Abstract
Background
There is limited and conflicting evidence on the association between selenium and non-alcoholic fatty liver disease (NAFLD). Therefore, the present population-based cross-sectional study aimed to explore the relationship between dietary selenium intake and the risk of NAFLD.
Methods
A total of 3026 subjects from the PERSIAN (Prospective Epidemiological Research Studies in IrAN) Kavar cohort study were included in the analysis. The daily selenium intake was evaluated using a semi-quantitative food frequency questionnaire, and energy-adjusted quintiles of selenium intake (µg/day) were calculated. NAFLD was defined as the fatty liver index (FLI) ≥ 60 or the hepatic steatosis index (HSI) > 36. The association between dietary selenium intake and NAFLD was evaluated using logistic regression analysis.
Results
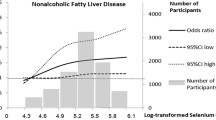
The prevalence rates of NAFLD were 56.4% and 51.9%, based on the FLI and HSI markers, respectively. The odds ratios (ORs) for FLI-defined NAFLD were 1.31 (95% confidence interval (CI): 1.01–1.70) and 1.50 (95% CI: 1.13–1.99) for the fourth and fifth quintiles of selenium intake, respectively, after adjustment for sociodemographic variables, smoking status, alcohol drinking, physical activity, and dietary factors (P trend = 0.002). There was also a similar association between selenium intakes and HSI-defined NAFLD (OR = 1.34 (95% CI: 1.03–1.75) for the fourth quintile and OR = 1.50 (95% CI: 1.12–2.01) for the fifth quintile of selenium intake) (P trend = 0.006).
Conclusion
In this large sample study, we observed a weak positive association between dietary selenium intake and NAFLD risk.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a variety of hepatic disorders, including steatosis, steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma [1]. The global prevalence of NAFLD is 25.24%, with the highest rates related to the Middle East and South America [2]. This disorder is also becoming one of the most common causes of liver transplantation [3]. NAFLD is a hepatic manifestation of metabolic syndrome and is strongly associated with obesity, insulin resistance, type 2 diabetes mellitus, and dyslipidemia [4]. Previous studies suggest that some dietary and lifestyle factors could influence NAFLD pathogenesis, prevention, and treatment [5, 6].
Selenium is a trace mineral and an essential component of the active sites of several proteins, such as glutathione peroxidase, thioredoxin reductase, selenoprotein P, and iodothyronine deiodinase [7, 8]. Therefore, this micronutrient participates in numerous body functions, including cell signaling systems, defending against free radicals, modulation of inflammatory responses, and immune and reproductive systems regulations [7, 9]. There are two forms of selenium in nature and organisms. Selenomethionine and selenocysteine are organic, and selenide, selenite, selenate, and elemental selenium are inorganic forms [8, 10]. The primary source of dietary selenium in humans is selenomethionine [7]. The Recommended Dietary Allowance (RDA) for selenium is 55 µg/ day for adults [11]. The amount of selenium in foods, especially plant-based foods, depends on the selenium content of the soil in a specific geographical area; therefore, dietary selenium intake varies significantly between countries. However, meat and dairy products, eggs, cereals, fish, poultry, seafood, and Brazil nuts are the main sources, and plants are the poor sources of selenium [8].
There are conflicting epidemiological studies on the association between selenium and metabolic disorders. Higher selenium intake and blood levels have been associated with an elevated risk of diabetes [12,13,14,15,16], hyperlipidemia [17, 18], hypertension [19], and NAFLD [20, 21]. Nonetheless, some evidence suggested no or a negative association between selenium and the risk of NAFLD or diabetes [22,23,24]. Therefore, due to the limited evidence and conflicting data, we aimed to perform the present cross-sectional study to investigate the association between dietary selenium intake and NAFLD in the general population of Kavar County.
Materials and methods
Study design and population
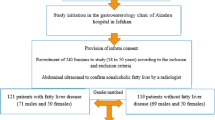
The data utilized in the current cross-sectional study was obtained from the baseline phase of the PERSIAN Kavar cohort study (PKCS), a prospective cohort aimed to assess the prevalence, trends, and risk factors of non-communicable diseases with a baseline phase between 2017 and 2019. The PKCS involves 4997 individuals comprising 2419 men and 2578 women aged 35 to 70 living in the urban area of Kavar County, Fars province, Iran [25]. All participants signed informed written consent. The present study was performed in line with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Shiraz University of medical sciences, Shiraz, Iran (Code: IR.SUMS.REC.1401.142).
