bias analysis resulted a moderate methodological quality of the included studies (See details in Supplementary Fig. 1).
Effect of exercise intervention on body composition
Four studies reported differences in the BMI of overweight/obese breast cancer survivors between the exercise and control groups, and there was no significant heterogeneity (I2 = 18%, P = 0.30). The forest plot revealed that the exercise intervention markedly decreased the BMI [WMD (95% CI) = -1.37 (-2.50, -0.23) kg/m2, P = 0.02] in overweight/obese breast cancer survivors (Fig. 2A). There was significant heterogeneity among the four studies reporting body fat (I2 = 75%, P = 0.007), and the pooled results indicated that exercise intervention markedly decreased body fat [WMD (95% CI) = -3.80 (-6.59, -1.01) %, P = 0.008] in overweight/obese breast cancer survivors (Fig. 2B). Nevertheless, the exercise intervention did not exert a significant effect on the body weight [WMD (95% CI) = -1.60 (-3.78, 0.57) kg, P = 0.15], fat mass [WMD (95% CI) = -1.07 (-4.95, 2.81) kg, P = 0.59], lean mass [WMD (95% CI) = 2.38 (-0.70, 5.46) kg, P = 0.13], and bone mineral density [WMD (95% CI) = 0.04 (-0.01, 0.08) g/m2, P = 0.13] of overweight/obese breast cancer survivors. The heterogeneity of the included studies was as follows: body weight (I2 = 0%, P = 0.8), fat mass (I2 = 88%, P<0.00001), lean mass (I2 = 73%, P = 0.006), and bone mineral density (I2 = 77%, P = 0.01) (Fig. 2C-F). In summary, in comparison to routine care, exercise intervention significantly decreased the BMI and body fat of overweight/obese breast cancer survivors but had no significant influence on body weight, fat mass, lean mass, and bone mineral density.
Forest plots showing the pooled results of exercise intervention on BMI (A), body fat (B), body weight (C), fat mass (D), lean mass (E), and bone mineral density (F) of overweight/obese breast cancer survivors.
Effect of exercise intervention on quality of life
There were only two studies reporting the differences in the physical health summary score (PHS) or the mental health summary score (MHS) of overweight/obese breast cancer survivors between exercise and control groups, and there was significant heterogeneity (I2 = 92%, P = 0.0003 for PHS; I2 = 58%, P = 0.12 for MHS). Forest plots revealed that exercise intervention had no significant influence on PHS [WMD (95% CI) = 2.65 (-10.19, 15.48), P = 0.69] and MHS [WMD (95% CI) = 1.38 (-4.18, 6.95), P = 0.63] in overweight/obese breast cancer survivors (Fig. 3).
Subgroup analysis
We conducted analyses for all covariates and observed significant heterogeneity among the remaining covariates, except for exercise duration. Consequently, it was deemed inappropriate to convert them into grouping variables for subgroup analysis. Therefore, we solely performed subgroup analysis on exercise duration. Table 2 shows the results of the subgroup analyses based on the duration of intervention (12 weeks, 16 weeks, and 52 weeks). There was significant heterogeneity among the studies for BMI, body fat, fat mass, lean mass, and bone mineral density, and the heterogeneity test in each subgroup suggested that intervention duration was one of the sources of heterogeneity (Table 2). Specifically, in the 16-week subgroup, exercise intervention decreased fat mass [WMD (95% CI) = -6.90 (-8.85, -4.95) kg, P < 0.001], while increased lean mass [WMD (95% CI) = 7.70 (4.58, 10.82) kg, P < 0.001], and bone mineral density [WMD (95% CI) = 0.08 (0.04, 0.12) g/m2, P < 0.001] of overweight/obese breast cancer survivors (Table 2). Long-term exercise intervention (52 weeks) also revealed to be effective in increasing the lean mass [WMD (95% CI) = 1.91 (0.09, 3.73) kg, P = 0.04]. The subgroup analyses revealed significant exercise intervention effect on BMI in the 16-week subgroup [WMD (95% CI) = -1.89 (-3.26, -0.52) kg/m2, P = 0.007], but there were no significant effects on BMI at 12-week [WMD (95% CI) = 0.40 (-1.84, 2.64) kg/m2, P = 0.726] and 52-week groups [WMD (95% CI) = -2.90 (-7.57, 1.77) kg/m2, P = 0.224]. A significant exercise intervention effect on body fat was detected in the 12-week subgroup [WMD (95% CI) = -4.50 (-8.59, -0.42) %, P = 0.031] but not in the 16-week [WMD (95% CI) = -4.39 (-8.97, 0.19) %, P = 0.061] and 52-week groups [WMD (95% CI) = -1.80 (-4.58, 0.98) %, P = 0.205]. There was only one study in the 12- and 52-week subgroups for BMI and body fat, respectively (Table 2). Only two studies examined PHS and MHS as outcomes; therefore, subgroup analysis was not performed for these two outcomes.
