Abstract
Background
The purpose of this study was to investigate the relationships between generalized, abdominal, and visceral fat obesity and the prevalence of gout in patients with type 2 diabetes mellitus (T2DM).
Methods
Data were obtained from the electronic medical databases of the National Metabolic Management Center (MMC) of Yuhuan Second People’s Hospital and Taizhou Central Hospital (Taizhou University Hospital) between September 2017 and June 2023. Four obesity indicators were analyzed: waist circumference (WC), waist-to-hip ratio (WHR), body mass index (BMI), and visceral fat area (VFA). The relationships between these parameters and gout prevalence were analyzed using multivariate logistic regression and restricted cubic spline (RCS) analyses. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic efficacy of the four parameters for gout.
Results
This cross-sectional study enrolled 10,535 participants (600 cases and 9,935 controls). Obesity was more common in patients with gout, and the obesity indicators were markedly higher in this group. After adjustment for confounders, obesity, as defined by BMI, WC, WHR, and VFA, was found to be associated with greater gout prevalence, with odds ratios (OR) of 1.775, 1.691, 1.858, and 1.578, respectively (P < 0.001). The gout odds ratios increased markedly in relation to the obesity indicator quartiles (P-value for trend < 0.001), and the obesity indicators were positively correlated with gout prevalence, as shown using RCS. The area under the ROC curve values for BMI, WC, WHR, and VFA were 0.629, 0.651, 0.634, and 0.633, respectively.
Conclusion
Obesity—whether general, abdominal, or visceral fat obesity—was positively linked with elevated gout risk. But uncovering the causality behind the relationship requires further prospective study. Obesity indicators (BMI, WC, WHR, and VFA) may have potential value for diagnosing gout in clinical practice.
Similar content being viewed by others
Background
Gout is an inflammatory disease affecting the joints [1]. Over the last few decades, gout has become increasingly prevalent in various regions and countries [2]. Between 2010 and 2020, the prevalence of gout increased globally from 0.08% to 2–4% [3]. A nationwide survey in China reported a gout prevalence of 3.2% between 2015 and 2017 (4.4% in men and 2.0% in women), translating into approximately 25.56 million people being affected [4].
Type 2 diabetes mellitus (T2DM) is a co-morbidity of gout. When T2DM coexists with gout, it can worsen the effects of the disease, lower the quality of life, and increase financial burdens on society [5]. Therefore, research on gout- and diabetes-related risk factors is crucial.
Obesity is a significant global health concern and is associated with gout [6,7,8]. Obesity is often thought to impact gout risk via elevated levels of serum uric acid. It has been associated with hyperuricemia possibly due to increasing production and reduced renal excretion of urate [9, 10].
However, most previous studies have chosen body mass index (BMI) as a measure of obesity, which is widely applied in clinical and epidemiological research [11]. Nevertheless, the accumulation of body fat, rather than weight, is the key factor determining obesity. Waist circumference (WC) [12], waist-to-hip ratio (WHR) [13], and visceral fat area (VFA) [14] are also indicators of obesity. These parameters reflect the adipose tissue distribution throughout the body. Both WC and WHR are indicators of abdominal obesity, while visceral fat obesity (VFO) is indicated by VFA.
This study evaluated the relationship between different obesity indicators (BMI, WC, WHR, and VFA) and gout prevalence in patients with T2DM using cross-sectional data from the National Metabolic Management Center (MMC) of The Second People’s Hospital of Yuhuan and Taizhou Central Hospital (Taizhou University Hospital).
Methods
Study design and participants
In this cross-sectional population-based study, a total of 10,848 participants was recruited from the MMC electronic medical database [15] of The Second People’s Hospital of Yuhuan and Taizhou Central Hospital (Taizhou University Hospital) between September 2017 and June 2023. Based on the idea of “One Center, One Step, and One Standard Model”, MMC is a national project to manage patients with metabolic conditions. Previous publications provide a thorough introduction to the MMC program [15]. Every patient consented to a thorough physical examination, blood sample collection, and interviews using an oral questionnaire. All data were gathered at the time of recruitment at local MMCs by personnel who had received training and followed a standard protocol. Additionally, some indicators, including visceral fat area (VFA), were detected using the same machine model. Additional quality control procedures could be seen in the MMC protocol. The inclusion criterion was to satisfy the 1999 T2DM diagnostic criteria established by the World Health Organization [16]. Those who met the following criteria were excluded: (1) T1DM or other type diabetes patients, (2) age < 18y, and (3) missing any values of obesity parameters. After screening, 10,535 people were enrolled (Fig. 1).