For the current analysis, we first excluded a total of 1971 participants who met the following exclusion criteria: missing data for laboratory tests (n = 9), being pregnant (n = 43), or having a history of hepatitis (n = 6), cardiovascular diseases (n = 414), hypertension (n = 935), diabetes (n = 785), thyroid diseases (n = 619), or malignancies (n = 39). None of the individuals reported implausible total energy intake (< 800 or > 8000 kcal/d for men and < 600 or > 6000 kcal/d for women) [26] or heavy alcohol intake (> 21 drinks per week in men and > 14 drinks per week in women) [27]. Finally, 3026 participants were included in our study (Fig. 1).
Dietary intake and outcome assessment
Dietary intakes were evaluated using a validated and semi-quantitative food frequency questionnaire (FFQ) with 130 items. Four trained nutritionists conducted the nutritional interview and completed the questionnaire regarding the participant’s usual dietary intakes during the previous year, using a food album and scales. The participants were requested to report the amount and frequency of consumption of each food item on a day, week, month, or year, according to the standard serving sizes. The daily intake of nutrients were calculated by multiplying the frequency of consumption of each food item and the nutrient content of that specific item and then summing amounts across all relevant food items [28]. Selenium intake was adjusted for total energy intake using the residual method [29].
FLI and HSI, two valid markers defined below, were calculated for NAFLD prediction. The FLI ≥ 60 or HSI > 36 was considered NAFLD.
FLI=(e0·953×loge(triglycerides (TG))+0·139×body mass index (BMI)+0·718×loge(gamma−glutamyltransferase(GGT))+0·053×waist circumference–15·745)/(1 + e0·953×loge(TG)+0·139×BMI+0·718×loge(GGT)+0·053×waist circumference–15·745)×100 [30].
HSI = 8×(alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ratio) + BMI (+ 2, if female) [31].
Other variables
Information about sociodemographic features, medical history, physical activity (in the past year), alcohol intake, and smoking was collected through interviews using general and medical questionnaires. Socioeconomic status was assessed by the wealth score index (WSI) based on households’ assets. The anthropometric components (height, weight, and waist circumference) and blood pressure were measured by a physician and trained staff [25, 32]. BMI also was calculated as weight (kg) divided by height (meter) squared. Venous blood samples were obtained after 10–14 h of fasting state. The measures of serum biochemical parameters (lipid profile, liver enzymes, and fasting plasma glucose (FPG)) were conducted using commercial kits (Pars Azmoon, Iran) by the auto-analyzer (model BT3000 Plus, Biotecnica®, Italy).
Statistical analysis
IBM SPSS (version 26.0) was used for data analysis. The normality of data distribution was assessed by descriptive statistics. Parametric, non-parametric, and qualitative data are expressed as mean ± standard deviation (SD), median (range), or frequency (percentages), respectively. Between-group differences were determined using the independent sample t-test or analysis of variance (ANOVA) test for parametric variables, Mann–Whitney U or Kruskal–Wallis tests for non-parametric parameters, and Chi-square test for categorical variables. Logistic regression analysis was carried out to disclose the independent association between quintiles of energy-adjusted selenium intake and NAFLD risk according to the two adjusted models. A test for linear trend was done by including dietary selenium as a continuous variable in the previous models. Values are expressed as odds ratio (OR) and 95% confidence interval (CI). A two-sided P-value < 0.05 was considered significant.
Results
Table 1 shows the characteristics of the study participants by quintiles of dietary energy-adjusted selenium intake. The median age of the total study population was 45 years (minimum: 35, maximum: 70). Subjects in the highest quintile of energy-adjusted selenium intake were more likely to be male, Turk Nomad, to consume lower fiber, fructose, and saturated fatty acids, and to have lower age and higher physical activity, blood pressure, TG, ALT, AST, and GGT levels compared to those in the lowest quintile. Furthermore, participants with higher selenium intake had lower high-density and low-density lipoprotein cholesterol (HDL-C and LDL-C). The prevalence of NAFLD in the total population was 56.4% according to the FLI and 51.9% based on the HSI markers. The NAFLD prevalence was not significantly different between quintiles of selenium intake.