Sensitivity analysis and publication bias
In sensitivity analysis, the pooled results for BMI changed from significant [WMD (95% CI) = -1.97 (-3.28, -0.66) kg/m2] to non-significant [WMD (95% CI) = -0.55 (-2.19, 1.10) kg/m2] after eliminating studies one by one, indicating an unstable result. For the other five outcomes, sensitivity analysis revealed stable results (Table 3). In addition, no publication bias was detected among the studies for any of the six outcomes (Table 3). Moreover, the funnel plot and scatter distribution of each outcome measure appear to be relatively symmetrical, indicating the absence of publication bias (Fig. 4A-H). There were only two studies on PHS and MHS; therefore, sensitivity analysis and publication bias tests were not performed for these two outcomes.
Discussion
This meta-analysis presented several important findings regarding the exercise effects on body composition and quality of life among overweight/obese breast cancer survivors. Compared to routine care, exercise intervention significantly decreased the BMI and body fat of overweight/obese breast cancer survivors. Meanwhile, exercise intervention decreased fat mass and increased lean mass and bone mineral density in overweight/obese breast cancer survivors while only under certain intervention periods (i.e., 16 weeks). In terms of the quality of life, exercise intervention showed a tendency to increase PHS and MHS, but this did not reach statistical significance. Taken together, these findings indicate that overweight/obese breast cancer survivors may benefit from exercise intervention for weight management.
Weight control is essential for patients with breast cancer, as overweight and obesity have been found to contribute to the recurrence and progression of breast cancer, negatively affecting its prognosis [39]. A high BMI is linked to higher risk and worse clinical outcomes in patients with breast cancer [40], while over half of the breast cancer survivors are overweight or obese [18, 41]. Exercise intervention has been shown to reduce BMI in patient with breast cancer [41]. Among breast cancer survivors, exercise significantly reduces body weight and waist circumference [33] and illustrated positive outcomes with BMI, lean mass, and muscle strength [42]. In the present study, we have extended these findings showing a favorable effect of exercise intervention on BMI among overweight/obese breast cancer survivors.
Given that BMI only reflects the relationship between height and weight and not the distribution of body fat, this study further demonstrated the favorable effect of exercise on body fat among overweight/obese breast cancer survivors. Body fat is positively correlated with the activation of the mTOR pathway, which is associated with tumor growth in breast cancer patients [43]. Fat body mass and lean body mass synergistically predict the risk of morphometric vertebral fractures in breast cancer patients who received aromatase inhibitors, a drug commonly used after chemotherapy that may lead to bone loss and elevated fracture risk [44, 45]. Lauby-Secretan et al. suggested that lower body fat may reduce the risk of various cancers [46]. As such, the synergistic results from this study, exercise reduces body fat, may provide practical guidance for the recovery plans for breast cancer survivors.
It is noteworthy that such exercise effects on these outcomes were moderated by the intervention durations. The current meta-analysis indicates the effects of exercise were better on BMI, fat mass, and bone mineral density at the relatively shorter program (12-week, 16-week) but not at long-term program (52-week); while the effects on lean mass were better in both short-term and long-term program. For example, Carayol et al. showed that adapted physical activity and diet intervention significant decreased BMI and fat mass at 26 weeks, but such effects did not persist afterward [47]. Juvet et al. indicated that exercise intervention could produce short-term improvements in physical functioning, and the time-dependent observations should be confirmed based on more studies [48]. The mechanism of different effects of intervention durations is not completely clear yet. Some researchers comment that the cessation of supervision and support may contribute to the difficulties of maintaining exercise in long-term exercise intervention [49]. The unique conditions of breast cancer survivors (e.g., breast cancer type, course of disease, treatment methods, and adverse reactions), may also interact with environmental influences to facilitate or hinder the weight management progress [50].
