Abstract
Background
Diabetes mellitus (DM) leads to multiple complications, including severe hypoglycaemia events (SHEs). SHEs can impact a patient’s quality of life and compliance and may directly result in additional costs to the health care system. The aim of this review was to evaluate the risk of severe hypoglycaemia in patients with type 1 (T1) and 2 (T2) DM as observed in everyday clinical practice for various drug regimens.
Methods
We conducted a systematic review of observational (retrospective or prospective) studies in the MEDLINE, Embase, and Cochrane Library databases that covered at least 100 children or adults with T1/T2 DM. In T1 DM, basal-bolus/pre-mix insulin (human or analogue) and insulin pump were reviewed, and in T2 DM, basal-bolus/pre-mix insulin (human or analogue), oral antidiabetic drugs supported with basal insulin (human or analogue), sulfonylureas in monotherapy, and combined oral treatment were reviewed. In order to estimate SHE rates, we extracted data on the time horizon of the study, number of patients, number of SHEs, and number of patients experiencing at least one SHE. We used a random effects model to estimate the annual SHE rate. We considered the risk for other antidiabetic medications in T2 DM to be negligible and the results of our main review yielded no observational data for premixes in T1 DM so they were assessed based on relative rates taken from additional systematic reviews. The study, being a desk research, did not involve any human subjects (including human material or human data) and no ethical committee approval was asked for. For the same reason there was no need to collect informed consent for participation in the study.
Results
We identified 76 observational studies encompassing 707,722.30 patient-years. The estimated annual SHE rate varied from 0.168 (95 % CI 0.123–0.237) for insulin pump up to 1.628 for biphasic human insulin in T1 DM patients, and from 0.0035 for oral antidiabetic drugs up to 0.554 (95 % CI 0.157–7.534) for basal-bolus with human insulin in T2 DM patients.
Conclusions
Our review indicates that SHE rates differ between patients depending on treatment regimen. However, SHEs are also driven by other factors. Proper modelling techniques are needed to use various types of information in published studies.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a common disease with increasing prevalence [1]. DM can lead to microvascular complications, including retinopathy, nephropathy, and neuropathy, as well as macrovascular complications caused mainly by atherosclerotic changes that cause myocardial infarction, stroke, TIA, and peripheral vascular disease. Hyperglycaemia is an important pathophysiological factor in the development of these complications [2, 3] and key to keeping the glucose concentration close to euglycaemic levels in the treatment of diabetes. This is very challenging in clinical practice because of the risk of treatment-induced hypoglycaemia. Hypoglycaemia can be perceived from clinical, physiological, or pathophysiological perspectives through risk factors and preventive measures. Clinical hypoglycaemia is described by the Whipple triad [4]. A plasma glucose concentration < 3.9 mmol/l (<70 mg/dl) with (documented symptomatic) or without symptoms (asymptomatic) is regarded as the threshold for hypoglycaemic events [5]. A severe hypoglycaemic event (SHE) is defined as one requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Plasma glucose measurements may not be available during such an event, but neurological recovery attributable to the restoration of normal plasma glucose levels is considered sufficient evidence that the event was induced by a low plasma glucose concentration [5]. Severe hypoglycaemia is common in both types of DM, but less frequent in type 2 (T2). The number of events depends on many factors, including type, duration of diabetes, and types of treatment. The event rates for severe hypoglycaemia in insulin-treated patients as reported in the literature and expressed as episodes per 100 patient-years range from 62 [3] to 320 [6] in type 1 (T1) DM, and from 0 [7] to 73 [8] in T2 DM.
The objective of this meta-analysis was to assess the SHE rate in patients with T1 and T2 DM in real life settings for various anti-diabetic treatment regimens. As data derived from randomized controlled trials (RCTs) have limited external validity and cannot be used directly to reflect everyday clinical practice, we decided to focus on observational studies. One can argue that many confounders associated with usual clinical practice are not appropriately addressed in randomized settings and can affect the risk of hypoglycaemic events.
The present study is an extension of previously published material [9], and here we include additional systematic reviews from the last 2 years. We also used an estimation method that is likely closer to actual clinical mechanisms, i.e., we accounted for some patients having a bigger risk of SHE and that the events may cluster in a subgroup of patients. The current review is supplemented with two additional systematic reviews targeted to identify additional studies because some treatment regimens were not covered by our primary review.
Methods
Main systematic review of observational studies
We conducted a systematic review in the MEDLINE, Embase, and Cochrane Library databases in stages. The original search covered observational studies published between 1 January 2002 and 1 October 2012 and was last updated 18 September 2014. In all of the updates, we consistently used the same methodology described in Online Resource Additional file 1 (and in [9]). Although the systematic review did not have a registered protocol, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10].
We included both T1 and T2 DM patients (children or adults) in our analysis. We used a consistent definition of SHE as an event with low plasma glucose levels that requires help from another person to manage. This definition has been used in numerous publications [11–15] and seems to be an attractive and clinically sound choice for results used in cost-measuring studies because it directly links SHEs to resource use.