The characteristics of the participants according to the NAFLD status are reported in Table 2. The NAFLD patients were more likely to be Persian, female, non-smoker, non-drinker, and have higher socioeconomic status than the healthy subjects. They also had higher BMI, waist circumference, blood pressure, TG, total cholesterol, LDL-C, FPG, ALT, AST, and GGT levels. Significant differences also were observed between patients with and without NAFLD regarding physical activity, HDL-C levels, and fiber and fructose intakes. These results were similar between FLI- and HSI-defined NAFLD, except that patients with HSI-defined NAFLD were significantly younger than healthy subjects (45.11 ± 7.45 vs. 46.97 ± 8.78, P < 0.001).
The association between dietary energy-adjusted selenium intake and NAFLD risk is demonstrated in Table 3. As shown in model 1 (crude ORs), the relationship between selenium intake and NAFLD risk was non-significant in both NAFLD prediction models. After adjustment for age, sex, ethnicity, education levels, smoking status, alcohol intake, WSI, and physical activity (model 2), the adjusted OR and 95% CIs for FLI- and HSI- defined NAFLD comparing the fifth quintile of selenium intake with reference group were 1.42 (1.11–1.80) and 1.40 (1.09–1.79), respectively, with a progressive increase in risk across quintiles (P trend < 0.05).
After adjusting for energy, saturated fatty acids, cholesterol, fiber, and fructose intakes (model 3), the multivariable-adjusted ORs and 95% CIs for FLI-defined NAFLD were 0.98 (0.77–1.25), 1.15 (0.90–1.48), 1.31 (1.01–1.70), and 1.50 (1.13–1.99) from the second to the fifth dietary selenium quintile, respectively, compared to the lowest category (P trend = 0.002). Furthermore, the multivariable-adjusted ORs and 95% CIs for HSI-defined NAFLD from the second to the fifth quintile were 0.95 (0.74–1.22), 1.11 (0.86–1.43), 1.34 (1.03–1.75), and 1.50 (1.12–2.01), respectively (P trend = 0.006) (Table 3).
Discussion
We conducted a population-based cross-sectional study in a large sample of Kavar County with the primary objective of assessing the relationship between dietary selenium intake and NAFLD prevalence. Our results demonstrated a weak positive linear association between dietary selenium intake and NAFLD risk. The prevalence rates of NAFLD in our population were 56.4% and 51.9%, based on the FLI and HSI markers, respectively. In our study, the mean selenium intake (109.29 µg/day) was higher than the RDA level as well as its intake by other Iranian populations [33]. Moreover, all subjects consumed below the tolerance limits (400 µg/day), and only 3.4% ingested less than the RDA level for selenium intake.
In our study, participants with the highest selenium intake had 50% higher NAFLD risk after adjustment for major confounders. Limited epidemiological investigations have explored the association between selenium and NAFLD prevalence. Liu et al. detected a positive association between more than 121.90 µg/d selenium intake and the odds of steatosis [34]. In another study of 8550 Chinese adults, participants in the third and fourth quartiles of plasma selenium levels had a 72% and 54% increased NAFLD risk compared with those in the reference quartile [20]. Inconsistent with our findings, Wu et al. revealed a positive dose-response relationship between dietary selenium intake, below the recommendations, and the prevalence of NAFLD, detected by ultrasonography, in the general population of China [21]. In another cross-sectional study of 42 adults with NAFLD, a negative and null correlation between selenium intake and liver fat was observed in females and males, respectively [23]. This conflicting evidence could be due to the differences in the population, method of exposure assessment, amounts of selenium intake, method of NAFLD diagnosis, and considered confounding variables.
Many observational investigations demonstrated a positive association between dyslipidemia and diabetes risk with different selenium levels [14, 15, 35, 36]. Insulin resistance and dyslipidemia have fundamental roles in NAFLD development and progression [37]. Therefore, high dietary selenium intake may increase NAFLD risk by dysregulating insulin biosynthesis and secretion and stimulating glucagon secretion, insulin resistance, and dyslipidemia [38]. The increment of liver protein tyrosine phosphatase 1B activity, an enzyme antagonizing insulin signaling and stimulating fatty acid synthesis, is also reported following selenium supplementation [39]. Furthermore, the high intake of this trace element increased hepatic TG by upregulating gluconeogenesis and lipogenesis and downregulating lipolysis in pigs [40]. However, more studies are warranted to clarify other related mechanisms.