Another reason for the different effects of intervention durations might be the confounding effects of exercise intensities. Exercise intensity is a key factor in exercise intervention, which determines the safety and effectiveness of exercise interventions for patients. The ideal exercise effect cannot be achieved when exercise intensity does not reach the minimum threshold. In contrast, it may lead to overtraining and joint damage when the intensity exceeds the maximum threshold. The American College of Sports Medicine roundtable recommends moderate-intensity (> 30 min for > 3 times per week) aerobic exercise for at least 8–12 weeks for cancer survivors to obtain health-related outcomes, or similar benefits are also obtained by combining resistance exercise with aerobic exercise at least 2 times per week, using at least 2 sets of 8–15 repetitions at least 60% of one repetition maximum [51]. In this meta-analysis, exercise prescriptions differed among RCTs and some did not provide specific intensities. Given the limited studies and the inconsistent effect of exercise durations, we should be with cautious when treating the association between exercise and the change of body compositions for overweight/obese breast cancer survivors, and more research is needed before making a confirmed conclusion.
The potential benefits of exercise in improving quality of life of breast cancer survivors have been reported in studies [18, 52]. In a systematic review based on 26 RCTs, Hong et al. concluded that exercise intervention substantially improved the quality of life of breast cancer survivors, and the improved quality of life was associated with “time of session” [53]. Another two meta-analyses also found that exercise intervention improved the quality of life in breast cancer survivors, including social well-being, functional well-being, emotional well-being, and physical well-being [54], mental health and general health [42]. However, our meta-analysis indicated that exercise intervention tended to increase PHS and MHS of overweight/obese breast cancer survivors, but did not reach statistical significance. The differences on the number of included studies might partly explain such inconsistent conclusion as only two studies reporting the differences in PHS or MHS of overweight/obese breast cancer survivors between exercise and control groups were included. On the other hand, the quality of life of breast cancer survivors is heavily influenced by the treatment (e.g., selective estrogen receptor modulator), which may cause a series of physical and psychological impairment [55]. Sexual health can also be negatively impacted after breast cancer and therefore influence their quality of life [56]. Specific treatment modalities has been suggested to improve sexual health and quality of life collaboratively for breast cancer survivors [57]. Furthermore, exercise can improve sleep quality in breast cancer survivors [58], and therefore improve their overall quality of life [59]. Thus, understanding breast cancer survivors’ conditions and treatment plans is critical while prescribing exercise treatment.
This meta-analysis has several limitations. First, although there was low methodological heterogeneity among the included RCTs, most of the RCTs were conducted in the United States, which limited the extrapolation of the results. Second, meta-analysis indicated that exercise intervention could significantly decrease the BMI of breast cancer survivors in comparison with that of routine care. However, the study of Dieli-Conwright et al. [24] weighted as high as 52.6% among the four studies, and revealed the significant exercise intervention effect on BMI [WMD (95% CI) = -2.10 (-3.66, -0.54) kg/m2, P < 0.05], while there were no significant effects on BMI in the other studies. In the sensitivity analysis, the pooled results for BMI changed from significant to non-significant after eliminating the study of Dieli-Conwright et al. [24], indicating an unstable result. Third, the number of included RCTs was small, and significant heterogeneity among studies was observed for multiple outcome indexes. Therefore, more evidence from high-quality RCTs with larger sample sizes is needed to confirm the benefits of exercise interventions for overweight/obese breast cancer survivors.
Conclusion
This meta-analysis indicated that exercise intervention could significantly decrease BMI and body fat of overweight/obese breast cancer survivors. Although there was no statistical significance, exercise intervention decreased body weight and fat mass, and increased lean mass and bone mineral density under different exercise intervention duration. A tendency of improved quality of life was also detected. These findings suggest the benefits of exercise interventions in overweight/obese breast cancer survivors, while more evidence is needed for a conclusive result.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
27 September 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12905-023-02661-0
References
Trayes KP, Cokenakes SEH. Breast Cancer Treatment. Am Fam Physician. 2021;104(2):171–8.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. 2021;71(3):209–49.
Ganz PA, Goodwin PJ. Breast Cancer Survivorship: where are we today? Adv Exp Med Biol. 2015;862:1–8.
Desantis CE, Ma J, Sauer AG, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca Cancer J Clin 2017, 67(6).
Wang X, Lai Q, Tian Y, Zou LJM. Effect of evidence-based nursing intervention on upper limb function in postoperative radiotherapy patients with breast cancer. Med (Baltim). 2020;99(11):e19183.