Patient data collection
Using the MMC specialist electronic medical record system, we gathered patient data through standardized questionnaires and clinical and laboratory assessments. The following variable data were collected: age, sex (male or female), smoking and drinking status, height (H), weight (W), WC, hip circumference (HC), diastolic blood pressure (DBP), systolic blood pressure (SBP), urea nitrogen (UN), serum creatinine (Scr), uric acid (UA), fasting blood glucose (FBG), glycated hemoglobin (HbA1c), alanine transaminase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and estimated glomerular infiltration rate (eGFR), VFA, hypertension (no/yes), and hyperlipidemia (no/yes). H, W, WC and HC were measured with a standard protocol, and BMI was calculated as W/H2 (kg/m2), while WHR was determined as WC/HC. VFA were measured at the level of umbilicus by a dual bioelectrical impedance analyzer (HDS2000, Omron Healthcare Co).
Variable definitions
Gout was diagnosed according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) clinical classification criteria for gout [17]. Hypertension was described as an SBP or DBP value of ≥ 140 or ≥ 90 mmHg, respectively [18], or physician diagnosis. Hyperlipidemia was determined by at least one of the following: TC ≥ 5.7 mmol/L, TG ≥ 1.7 mmol/L, LDL-C ≥ 3.6 mmol/L, and HDL-C < 1.29 mmol/L in women and < 1.03 mmol/L in men [19] or physician diagnosis. Those who smoked cigarettes were considered current smokers, whereas those who were currently drinking alcohol were considered current drinkers. The eGFR was determined as described [20].
Four obesity indicators were analyzed, namely, BMI, WC, WHR, and VFA. The obesity thresholds were: (1) general, BMI ≥ 28.0 kg/m2 [11], (2) abdominal, WC > 90 cm for men or > 85 cm for women [12], or WHR > 0.90 for men or > 0.85 for women [13], (3) VFO: VFA ≥ 100 cm2 [14].
Statistical analysis
Data are shown as numbers (%) or medians (interquartile range). Non-normally distributed continuous variables were evaluated using the Mann–Whitney U test, and categorical variables were analyzed with the Chi-squared test. Two-sided p-values < 0.05 were deemed to indicate statistical significance. Statistical analysis was undertaken with SPSS, version 23.0, and R, version 4.1.3.
Multivariate logistic regression was used to assess the independent effects of general obesity, abdominal obesity, VFO, and obesity indicators (BMI, WC, WHR, and VFA) on the presence of gout. Obesity indicators were divided into quartiles; the lowest quartile was used as a reference. Analyses were adjusted for multiple variables. We considered the the baseline difference, clinical significance, and the results of previous studies to determine the adjusted variables [7, 21]. We performed a number of different statistical models to verify the stability of the results: Age and sex were adjusted in Model 1, with further adjustments for HbA1c, hyperlipidemia, hypertension, smoking status, drinking status, and eGFR in Model 2. We conducted Restricted cubic spline (RCS) models fitted for the logistic regression model to assess the potential nonlinear relationships between levels of obesity indicators and gout. In this model, obesity indicators was used as a continuous variable with four knots (5th, 35th, 65th and 95th) suggested by Harrell. The diagnostic efficacies of the indices for gout were analyzed with Receiver operating characteristic (ROC) curves.
Ethics statement
The study protocol conforms to STROBE guidelines [22], and was approved by the Ethics Committees of Yuhuan Second People’s Hospital and Taizhou Central Hospital (Taizhou University Hospital). Written informed consent was obtained from all participants.
