Abstract
Background
There are no studies done to evaluate the distribution of mammographic breast density and factors associated with it among Pakistani women.
Methods
Participants included 477 women, who had received either diagnostic or screening mammography at two hospitals in Karachi Pakistan. Mammographic breast density was assessed using the Breast Imaging Reporting and Data System. In person interviews were conducted using a detailed questionnaire, to assess risk factors of interest, and venous blood was collected to measure serum vitamin D level at the end of the interview. To determine the association of potential factors with mammographic breast density, multivariable polytomous logistic regression was used.
Results
High-density mammographic breast density (heterogeneously and dense categories) was high and found in 62.4% of women. There was a significant association of both heterogeneously dense and dense breasts with women of a younger age group < 45 years (OR 2.68, 95% CI 1.60–4.49) and (OR 4.83, 95% CI 2.54–9.16) respectively. Women with heterogeneously dense and dense breasts versus fatty and fibroglandular breasts had a higher history of benign breast disease (OR 1.90, 95% CI 1.14–3.17) and (OR 3.61, 95% CI 1.90–6.86) respectively. There was an inverse relationship between breast density and body mass index. Women with dense breasts and heterogeneously dense breasts had lower body mass index (OR 0.94 95% CI 0.90–0.99) and (OR 0.81, 95% CI 0.76–0.87) respectively. There was no association of mammographic breast density with serum vitamin D levels, diet, and breast cancer.
Conclusions
The findings of a positive association of higher mammographic density with younger age and benign breast disease and a negative association between body mass index and breast density are important findings that need to be considered in developing screening guidelines for the Pakistani population.
Similar content being viewed by others
Background
Mammography (MMG) is the baseline investigation used for screening and diagnosis of breast cancer, which remains responsible for more than 500,000 deaths each year worldwide [1]. Mammographic breast density (MBD) refers to the relative amount of dense tissue in an entire breast. Dense tissue comprises of connective and epithelial tissue including glandular parenchyma and hinders X-Ray transmission and therefore, appears dense/white on mammography. Fatty parenchyma allows unhindered X-ray transmission and hence appears darker/lucent on a mammogram. Dense breast tissue results in masking for breast cancer and hence the mammographic sensitivity is reduced with increasing MBD [2]. Increased MBD is reported to decrease the sensitivity of screening mammography by 48%, in comparison to the 78% mammographic sensitivity of the entire sample of the study [3]. Moreover, MBD has been recognized as an independent risk factor for breast cancer incidence and recurrence [4]. More than 50% of women in less than 50 years of age in the USA are reported to have dense breasts [5]. Therefore, there has been a lot of interest in the evaluation of factors associated with MBD including the role of environment and genetics and the causal relationship between breast cancer and MBD.
Several factors can affect MBD like age, heredity, parity, ethnicity, diet, hormonal replacement therapy (HRT), and the molecular subtypes of breast cancer [6, 7]. An increasing parity status has been inversely associated with MBD and the percent breast density [8]. MG del Carmen et al. compared breast density among different races and reported MBD to be lowest in white and African American women and highest in Asian women [9]. Race and ethnicity have been explored and identified as important determinants of MBD in another study too [10]. Though race and ethnicity are relevant to breast density, other factors such as diet, and environmental exposures are also important determinants of mammographic density and hence the risk of breast cancer is different in different ethnic groups [11]. There is an inverse association of body weight with the percentage of MBD in both pre and post-menopausal women due to more fat in the breast. It was recently confirmed in another retrospective study showing association of surgical weight loss with a decrease in breast density [12]. Few other studies reported a higher intake of fats, protein, alcohol, and hormonal replacement therapy (HRT) with higher MBD [13, 14]. Tamoxifen, however, reduced MBD in short term, and in a randomized control trial, a 10% reduction in MBD with a 63% breast cancer risk reduction was reported [15]. MBD is also reported to be heritable and has shown a correlation coefficient of 0.74 in monozygotic twins in comparison to 0.38 in dizygotic twins [16]. However, the exact influence of the environment and individual’s behavior on this heritable effect is still not clearly known.
There is no organized (service) screening program in Pakistan and most of the mammography done is opportunistic (individual) screening [17]. Due to limited screening mammography units and screening practices, there is a paucity of epidemiological data on MBD prevalence and factors associated with MBD in Pakistan. Therefore, the joint effects of sociodemographic and other factors associated with MBD among Pakistani women remain unclear. The main objective of this study was to evaluate the factors associated with MBD including serum level of vitamin D, intake of different food categories, physical activity, body mass index BMI, and other risk factors of breast cancer. Another objective was to assess the association of MBD with breast cancer among Pakistani women.