Early detection of NAFLD may be helpful for the recognition of those with probably silent progressive NAFLD. Diagnostic routes are different and include clinical, biochemical, and radiographic tests. The liver biopsy remains the gold standard for NAFLD confirmation, but it is practically infeasible as a diagnostic instrument [41]. In our study, NAFLD was predicted by computing FLI and HSI biomarkers. These validated indicators can be used for detecting participants to be referred for lifestyle counseling, ultrasonography, and conducting epidemiologic studies [30, 31]. According to the study by Hsu et al., FLI was a stronger predictor than sex, liver function tests, BMI, body fat, FPG, uric acid, and triglyceride for NAFLD diagnosis in lean patients [42]. In a previous study, a good agreement between NAFLD prevalence by FLI (47.6%) and HSI (53.5%) vs. controlled attenuation parameter derived via transient elastography (CAP-TE) (48.1%) was detected [43]. However, a higher NAFLD prevalence was reported in studies using FLI than ultrasound in obese and diabetic patients [44]. The NAFLD prevalence rates in our studied population were 56.4% and 51.9%, based on the FLI and HSI markers, respectively. These estimations were higher than the prevalence of ultrasonography or liver biopsy-diagnosed NAFLD in Iran (33.95%), yielded by a meta-analysis study published in 2016 [45]. Therefore, differences in NAFLD diagnosis methods and increased incidence of NAFLD in recent years could partly describe the high prevalence of NAFLD in the present study.
The population-based sampling, large sample size, and assessing the association between selenium and NAFLD in the Iranian population for the first time are some strengths of the present study. Nonetheless, there are several limitations. First, due to the specific characteristics of the cross-sectional studies, supposing a causal connection between dietary selenium intake and NAFLD prevalence is impossible. Second, we used FLI and HSI markers but not liver biopsy as the gold standard of NAFLD diagnosis. Third, we did not assess the blood selenium concentration, which provides more reliable evidence regarding selenium’ status in the body. Using FFQ for selenium intake estimation in the current study may cause recall bias and errors in exposure assessment. Fourth, because of the social stigma associated with alcohol consumption in Iranian society, the actual amount of alcohol intake may be biased. Further well-designed prospective cohort studies on the association between blood serum biomarkers and NAFLD risk should be carried out to clarify this association.
Conclusion
In this study, dietary selenium intake was associated with the prevalence of NAFLD after controlling for major confounders.
Data availability
Data supporting the results of this study is available from the author [A.R.S.] upon reasonable request.
Abbreviations
- ALT:
-
alanine aminotransferase
- ANOVA:
-
analysis of variance
- AST:
-
aspartate aminotransferase
- BMI:
-
body mass index
- CI:
-
confidence interval
- FFQ:
-
food frequency questionnaire
- FLI:
-
fatty liver index
- FPG:
-
fasting plasma glucose
- GGT:
-
gamma-glutamyltransferase
- HDL-C:
-
high-density lipoprotein cholesterol
- HSI:
-
hepatic steatosis index
- LDL-C:
-
low-density lipoprotein cholesterol
- NAFLD:
-
non-alcoholic fatty liver disease
- OR:
-
odds ratio
- PERSIAN:
-
prospective epidemiological research studies in IrAN
- RDA:
-
recommended dietary allowance
- SD:
-
standard deviation
- TG:
-
triglycerides
- WSI:
-
wealth score index
References
Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016. https://doi.org/10.1016/j.cld.2015.10.001.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016. https://doi.org/10.1002/hep.28431.
Shaker M, Tabbaa A, Albeldawi M, Alkhouri N. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol. 2014. https://doi.org/10.3748/wjg.v20.i18.5320.
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015. https://doi.org/10.1053/j.gastro.2014.11.039.
Ullah R, Rauf N, Nabi G, Ullah H, Shen Y, Zhou YD, et al. Role of Nutrition in the pathogenesis and Prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci. 2019. https://doi.org/10.7150/ijbs.30121.
Shojaei-Zarghani S, Safarpour AR, Fattahi MR, Keshtkar A. Sodium in relation with nonalcoholic fatty liver disease: a systematic review and meta-analysis of observational studies. Food Sci Nutr. 2022. https://doi.org/10.1002/fsn3.2781.
Kieliszek M, Blazejak S. Current knowledge on the importance of Selenium in Food for living organisms: a review. Molecules. 2016. https://doi.org/10.3390/molecules21050609.