Results
Characteristics of study participants
Figure 1 illustrates a flowchart of the study cohort, and Table 1 lists the features of the cohort. The median (IQR) age of the participants was 55(47–63) years, and 6342(60.2%) of them were men. The median (IQR) obesity indicators(BMI, WC, WHR, VFA) was 25.2(23.07,27.55), 89.4(83.1,96), 0.94(0.9,0.98), 94(66,124), respectively. Out of 10,535 participants. 600 had gout, while 9,935 did not. The group with gout was predominately male (n = 503, 83.8%) compared to the group without gout. The distribution of obesity indicators is shown in Figure S1 (Supplementary Fig. S1). Patients with gout had markedly higher values of BMI, WC, WHR, VFA, SBP, and DBP compared to those without gout. Additionally, they exhibited markedly higher levels of AST, ALT, UN, Scr, UA, and TG, and lower levels of FBG, HbA1c, HDL-C, and e-GFR. Moreover, patients with gout exhibited higher proportions of obesity (general obesity, abdominal obesity, and VFO) and comorbidities such as hypertension and hyperlipidemia. Additionally, patients with gout had higher rates of smoking and alcohol consumption compared to those without gout.
Association between obesity indicators and gout prevalence
After gradually adjusting for all potential confounding factors, obesity defined by BMI, WC, WHR, and VFA was linked with a higher prevalence of gout, with ORs (95% CI) of 1.775 (1.468–2.145), 1.691 (1.394–2.053), 1.858 (1.367–2.524), and 1.578 (1.317–1.890), respectively (Table 2).
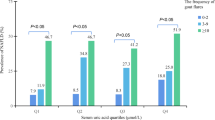
Table 2 illustrates the relationships between obesity parameters and gout. In the multivariate regression model, adjusting for sex, age, HbA1c, hyperlipidemia, hypertension, smoking status, drinking status, and eGFR, a significant increase in the ORs of gout from the lowest to the highest quartiles of obesity indicators (BMI, WC, WHR, and VFA) was observed (trend P-value < 0.001). The ORs (95% CI) of gout were 2.751 (2.034–3.720), 2.999 (2.185–4.118), 2.127 (1.561–2.896), and 2.259 (1.730–2.950) for the highest relative to the lowest quartiles, respectively.
Furthermore, the RCS analysis indicated positive correlations between the four obesity indices and gout prevalence in both males and females (P-value for nonlinearity = 0.1453, 0.1118, 0.0374, and 0.8171 in males, and 0.4902, 0.2890, 0.0072, and 0.3472 in females, for BMI, WC, WHR, and VFA, respectively) (Fig. 2).
RCS analysis of the relationships between obesity indices (BMI, WC, WHR, and VFA) and gout prevalence. The solid line represents the OR, with the shaded area representing the 95% CI. Adjustments were made to the model for age, HbA1c, hyperlipidemia, hypertension, smoking status, drinking status, and eGFR
RCS, restricted cubic spline; BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; VFA, visceral fat area; OR, odds ratio; CI, confidence interval; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate.
ROC curve analysis of gout
The ability of obesity parameters to diagnose gout was investigated with ROC curves. The analysis showed a slightly higher area under the ROC curve (AUC) for WC (0.651, 95% CI: 0.510–0.707) and WHR (0.634, 95% CI: 0.417–0.785) than for BMI (0.629, 95% CI: 0.370–0.812) and VFA (0.633, 95% CI: 0.477–0.726) (Fig. 3). The optimum thresholds of BMI, WC, WHR, and VFA for gout were 24.055, 89.25, 0.925, and 118.5, respectively.
Discussion
To our knowledge, this is the first study to investigate the relationship between different obesity indicators (BMI, WC, WHR, and VFA) and gout in T2DM. In this study, associations were observed between increased risk of gout and general obesity, abdominal obesity, and VFO in patients with T2DM. Levels of obesity indicators (BMI, WC, WHR, and VFA) were markedly higher in patients with gout compared to those without. Lastly, ROC curves indicated increased—although non-significantly—AUCs for indicators of abdominal obesity (WC and WHR) compared to indicators of general obesity (BMI) and VFO (VFA), suggesting similar predictive abilities of these parameters for the development of gout.