Materials and methods
The source population of this study is from a multicenter hospital-based case-control study which was conducted at two large tertiary care hospitals of Karachi, the details of the study and the questionnaire used are described in the previously published article [18]. Briefly, 477 women with complete records of MBD were extracted from the main study. We included women of ages 30–74 years subjects from the main study who underwent a screening mammogram between 2015 and 2019. There were 178 breast cancer cases and 299 controls who went diagnostic and screening (annual and biennial) mammography. Women were included even if they had multiple mammograms. Women were excluded if they were unable to complete the interview due to any sickness or been living outside Pakistan for more than a year. We also excluded women if mammographic breast density information was unavailable. All mammograms were taken before breast cancer diagnosis among cases. All subjects completed an interview-based questionnaire that included information on age, education, socioeconomic status, parity, age of mother at first birth, breastfeeding, age at menarche and menopause, age of mother at first birth, history of any comorbid or benign breast disease, family history of breast cancer. Menopausal status was either premenopausal or postmenopausal. Participants also reported the average number of hours per week, engaged in physical activity of different intensities for at least 10 min, like vigorous exercise or moderate exercise of household activities like mopping, etc., and walking. BMI (kg/m2) and tumor characteristics were recorded from medical files and reports. Women with missing information of BI-RADS (Breast Imaging-Reporting and Data System) density were excluded. All consenting participants were interviewed, after informed consent, in a separate room to ensure privacy using a structured questionnaire.
Mammography measurement details
Two view mammography was performed for all patients comprising of medio-lateral oblique (MLO) and cranio-caudal (CC) views on a computed radiography (CR) system. In the CR system, the X- Rays passing through the breast, grid, and cassette cover are absorbed by the plate reader system that comprises photostimulable storage phosphor (PSP). An electronic latent image is produced on the PSP due to the local absorption of X-ray energy that varies with the anatomical variation of breast parenchyma. The cassette is subsequently placed in the reader that captures the information and converts it to a digital signal that is finally displayed at the workstation [19]. The soft copies of the mammographic images were downloaded and reviewed at picture archiving and communication system (PACS) by breast imagers, and qualitative assessment of mammographic density was done by dedicated radiologists with years of experience in interpreting mammography and breast density.
Assessment of mammographic breast density (MBD)
Using the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS 5th edition), the mammographic breast density on mammograms was categorized as; Category 1, Predominantly fatty (less than 25% glandular); category 2 for scattered fibroglandular (25–50% glandular); category 3 for heterogeneously dense (51–75% glandular) and category 4 for dense breast parenchyma (more than 75% glandular) [20]. Categories 3 and 4 both are high MBD (Figs. 1 and 2 showing fatty and dense breast parenchyma). Analyses were restricted to patients with an available BI-RADS measurement. The density measurements of the breast contralateral to the tumor were used to avoid a distortion of measurements due to the tumor itself.
Dietary intake assessment with a Food Frequency Questionnaire (FFQ)
Dietary intake assessment was done by using the validated food frequency questionnaire FFQ [21]. Intake frequency was categorized into 7 groups and category for each food item was converted to daily intake. Each participant was also asked about their average portion size/common serving size of the food. The intake frequencies were multiplied by standard portion size to calculate servings per day of all food items. All the food items were grouped into six components including fruits, vegetables, dairy, grains, white meat, red meat, and plant proteins and total servings/day was calculated for each food category.
Measurement of serum 25 (OH)D level
After the interview, blood samples were collected from the study participants and serum 25 (OH)D level was measured using ELISA.
Tumor characteristics
Histopathology and estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2/neu) status of breast cancer cases were retrieved from medical records [22].
The ethical approval was obtained by the Human Research Ethics Committee of the University of Adelaide and the Ethical Review Committees of two hospitals in Karachi Pakistan: Aga Khan University Hospital AKUH and Karachi Institute of Radiation and Nuclear Medicine Hospital KIRAN. Patients who were literate read and signed the informed consent form and informed consent was obtained verbally from those who could not read or write.