Kieliszek M. Selenium–fascinating microelement, properties and sources in food. Molecules. 2019. https://doi.org/10.3390/molecules24071298.
Bhattacharya PT, Misra SR, Hussain M. Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica. 2016. https://doi.org/10.1155/2016/5464373.
Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013. https://doi.org/10.3390/molecules18033292.
Tóth RJ, Csapó J. The role of selenium in nutrition: a review. Acta Univ Sapientiae Aliment. 2018. https://doi.org/10.2478/ausal-2018-0008.
Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, et al. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010. https://doi.org/10.1186/1471-2458-10-564.
Siddiqi SM, Sun C, Wu X, Shah I, Mehmood A. The correlation between dietary selenium intake and type 2 diabetes: a cross-sectional population-based study on North Chinese adults. Biomed Res Int. 2020. https://doi.org/10.1155/2020/8058463.
Wei J, Zeng C, Gong Q-y, Yang H-b, Li X-x, Lei G-h, et al. The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutr J. 2015. https://doi.org/10.1186/s12937-015-0007-2.
Lin J, Shen T. Association of dietary and serum selenium concentrations with glucose level and risk of diabetes mellitus: a cross sectional study of national health and nutrition examination survey, 1999–2006. J Trace Elem Med Biol. 2021. https://doi.org/10.1016/j.jtemb.2020.126660.
Cardoso BR, Braat S, Graham RM. Selenium Status is Associated with insulin resistance markers in adults: findings from the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.696024.
Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas-Acien A, Ordovas JM, et al. Higher selenium status is associated with adverse blood lipid profile in british adults. J Nutr. 2010. https://doi.org/10.3945/jn.109.111252.
Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis. 2010. https://doi.org/10.1016/j.atherosclerosis.2010.01.005.
Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and hypertension in the US Population. Circ Cardiovasc qual outcomes. Circ Cardiovasc Qual Outcomes. 2009. https://doi.org/10.1161/CIRCOUTCOMES.108.831552.
Yang Z, Yan C, Liu G, Niu Y, Zhang W, Lu S, et al. Plasma selenium levels and nonalcoholic fatty liver disease in chinese adults: a cross-sectional analysis. Sci Rep. 2016. https://doi.org/10.1038/srep37288.
Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, et al. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study. J Am Coll Nutr. 2020. https://doi.org/10.1080/07315724.2019.1613271.
Dias JPV, Costa Sobrinho PS, Pimenta AM, Hermsdorff HHM, Bressan J, Nobre LN. Dietary selenium intake and Type-2 diabetes: a cross-sectional Population-Based study on CUME Project. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.678648.
Aktary ML, Eller LK, Nicolucci AC, Reimer RA. Cross-sectional analysis of the health profile and dietary intake of a sample of canadian adults diagnosed with non-alcoholic fatty liver disease. Food Nutr Res. 2020. https://doi.org/10.29219/fnr.v64.4548.
Vinceti M, Grioni S, Alber D, Consonni D, Malagoli C, Agnoli C, et al. Toenail selenium and risk of type 2 diabetes: the ORDET cohort study. J Trace Elem Med Biol. 2015. https://doi.org/10.1016/j.jtemb.2014.07.017.
Safarpour AR, Fattahi MR, Niknam R, Tarkesh F, Mohammadkarimi V, Boogar SS, et al. Alarm of non-communicable disease in Iran: Kavar cohort profile, baseline and 18-month follow up results from a prospective population-based study in urban area. PLoS ONE. 2022. https://doi.org/10.1371/journal.pone.0260227.
Xun P, Hou N, Daviglus M, Liu K, Morris JS, Shikany JM, et al. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J Intern Med. 2011. https://doi.org/10.1111/j.1365-2796.2010.02338.x.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the american Gastroenterological Association, American Association for the study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012. https://doi.org/10.1053/j.gastro.2012.04.001.
Ariya M, Shahraki HR, Farjam M, Ehrampoush E, Bahramali E, Homayounfar R, et al. Dietary inflammatory index and metabolic syndrome in iranian population (Fasa Persian Cohort Study). Sci Rep. 2020. https://doi.org/10.1038/s41598-020-73844-0.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997. https://doi.org/10.1093/ajcn/65.4.1220S.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006. https://doi.org/10.1186/1471-230X-6-33.
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010. https://doi.org/10.1016/j.dld.2009.08.002.