Lovelace DL, Mcdaniel LR, Golden D. Long-Term Effects of breast Cancer surgery, treatment, and Survivor Care. J Midwifery Wom Heal 2019, 64(6).
SM J, SM P. Care for breast Cancer survivors. Adv Exp Med Biol. 2021;1187:511–24.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galv?O DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exer. 2010;42(7):1409–26.
MT JR. Physical activity and physiotherapy: perception of women breast cancer survivors. Breast cancer (Tokyo Japan). 2019;26(3):333–8.
Supa P, Remco P, Meron P, Melanie F, Nanthaphan C, Lily S, Vasso A. Physical activity and breast cancer survivors: importance of adherence, motivational interviewing and psychological health. Maturitas. 2018;116:037851221830389X.
CJ C, KE P, GM C. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Campbell KL, Winters-Stone, Kerri M, Wiskemann, Joachim AM, Schwartz AL. Courneya: Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Db Syst Rev 2010(6).
Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15(1):1–13.
LX HSPB, SYC M. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;12(12):Cd012110.
World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 1998.
Health NIo N, Heart L, Institute B. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Volume 6. National Institutes of Health, National Heart, Lung, and Blood Institute; 1998.
Paxton RJ, Phillips KL, Jones LA, Chang S, Taylor WC, Courneya KS, Pierce JP. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–31.
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, Mctiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14.
Wendy DW, Schmitz KH, Alfano CM, Bail JR, Goodwin PJ, Thomson CA, Bradley DW, Courneya KS, Befort CA, Denlinger CS. Weight management and physical activity throughout the cancer care continuum. CA-Cancer J Clin. 2018;68(3):64–89.
Senie RT, Rosen PP, Rhodes P, Lesser ML, Kinne DW. Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med. 1992;116(1):26–32.
Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I. The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Cancer Res Ther. 1900;10(3):658–64.
Brown JC, Sarwer DB, Troxel AB, Sturgeon K, DeMichele AM, Denlinger CS, Schmitz KH. A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Res Tr. 2021;189(1):145–54.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, Spicer DV, Tripathy D, Bernstein L, Mortimer JE. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol. 2018;36(9):875.
Hooshmand Moghadam B, Golestani F, Bagheri R, Cheraghloo N, Eskandari M, Wong A, Nordvall M, Suzuki K, Pournemati P. The effects of high-intensity interval training vs. moderate-intensity continuous training on inflammatory markers, body composition, and physical fitness in overweight/obese survivors of breast cancer: a randomized controlled clinical trial. Cancers (Basel). 2021;13(17):4386.
Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–12.
Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, Yanosik MA, Vona-Davis L. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23:2995–3003.
Thomas GA, Cartmel B, Harrigan M, Fiellin M, Capozza S, Zhou Y, Ercolano E, Gross CP, Hershman D, Ligibel J. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obes (Silver Spring). 2017;25(2):346–51.
JC B, DB S, AB T, AM KS, CS D, KH D. A randomized trial of exercise and diet on health-related quality of life in survivors of breast cancer with overweight or obesity. Cancer. 2021;127(20):3856–64.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20(1):1–10.
Drenowatz C, Jakicic JM, Blair SN, Hand GA. Differences in correlates of energy balance in normal weight, overweight and obese adults. Obes Res Clin Pract. 2015;9(6):592–602.
LeCheminant JD, Heden T, Smith J, Covington NK. Comparison of energy expenditure, economy, and pedometer counts between normal weight and overweight or obese women during a walking and jogging activity. Eur J Appl Physiol. 2009;106:675–82.
Joaquim A, Leão I, Antunes P, Capela A, Viamonte S, Alves AJ, Helguero LA, Macedo A. Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: evidence from systematic reviews and meta-analyses. Front Oncol. 2022;12:1–15.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed). 2011;343:d5928.
Schmidt FL, Oh IS, Hayes TL. Fixed-versus random‐effects models in meta‐analysis: Model properties and an empirical comparison of differences in results. Brit J Math Stat Psy. 2009;62(1):97–128.
Aurelio T. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Tech Bull 1999, 8(47).
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Sterne JA, Harbord RM. Funnel plots in meta-analysis. Stata J. 2004;4(2):127–41.
Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast Cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41.
Vernaci G, Dieci MV, Manfrin S, Mantiero M, Falci C, Faggioni G, Mioranza E, Menichetti A, Tasca G, Griguolo G, et al. BMI is an independent prognostic factor for late outcome in patients diagnosed with early breast cancer: a landmark survival analysis. Breast. 2019;47:77–84.