Statistical analysis
Multinomial logistic regression models were applied to compute odds ratios (ORs) and 95% confidence intervals (CIs) for the MBD categories. The fatty and scattered fibroglandular tissue categories were merged and used as a reference. Multivariate models were adjusted for variables found to be significantly associated with breast density and known risk factors for breast cancer such as age, BMI, age at menarche and menopause in the postmenopausal group, parity, and family history of breast cancer among first-degree relatives, BMI, and the food categories. SPSS 22.0 software (IBM Statistics, Armonk, New York, USA) was used for the analysis.
Results
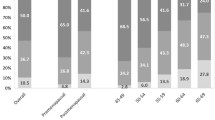
The mean age of the study participants was 46.2 years (SD 11.7). Hundred and eighty (37.3%) women had breast biopsy-proven breast cancer and 297 (62.7%) of the women had normal or benign breast disease. Figure 3 shows that high density MBD (heterogeneously and dense categories) accounted for 62.4% of all participants.
Characteristics of study participants
Table 1 shows that there were significant differences in age, education, parity, breastfeeding, body weight, BMI, parity, history of breastfeeding, history of comorbid, menopausal status, among women of different breast densities. In both heterogeneously dense and dense groups, there was a higher percentage of women who were of younger age categories, nullipara, lower parity, higher education, and had a family history of breast cancer.
Tumor characteristics
There was no significant difference observed in the tumor characteristics among different MBD categories (Table 2).
There was a positive association of MBD with young age, nulliparity and low parity, family history of breast cancer and history of benign breast disease BBD. Significant protective association of MBD was observed for women of younger age, menopausal status, younger age at first birth (<20 years AFB) and BMI (Table 3).
Diet and breast density
There was no association reported between different MBD and food categories of grains, fruits, vegetables, plant proteins, dairy products, white and red meat as shown in Table 4.
Multivariable analysis using multinomial logistic regression (Table 5) shows that women with dense and heterogeneously dense breasts versus fatty and fibroglandular breasts were of a younger age group and had higher benign breast disease BBD. An inverse relationship of BMI with MBD was also observed. There was no association of serum vitamin D levels or breast cancer with breast density.
Discussion
The study findings of significant association of younger age and BBD with higher MBD and protective association of higher BMI are consistent with other studies. Younger age as an important determinant of a high MBD is similar to other studies done in different populations [23,24,25,26,27]. In another study, there was a decline in density reported with age in women with and without breast cancer [28]. It supports the hypothesis that breast density declines with age as younger women have a higher proportion of dense breast tissue compared to older women [6]. In a study done on data from the San Francisco Mammography Registry, breast density decreased with age on an annual basis over the perimenopausal stage [29]. Our findings provide important data on the association of benign breast disease with higher mammographic breast density and are similar to other studies [30,31,32].
In our study, the inverse relationship between MBD and higher BMI remained the same in both menopausal and premenopausal women. The inverse relationship between BMI and MBD has been reported in previous studies among both menopausal and premenopausal women [23, 33, 34]. In the Minnesota Breast Cancer Family Study, higher BMI was associated with lower breast density among postmenopausal women only [35]. BMI was also inversely related to MBD in Chinese women [36]. An inverse relationship of greater body weight with percentage mammographic density was also reported in premenopausal women, with mammogram showing a larger area of fatty tissue caused by an increased quantity of fat in the breast [37].
Similar to our study findings, a study among Japanese women reported that younger age and BMI were inversely related to high MBD [38]. In another breast cancer family study of 426 families, younger age and lower BMI were associated with increased MBD in both premenopausal and postmenopausal women. Hormone replacement therapy among postmenopausal women was also associated with MBD but this association of MBD with hormone replacement therapy was not seen in the current study as HRT use is very small among Pakistani women. In a study in Norway, volumetric mammographic density (VMD) was inversely associated with age and BMI which is consistent to our study findings [31, 39]. A negative association of high MBD with age, low parity and BMI, was similarly reported by other studies [40, 41].
However, this study observed no association between MBD and breast cancer, and this lack of association has also been reported in a study done in the USA [42] and one in China [42]. A nested case-control study in Ontario, Canada also reported no association of MBD with breast cancer among women with BRCA mutation[43]. Another study at the Massachusetts General Hospital showed that while Asians had the highest breast density, the incidence of breast cancer among them was lesser than that among white women [13]. An observational study of the Mayo Clinic Benign Breast Disease (BBD) cohort failed to find any evidence of an association between MBD and breast cancer risk in women diagnosed with BBD of atypical hyperplasia (AH) type [44]. Similarly, another study done at Johns Hopkins showed that breast cancer was not associated with MRI breast density [45]. The study findings did not show any association of MBD with vitamin D levels, similar to the Nurses’ Health Study [46]. There was also no association observed between MBD and diet.