Moradinazar M, Najafi F, Jalilian F, Pasdar Y, Hamzeh B, Shakiba E, et al. Prevalence of drug use, alcohol consumption, cigarette smoking and measure of socioeconomic-related inequalities of drug use among iranian people: findings from a national survey. Subst Abuse Treat Prev Policy. 2020. https://doi.org/10.1186/s13011-020-00279-1.
Stoffaneller R, Morse NL. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015; doi: https://doi.org/10.3390/nu7031494.
Liu X, Shen H, Chen M, Shao J. Clinical relevance of selenium with liver stiffness and steatosis detected by transient elastography in adults. Biol Trace Elem Res. 2022. https://doi.org/10.1007/s12011-021-02912-x.
Su L, Gao S, Unverzagt FW, Cheng Y, Hake AM, Xin P, et al. Selenium level and dyslipidemia in rural elderly chinese. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0136706.
Christensen K, Werner M, Malecki K. Serum selenium and lipid levels: Associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ Res. 2015. https://doi.org/10.1016/j.envres.2015.03.020.
Zhang Q-Q, Lu L-G. Nonalcoholic fatty liver disease: dyslipidemia, risk for cardiovascular complications, and treatment strategy. J Clin Transl Hepatol. 2015. https://doi.org/10.14218/JCTH.2014.00037.
Steinbrenner H, Speckmann B, Pinto A. Sies HJJocb, nutrition. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2010. https://doi.org/10.3164/jcbn.11-002FR.
Mueller AS, Klomann SD, Wolf NM, Schneider S, Schmidt R, Spielmann J, et al. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008. https://doi.org/10.3945/jn.108.089482.
Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr. 2016. https://doi.org/10.3945/jn.116.229955.
Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014. https://doi.org/10.3748/wjg.v20.i2.475.
Hsu C-L, Wu F-Z, Lin K-H, Chen Y-H, Wu P-C, Chen Y-H, et al. Role of fatty liver index and metabolic factors in the prediction of nonalcoholic fatty liver disease in a lean population receiving health checkup. Clin Transl Gastroenterol. 2019. https://doi.org/10.14309/ctg.0000000000000042.
Jones GS, Alvarez CS, Graubard BI, McGlynn KAJCG. Hepatology. Agreement between the prevalence of nonalcoholic fatty liver disease determined by transient elastography and fatty liver indices. Clin Gastroenterol Hepatol. 2022. https://doi.org/10.1016/j.cgh.2020.11.028.
Cholongitas E, Pavlopoulou I, Papatheodoridi M, Markakis GE, Bouras E, Haidich AB, et al. Epidemiology of nonalcoholic fatty liver disease in Europe: a systematic review and meta-analysis. Ann Gastroenterol. 2021. https://doi.org/10.20524/aog.2021.0604.
Moghaddasifar I, Lankarani KB, Moosazadeh M, Afshari M, Ghaemi A, Aliramezany M, et al. Prevalence of non-alcoholic fatty liver Disease and its related factors in Iran. Int J Organ Transplant Med. 2016;7(3):149–60.
Acknowledgements
The Iranian Ministry of Health and Medical Education has contributed to the funding used in the PERSIAN cohort through Grant no 700/534.
Funding
This study was supported by the Vice-Chancellor for Research and Technology of Shiraz University of Medical Sciences (Code: 25712). The funder had no role in the study design, analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.S.Z., A.R.S; Methodology: S.S.Z., A.R.S, N.R.K., Z.B., M.T., M.R.F.; Data curation, Formal analysis, Software: S.S.Z., A.R.S., Z.B.; Project administration, Validation, and Supervision: A.R.S., M.R.F.; Funding acquisition: A.R.S.; Writing – original draft: S.S.Z., N.R.K., M.T.; Writing – review & editing: A.R.S., M.R.F., Z.B.; All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shiraz University of medical sciences, Shiraz, Iran (Code: IR.SUMS.REC.1401.142). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shojaei Zarghani, S., Rahimi Kashkooli, N., Bagheri, Z. et al. Dietary selenium intake in relation to non-alcoholic fatty liver disease assessed by fatty liver index and hepatic steatosis index; a cross-sectional study on the baseline data of prospective PERSIAN Kavar cohort study.. BMC Endocr Disord 23, 51 (2023). https://doi.org/10.1186/s12902-023-01307-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01307-4