Significant associations have been reported between obesity, hyperuricemia, and gout incidence. A study of 29,310 participants reported a 5% increase in the incidence of gout for every unit increase in BMI [8]. According to a 2018 meta-analysis, BMI values of 30 kg/m2 were linked to a 2.24-fold increased risk of developing gout [7]. A health professional follow-up study revealed that weight gain and adiposity are linked with elevated gout risk in male patients, while weight reduction was found to be preventive [6]. A study of patients with obesity in Sweden showed that, over 26 years of follow-up, gout incidence was reduced by 40% after bariatric surgery [23]. Guo et al.’s study [24] revealed an interesting finding that WHR and UA target achievement were correlated, although BMI was not. Furthermore, abdominal obesity has been found to be more common in patients with gout [25]. Choi et al. [26]. reported a gout risk of 1.82 (95% CI 1.39–2.39; P for trend < 0.001) in males in the top WHR quintile (0.98–1.39) relative to the bottom quintile (0.70–0.88). However, Takahashi et al. [27]. contended that increased visceral fat might be more detrimental to UA metabolism than BMI, suggesting that visceral rather than subcutaneous fat is linked to metabolic anomalies and hyperuricemia in gout [28]. According to a health examination study, VFO was more common in individuals with gout compared to healthy controls [21]. However, the aforementioned studies may havecertain shortcomings. These include the comparatively smaller sample size, the fact that only the general population was taken into account, rather than the so-called highly exposed population groups, and the absence of a comparative study of several obesity indicators. In contrast to other research, the focus of this study was on the relationship between indicators of various types obesity with gout in patients with T2DM.
The exact underlying processes of obesity in gout development remain unknown. It has been found that obesity influences gout by augmenting serum UA levels. Obesity, especially that associated with abdominal fat, has been linked to hyperuricemia, possibly resulting from increased urate synthesis and decreased renal clearance [29]. Increased body fat leads to increased total nucleic acid metabolism, which in turn promotes UA production via purine metabolism [30]. Moreover, obesity may cause abnormalities in glomerular hemodynamics and hyperactivate the renin-angiotensin-aldosterone pathway, resulting in obesity-linked nephropathy. Long-term exposure to these effects may lower renal UA excretion by inducing glomerular atherosclerosis [31]. The excess adipose tissues of obesity produce pro-inflammatory cytokines that reduce antioxidant levels and raise reactive oxygen species (ROS) and nitrogen species produced by macrophages and monocytes. As a result, this exacerbates the impact of urate excretion dysfunction, leading to urate resorption disequilibrium and ultimately to hyperuricemia and gout risk [32]. Additionally, it has been shown that the onset of hyperuricemia is correlated with several adipocytokines linked to obesity, including adiponectin and leptin [33, 34].
Hyperuricemia and gouty arthritis are interrelated because white blood cells phagocytize MSU crystals [35]. MSU crystals can interact with Toll-like receptors and interleukin-1(IL-1) receptors on macrophage surfaces, causing the NOD-like receptor family protein 3 (NLRP3) inflammasome to produce IL-1 and promote neutrophil and macrophage influx [35]. It has been demonstrated that obesity increases proinflammatory molecules, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [36, 37]. This intensifies inflammation brought on by obesity. Furthermore, previous studies have shown that soluble urate can promote NLRP3 inflammasome activation [38] and act as an alum to stimulate inflammation in an adjuvant capacity [35]. B cell antigen presentation to CD4 and CD8 T cells furthers the pathogenic function of IgM/IgG antibodies by facilitating urate crystallization, which in turn causes MSU crystals to be phagocytosed and the NLRP3 inflammasome to be sensed [39]. In addition, comorbidities related to obesity may raise the incidence of gout [40, 41].
This study has several limitations. First, causal links cannot be inferred owing to the cross-sectional design. Future studies should examine the longitudinal relationship between gout and obesity indicators. Second, by employing statistical adjustments, our study reduced other risk factors; however, unobserved confounders, such as alcohol intake and history of pertinent medication use, persisted. This limitation may have affected the robustness and reliability of our findings. Third, although we included multiple indicators of obesity for our study, there are also some better indicators associated with obesity and metabolic disease, such as body roundness index, triglyceride glucose index-waist circumference [42, 43]. Future study could be conducted on these indicators. Fourth, our study is the lack of data on specific endocrine factors such as plasma aldosterone concentrations. Recent research has highlighted the potential dual impact of elevated aldosterone levels on both hyperuricemia and gout risk in hypertensive patients [44]. Aldosterone, a hormone pivotal in blood pressure regulation, may contribute to the development of gout through its effects on uric acid metabolism and renal excretion. The exclusion of such endocrine data may have limited our ability to fully elucidate the mechanisms linking obesity with gout, particularly in the context of metabolic and cardiovascular comorbidities. Future studies should consider incorporating measurements of aldosterone and other related hormones to provide a more comprehensive understanding of the underlying pathophysiology. Finally, our investigation was limited to Chinese populations, which may restrict the general extrapolation of our findings. Further research on various ethnic groups is needed to determine the relationships between obesity and gout risk.