The high percentage of high MBD (62.4%) in Pakistani women is important information and will be helpful in planning or advising for an organized screening mammography program in Pakistan in future. This is higher than the reported percentage among women in the USA [47] and lower than the reported percentage among women in Jordon [48]. High density breast, on one hand, increases the rates of false-negative diagnosis and on the other hand, there is a requirement of additional tests. The need for additional tests like ultrasound, and MRI increases the sensitivity of screening programs [49] but the cost then becomes too high for the majority of the asymptomatic women to opt for regular screening as there is no health insurance and high poverty in Pakistan.
Significant association of younger age with high MBD in our study is an important finding because ACR guidelines are followed here in the absence of screening guidelines established for Pakistani women where mean age at the time of breast cancer diagnosis reported is also much younger compared to the women in America (46.1 years ± SD 10.1) [50]. Mammographic breast density was also found to be positively associated with BBD and negatively associated with BMI. In univariate analysis, lower parity, breastfeeding, and family history of breast cancer also showed a significant protective association with dense breast. However, in a multivariate analysis performed to adjust for confounding factors, only younger age, BBD, and BMI remained as significant factors associated with high breast density.
Limitations
Study limitations include small sample size and limited generalizability of the study population due to lack of any organized screening mammography program in Pakistan. Moreover, there was no availability of digital radiography (DR) which was installed in 2019, after the completion of the data collection period of the study. We could not calculate percentage density and absolute dense area due to the lack of availability of the software. There is still a lack of standardization in MBD assessment with high density definitions varying widely from 25 to 75% of dense tissues on mammograms in different studies. Though we tried our best to evaluate MBD with standard mammographic procedures, breast density classification as well as a standardized definition of MBD. Still, some misclassification of MBD could have affected our results since we used visual classification using BI RADS by two experienced radiologists. However, a study reported very good agreement between automatic assessment software of breast density based on artificial intelligence (AI) and visual assessment by a senior and a junior radiologist [51]. The strengths of the study are a good quality of data collection by medical doctors, and comprehensive assessment of all factors associated with MBD.
Conclusion and recommendations
To our knowledge, this study is the first to report unique distribution of MBD and identify factors associated with MBD in Pakistani women. The findings of positive association of higher mammographic density younger age and BBD and negative association between BMI and breast density are consistent with predictors of mammographic density observed in other populations; however, certain risk factors were not significantly associated with BMD. These are important findings which may be helpful to develop screening guidelines for Pakistani population. Given the current role of breast density in determining breast cancer screening protocols, public health policy, and future research directions, it is important to validate our findings in a larger scale investigation with the advanced technology of the DR system and assess if it is better than screen-film mammography in women with dense breasts.
Availability of data and materials
Only the data SPSS file has been submitted and mentioned as per requirement.
Abbreviations
- ACR:
-
American College of Radiology
- AH:
-
Atypical hyperplasia
- AFB:
-
Age at first birth
- BI-RADS:
-
Breast Imaging-Reporting and Data System
- BMI:
-
Body mass index
- BBD:
-
Benign breast disease
- CC:
-
Craniocaudal
- CR:
-
Computed radiography
- DR:
-
Digital radiography
- ER:
-
Estrogen receptor
- FFQ:
-
Food Frequency questionnaire
- HER2:
-
human epidermal growth factor 2
- MBD:
-
Mammographic breast density
- MLO:
-
mediolateral oblique
- PR:
-
Progesterone receptor
- SES:
-
Socioeconomic status
References
Freer PE. Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics. 2015;35(2):302–15.
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36.
Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225(1):165–75.
Park B, Cho HM, Lee EH, Song S, Suh M, Choi KS, et al. Does breast density measured through population-based screening independently increase breast cancer risk in Asian females? Clin Epidemiol. 2018;10:61–70.
Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255. https://doi.org/10.1093/jnci/dju255.
Woolcott CG, Koga K, Conroy SM, Byrne C, Nagata C, Ursin G, et al. Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case–control studies. Breast Cancer Res Treat. 2012;132(3):1163–71.
Boyd NF. Mammographic density and risk of breast cancer. Am Soc Clin Oncol Educ Book. 2013;33:e57–62.
Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomark Prev. 2005;14(2):343–9.
del Carmen MG, Halpern EF, Kopans DB, Moy B, Moore RH, Goss PE, et al. Mammographic breast density and race. AJR Am J Roentgenol. 2007;188(4):1147–50.
Moore JX, Han Y, Appleton C, Colditz G, Toriola AT. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4(2):pkaa010.
Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer. 2018;25(3):259–67.
Williams AD, So A, Synnestvedt M, Tewksbury CM, Kontos D, Hsiehm MK, et al. Mammographic breast density decreases after bariatric surgery. Breast Cancer Res Treat. 2017;165(3):565–72.
El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States). Cancer Causes Control. 2000;11(10):955–63.
Quandt Z, Flom JD, Tehranifar P, Reynolds D, Terry MB, McDonald JA. The association of alcohol consumption with mammographic density in a multiethnic urban population. BMC Cancer. 2015;15:1094.
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–52.
Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ, et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343–60.
Shamsi U. Cancer prevention and control in Pakistan: review of cancer epidemiology and challenges. Liaquat Natl J Prim Care. 2020;2(1):34–8.
Shamsi U, Khan S, Azam I, Habib Khan A, Maqbool A, Hanif M, et al. A multicenter case control study of association of vitamin D with breast cancer among women in Karachi, Pakistan. PLoS One. 2020;15(1):e0225402.
Heddson B, Ronnow K, Olsson M, Miller D. Digital versus screen-film mammography: a retrospective comparison in a population-based screening program. Eur J Radiol. 2007;64(3):419–25.
Balleyguier C, Ayadi S, Van Nguyen K, Vanel D, Dromain C, Sigal R. BIRADS classification in mammography. Eur J Radiol. 2007;61(2):192–4.
Safdar NF, Bertone-Johnson E, Cordeiro L, Jafar TH, Cohen NL. Dietary patterns of Pakistani adults and their associations with sociodemographic, anthropometric and life-style factors. J Nutr Sci. 2013;2:e42.
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–7.
Lee E, Doanvo N, Lee M, Soe Z, Lee AW, Van Doan C, et al. Immigration history, lifestyle characteristics, and breast density in the Vietnamese American Women’s Health Study: a cross-sectional analysis. Cancer Causes Control. 2020;31(2):127–38.
Mizukoshi MM, Hossain SZ, Poulos A. Mammographic breast density of japanese women living in Australia: implications for breast screening policy. Asian Pac J Cancer Prev. 2019;20(9):2811–7.
Liu J, Liu PF, Li JN, Qing C, Ji Y, Hao XS, et al. Analysis of mammographic breast density in a group of screening chinese women and breast cancer patients. Asian Pac J Cancer Prev. 2014;15(15):6411–4.
Titus-Ernstoff L, Tosteson AN, Kasales C, Weiss J, Goodrich M, Hatch EE, et al. Breast cancer risk factors in relation to breast density (United States). Cancer Causes Control. 2006;17(10):1281–90.
Gapstur SM, Lopez P, Colangelo LA, Wolfman J, Van Horn L, Hendrick RE. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Biomark Prev. 2003;12(10):1074–80.
Lokate M, Stellato RK, Veldhuis WB, Peeters PH, van Gils CH. Age-related changes in mammographic density and breast cancer risk. Am J Epidemiol. 2013;178(1):101–9.
Engmann NJ, Scott C, Jensen MR, Winham SJ, Ma L, Brandt KR, et al. Longitudinal changes in volumetric breast density in healthy women across the menopausal transition. Cancer Epidemiol Biomarkers Prev. 2019;28(8):1324–30.
Ghosh K, Vierkant RA, Frank RD, Winham S, Visscher DW, Pankratz VS, et al. Association between mammographic breast density and histologic features of benign breast disease. Breast Cancer Res. 2017;19(1):134.
Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137–43.
Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105(14):1043–9.
Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomark Prev. 2007;16(1):43–9.
Kawahara M, Sato S, Ida Y, Watanabe M, Fujishima M, Ishii H, et al. Factors influencing breast density in Japanese women aged 40–49 in breast cancer screening mammography. Acta Med Okayama. 2013;67(4):213–7.
Rice MS, Bertrand KA, Lajous M, Tamimi RM, Torres G, Lopez-Ridaura R, et al. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann Epidemiol. 2015;25(11):868–73.