Shaikh H, Bradhurst P, Ma LX, Tan SYC, Egger SJ, Vardy JL. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;12(12):Cd012110.
Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y, Sun F. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. Oncotargets Ther 2016:2153–68.
Cheng TD, Omilian AR, Yao S, Sanchez PV, Polk LZ, Zhang W, Datta S, Bshara W, Ondracek RP, Davis W, et al. Body fatness and mTOR pathway activation of breast cancer in the Women’s Circle of Health Study. NPJ Breast Cancer. 2020;6:45.
Monteverdi S, Pedersini R, Gallo F, Maffezzoni F, Dalla Volta A, Di Mauro P, Turla A, Vassalli L, Ardine M, Formenti AM, et al. The Interaction of lean body Mass with Fat Body Mass is Associated with vertebral fracture prevalence in women with early breast Cancer undergoing aromatase inhibitor therapy. JBMR Plus. 2021;5(2):e10440.
Artese AL, Whitney NJ, Grohbrugge KE, Panton LB. Assessment of Arm lean Mass, Fat Mass, and bone Mineral density in breast Cancer Survivors without Lymphedema. Oncol Nurs Forum. 2021;48(2):166–72.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and Cancer–viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8.
Carayol M, Ninot G, Senesse P, Bleuse JP, Jacot W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the APAD1 randomized controlled trial. BMC Cancer. 2019;19(1):737.
Juvet LK, Thune I, Elvsaas I, Fors EA, Lundgren S, Bertheussen G, Leivseth G, Oldervoll LM. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast (Edinburgh Scotland). 2017;33:166–77.
Suman OE. Herndon DNJAopm, rehabilitation: Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. 2007, 88(12):S24–9.
Nguyen V, Chen J, Lord R, Preda VJO. The impact of multidisciplinary weight management on body weight and body mass composition in women with breast cancer post-adjuvant chemotherapy: a retrospective chart review. 2022, 100(6):344–53.
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90.
Mandelblatt JS, Luta G, Kwan ML, Makgoeng SB, Ergas IJ, Roh JM, Sternfeld B, Adams-Campbell LL, Kushi LH. Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the Pathways Study. Breast Cancer Res Treat. 2011;129(2):521–9.
Hong F, Ye W, Kuo CH, Zhang Y, Qian Y, Korivi M. Exercise intervention improves clinical outcomes, but the Time of Session is crucial for better quality of life in breast Cancer survivors: a systematic review and Meta-analysis. Cancers (Basel). 2019;11(5):706.
Joaquim A, Leão I, Antunes P, Capela A, Viamonte S, Alves AJ, Helguero LA, Macedo A. Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: evidence from systematic reviews and meta-analyses. Front Oncol. 2022;12:955505.
Hamer J, McDonald R, Zhang L, Verma S, Leahey A, Ecclestone C, Bedard G, Pulenzas N, Bhatia A, Chow R, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer. 2017;25(2):409–19.
Vizza R, Capomolla EM, Tosetto L, Corrado G, Bruno V, Chiofalo B, Di Lisa FS, Filomeno L, Pizzuti L, Krasniqi E. Sexual dysfunctions in breast cancer patients: evidence in context. Eur J Obstet Gyn R B 2023:qead006.
D’Oria O, Giannini A, Buzzaccarini G, Tinelli A, Corrado G, Frega A, Vizza E, Caserta D. Fractional Co2 laser for vulvo-vaginal atrophy in gynecologic cancer patients: a valid therapeutic choice? A systematic review. Eur J Obste Gyb R B 2022.
Yang H, Yang Z, Pan H, Zhou Q. Effects of physical activity on sleep problems in breast cancer survivors: a meta-analysis. Support Care Cancer. 2021;29:4023–32.
Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manag. 2002;24(5):471–80.
Acknowledgements
None.
Funding
This work was supported by Project of Hohai university Discipline Action Plan (grant number 1013-418246).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: HY; acquisition of data: HY; analysis and interpretation of data: HY & XZ; statistical analysis: HY; obtaining funding: HY; drafting the manuscript: HY, XZ & LL; revision of the manuscript for important intellectual content. All authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: affiliation section has been updated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, H., Liu, L. & Zhang, X. Exercise interventions on body composition and quality of life of overweight/obese breast cancer survivors: a meta-analysis. BMC Women's Health 23, 484 (2023). https://doi.org/10.1186/s12905-023-02627-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02627-2