Conclusions
Obesity, particularly abdominal fat, contributes to the occurrence of gout. Obesity and gout risk were found to be significantly and positively linked in patients with T2DM. But uncovering the causality behind the relationship requires further prospective study. Obesity indicators (BMI, WC, WHR, and VFA) show potential predictive ability for gout development. Avoiding excessive gain in weight, WC, and VFA maybe an effective approach to prevent gout in patients with T2DM.
Data availability
The datasets of the study are available from the corresponding author upon reasonable request.
Abbreviations
- MSU:
-
Monosodium urate
- T2DM:
-
Type 2 diabetes mellitus
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- WHR:
-
Waist-to-hip ratio
- VFA:
-
Visceral fat area
- VFO:
-
Visceral fat obesity
- H:
-
Height
- W:
-
Weight
- WC:
-
Waist circumference
- HC:
-
Hip circumference
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- UN:
-
Urea nitrogen
- Scr:
-
Serum creatinine
- UA:
-
Uric acid
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Glycated hemoglobin
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- LDL:
-
C-Low-density lipoprotein cholesterol
- HDL:
-
C-High-density lipoprotein cholesterol
- eGFR:
-
Estimated glomerular infiltration rate
References
Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout Nat Rev Dis Primers. 2019;5(1):69.
Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–90.
Guan Y, Wei J, Meng L, Li Y, Wang T, Chen D, et al. Genetically predicted physical activity is associated with lower serum urate concentrations. Genes Genomics. 2022;44(7):843–53.
Song J, Jin C, Shan Z, Teng W, Li J. Prevalence and risk factors of Hyperuricemia and gout: a cross-sectional survey from 31 provinces in Mainland China. J Transl Int Med. 2022;10(2):134–45.
Li X, Li L, Xing Y, Cheng T, Ren S, Ma H. Diabetes Mellitus is Associated with a lower risk of gout: a Meta-analysis of Observational studies. J Diabetes Res. 2020;2020:5470739.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8.
Evans PL, Prior JA, Belcher J, Mallen CD, Hay CA, Roddy E. Obesity, hypertension and diuretic use as risk factors for incident gout: a systematic review and meta-analysis of cohort studies. Arthrit Res Ther. 2018;20(1):136.
Mao T, He Q, Yang J, Jia L, Xu G. Relationship between gout, hyperuricemia, and obesity-does central obesity play a significant role?-a study based on the NHANES database. Diabetol Metab Syndr. 2024;16(1):24.
Aune D, Norat T, Vatten LJ. Body mass index and the risk of gout: a systematic review and dose-response meta-analysis of prospective studies. Eur J Nutr. 2014;53(8):1591–601.
Juraschek SP, Miller ER 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res (Hoboken). 2013;65(1):127–32.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity Guidelines for the management of obesity in Korea. J Obes Metab Syndr. 2021;30(2):81–92.
Appropriate body-mass. Index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Jia W, Lu J, Xiang K, Bao Y, Lu H, Chen L. [Evaluation of abdominal visceral obesity from anthropometric parameters using receiver operating characteristic curves]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(1):20–3.
Zhang Y, Wang W, Ning G. Metabolic Management Center: an innovation project for the management of metabolic diseases and complications in China. J Diabetes. 2019;11(1):11–3.
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23(8):1108–12.
Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 gout classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Liu J, Grundy SM, Wang W, Smith SC Jr., Vega GL, Wu Z, et al. Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care. 2006;29(6):1414–6.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Lee J, Lee JY, Lee JH, Jung SM, Suh YS, Koh JH, et al. Visceral fat obesity is highly associated with primary gout in a metabolically obese but normal weighted population: a case control study. Arthrit Res Ther. 2015;17(1):79.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7.