Li T, Tang L, Gandomkar Z, Heard R, Mello-Thoms C, Xiao Q, et al. Characteristics of mammographic breast density and associated factors for chinese women: results from an automated measurement. J Oncol. 2019;2019:4910854.
Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomark Prev. 2006;15(11):2086–92.
Ishihara S, Taira N, Kawasaki K, Ishibe Y, Mizoo T, Nishiyama K, et al. Association between mammographic breast density and lifestyle in Japanese women. Acta Med Okayama. 2013;67(3):145–51.
Hjerkind KV, Ellingjord-Dale M, Johansson ALV, Aase HS, Hoff SR, Hofvind S, et al. Volumetric mammographic density, age-related decline, and breast cancer risk factors in a national breast cancer screening program. Cancer Epidemiol Biomark Prev. 2018;27(9):1065–74.
Wong CS, Lim GH, Gao F, Jakes RW, Offman J, Chia KS, et al. Mammographic density and its interaction with other breast cancer risk factors in an Asian population. Br J Cancer. 2011;104(5):871–4.
Dite GS, Stone J, Chiarelli AM, Giles GG, English DR, Cawson JC, et al. Are genetic and environmental components of variance in mammographic density measures that predict breast cancer risk independent of within-twin pair differences in body mass index? Breast Cancer Res Treat. 2012;131(2):553–9.
Li T, Tang L, Gandomkar Z, Heard R, Mello-Thoms C, Shao Z, et al. Mammographic density and other risk factors for breast cancer among women in China. Breast J. 2018;24(3):426–8.
Passaperuma K, Warner E, Hill KA, Gunasekara A, Yaffe MJ. Is mammographic breast density a breast cancer risk factor in women with BRCA mutations? J Clin Oncol. 2010;28(23):3779–83.
Byrne C, Schairer C, Brinton LA, Wolfe J, Parekh N, Salane M, et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes Control. 2001;12(2):103–10.
Zhu W, Huang P, Macura KJ, Artemov D. Association between breast cancer, breast density, and body adiposity evaluated by MRI. Eur Radiol. 2016;26(7):2308–16.
Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127(3):667–74.
Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198(3):W292–5.
Al-Mousa DS, Alakhras M, Spuur KM, Alewaidat H, Rawashdeh M, Abdelrahman M, et al. Mammographic breast density profile of jordanian women with normal and breast cancer findings. Breast Cancer (Auckl). 2020;14:1178223420921381.
Kim WH, Chang JM, Moon HG, Yi A, Koo HR, Gweon HM, et al. Comparison of the diagnostic performance of digital breast tomosynthesis and magnetic resonance imaging added to digital mammography in women with known breast cancers. Eur Radiol. 2016;26(6):1556–64.
Shamsi U, Khan S, Usman S, Soomro S, Azam I. A multicenter matched case control study of breast cancer risk factors among women in Karachi. Pakistan Asian Pac J Cancer Prev. 2013;14(1):183–8.
Le Boulc'h M, Bekhouche A, Kermarrec E, Milon A, Abdel Wahab C, Zilberman S, et al. Comparison of breast density assessment between human eye and automated software on digital and synthetic mammography: impact on breast cancer risk. Diagn Interv Imaging. 2020;101:811–9.
Acknowledgements
None.
Funding
This work was partially supported by the Deans fund Aga Khan University Hospital (Grant ID: 201 710550 20251 500 81001 0000). The funding bodies had no role in the design, collection of data, analysis, and interpretation of results or preparation of the manuscript of this study.
Author information
Authors and Affiliations
Contributions
US participated in the design of the study, acquisition of data, performed the statistical analysis and drafted the manuscript. SA made contributions to conception and design, acquisition of data, and interpretation of data. AS contributed to the drafting of the manuscript. DC and IA made contributions in the statistical analysis, interpretation of data. All authors participated in revising the manuscript critically for important intellectual content, and final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval was obtained by the Human Research Ethics Committee of the University of Adelaide and the Ethical Review Committees (ERC) of two hospitals in Karachi Pakistan: Aga Khan University Hospital AKUH and Karachi Institute of Radiation and Nuclear Medicine Hospital KIRAN. All participants provided written informed consent while informed consent was obtained verbally from those who could not read or write as approved by the ERC.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shamsi, U., Afzal, S., Shamsi, A. et al. Factors associated with mammographic breast density among women in Karachi Pakistan. BMC Women's Health 21, 438 (2021). https://doi.org/10.1186/s12905-021-01538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-021-01538-4