Maglio C, Peltonen M, Neovius M, Jacobson P, Jacobsson L, Rudin A, et al. Effects of bariatric surgery on gout incidence in the Swedish obese subjects study: a non-randomised, prospective, controlled intervention trial. Ann Rheum Dis. 2017;76(4):688–93.
Guo G, Dong C, Yin R, Yang Y, Zhao R, Wang Y, et al. Serum urate goal attainment and associated factors in Chinese gout patients. Psychol Health Med. 2020;25(8):931–9.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–15.
Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17(3):341–5.
Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46(10):1162–5.
Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Hada T. Increased visceral fat accumulation in patients with primary gout. Adv Exp Med Biol. 2000;486:131–4.
Gao B, Zhou J, Ge J, Zhang Y, Chen F, Lau WB, et al. Association of maximum weight with hyperuricemia risk: a retrospective study of 21,414 Chinese people. PLoS ONE. 2012;7(11):e51186.
Ding Y, Xu Z, Zhou X, Luo Y, Xie R, Li Y. Association between weight-adjusted-waist index and the risk of hyperuricemia in adults: a population-based investigation. Front Endocrinol (Lausanne). 2023;14:1236401.
Martínez-Montoro JI, Morales E, Cornejo-Pareja I, Tinahones FJ, Fernández-García JC. Obesity-related glomerulopathy: current approaches and future perspectives. Obes Rev. 2022;23(7):e13450.
Gong M, Wen S, Nguyen T, Wang C, Jin J, Zhou L. Converging relationships of obesity and hyperuricemia with Special Reference to Metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:943–62.
Fruehwald-Schultes B, Peters A, Kern W, Beyer J, Pfützner A. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism. 1999;48(6):677–80.
Tamba S, Nishizawa H, Funahashi T, Okauchi Y, Ogawa T, Noguchi M, et al. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47(13):1175–80.
Ea HK. [Mechanisms of gout inflammation]. Presse Med. 2011;40(9 Pt 1):836–43.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Sell H, Eckel J. Adipose tissue inflammation: novel insight into the role of macrophages and lymphocytes. Curr Opin Clin Nutr Metab Care. 2010;13(4):366–70.
Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev. 2010;233(1):218–32.
Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev. 2010;233(1):203–17.
McAdams-DeMarco MA, Maynard JW, Baer AN, Coresh J. Hypertension and the risk of incident gout in a population-based study: the atherosclerosis risk in communities cohort. J Clin Hypertens (Greenwich). 2012;14(10):675–9.
Chen JH, Pan WH, Hsu CC, Yeh WT, Chuang SY, Chen PY, et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care Res (Hoboken). 2013;65(1):133–40.
Cai X, Song S, Hu J, Zhu Q, Yang W, Hong J, et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clin Exp Hypertens. 2023;45(1):2259132.
Hu J, Cai X, Li N, Zhu Q, Wen W, Hong J, et al. Association between triglyceride glucose Index-Waist circumference and risk of first myocardial infarction in Chinese hypertensive patients with obstructive sleep apnoea: an Observational Cohort Study. Nat Sci Sleep. 2022;14:969–80.
Song S, Cai X, Hu J, Zhu Q, Shen D, Ma H, et al. Plasma aldosterone concentrations elevation in hypertensive patients: the dual impact on hyperuricemia and gout.Front endocrinol. (Lausanne). 2024;15:1424207.
Acknowledgements
We sincerely appreciate Clinical Scientists team for helping in this revision.
Funding
This study was supported by grants from the Medical Science and Technology Project of Zhejiang Province (Nos. 2019ZH012, 2022KY1402), Public Welfare Foundation Project of Zhejiang Province (LGF20H070005).
Author information
Authors and Affiliations
Contributions
YYW and NYC: Concept and design. PF and QDZ: Data acquisition and analysis. NYC and MDC: Interpretation of data and drafting of the manuscript. XPZ, SQY and ZBZ: Critical manuscript revision. All authors contributed to the manuscript, and read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committees of Yuhuan Second People’s Hospital and Taizhou Central Hospital (Taizhou University Hospital). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, N., Chen, M., Feng, P. et al. Relationships between obesity and prevalence of gout in patients with type 2 diabetes mellitus: a cross-sectional population-based study. BMC Endocr Disord 24, 137 (2024). https://doi.org/10.1186/s12902-024-01672-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01672-8