We decided to include observational studies in the systematic review in order to more accurately assess SHE rates in real-life settings. Moreover, to account for changes in clinical practice in recent years and the possible impact on treatment-related risk, only recent studies published no more than 10 years ago were included. Taking into account our updates, the earliest studies could have been published on 1 January 2002. To balance the number of studies included and the strength of evidence, we only included studies of some minimal reasonable size, which we defined as at least 100 patients in the total study, though possibly split between several groups.
We designed our review to differentiate between the following treatment regimens: insulin pumps, basal-bolus insulin therapy with long-acting insulin analogue as the basal component (BBA), basal-bolus insulin therapy with human insulin as the basal component (BBH), biphasic insulin analogue, and biphasic human insulin in T1 DM, and sulfonylurea (SU) with or without other oral drugs but excluding insulin, basal long-acting insulin analogue (BOTA), basal human insulin (BOTH), BBA, BBH, biphasic insulin analogue, and biphasic human insulin (all insulin regimens could be in combination with other antidiabetic drugs, OADs) in T2 DM. We defined basal-bolus insulin therapy as long-acting insulin analogue once or twice daily and short/ultrashort insulin at mealtime. In the main systematic review, OADs in T2 DM, especially oral antidiabetic medications different than SU, were neglected assuming that the risk of SHE is negligible. We planned to only assess risk indirectly using information on relative rates from studies searched for in a separate systematic review of secondary studies as described below.
Two authors independently conducted the selection process for relevant trials (basic search: J.P. and E.R.; 1st update: J.P. and E.R.; 2nd update: E.R. and M.J.). Protocol assumed that in the case of discrepancies between the authors, a discussion would be held until consensus was reached. We extracted the following parameters: time horizon at which hypoglycaemia was assessed, number of patients in the study group, number of hypoglycaemic episodes (absolute or mean per patient in a specified period of time, if available), and number of patients experiencing at least one SHE (if available). If one study was described in more than one manuscript, then the manuscript with the most appropriate and complete results was selected for extraction (e.g., data for a total study cohort instead of subpopulation, results presented separately for patients with T1 and T2 diabetes, or results split by insulin regimens of interest). Data from included studies were extracted by one of the reviewers and verified by the other.
We assessed the quality of the observational studies using the Newcastle-Ottawa Scale (NOS) [16], a tool developed for case–control and cohort studies that allows the quality of non-randomized studies (its design, content, and ease of use) to be assessed. Deeks et al. [17] pointed out that this scale is one of the two best for evaluating non-randomized interventional studies and can be used in systematic reviews as either a scale or a checklist. This tool is also mentioned in the Cochrane Handbook as a tool that can be used for assessing methodological quality or risk of bias in non-randomized studies [18]. Thus, in our systematic review we decided to use NOS for case–control and cohort studies, while in order to assess the quality of other types of non-interventional studies we focused on the following elements: patient selection methods, methods for recording outcomes (regarding only severe hypoglycaemia), study size, and study representativeness. According to a recent systematic review of tools used to assess the quality of observational studies [19], there are 97 tools (46 scales and 51 checklists) that can be used to evaluate observational studies, but a transparent objective assessment of the quality of observational research is still missing.
As it was a desk research, the study did not involve any human subjects (including human material or human data) and no ethical committee approval was asked for. For the same reason there was no need to collect informed consent for participation in the study.
Supplementary systematic reviews
As mentioned above, we planned to assess the risk related to OADs in T2 DM by calculating the relative rates compared to SU based on secondary studies and then impose these relative rates on the background SU-related SHE rate. Our main systematic review also yielded no observational studies for biphasic therapies in T1 DM; therefore, we decided to use similar methodology to assess the SHE rate for that treatment regimen.
In the first of the two additional systematic reviews we used the following approach. We searched MEDLINE, Embase, the Cochrane Library, and Centre for Reviews and Dissemination (CRD) to identify secondary studies (systematic reviews and meta-analyses) that can be used to estimate the relative rates (see search strategy in Additional file 1). Studies were included if the search was performed in at least two databases, including at least one of the above databases, by at least two authors (due to the need to confirm the search results) and if the search strategy was described. RCTs of T2 DM required at least one of the following: dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 agonist, OADs such as metformin, and TZD. In addition, severe hypoglycaemia had to be defined as an episode when a patient required help from another person. We used RCTs instead of observational studies because they more commonly provide data on relative rates.
We used the following approach in the second of the additional reviews. The MEDLINE database was searched on 25 February 2015 using the search strategy presented in Additional file 1. Only systematic reviews and RCTs (also those included in systematic reviews) were eligible. Studies performed in patients with T1 DM or T1 and T2 DM (with results presented separately) had to compare patients randomized to a group receiving biphasic insulin analogues to a group receiving biphasic human insulin, a group receiving a basal-bolus insulin regimen, or a group on insulin pumps.
Data synthesis methods for the main review
The data from included studies vary in structure, i.e., some studies present the total number of episodes in a given group of patients over some time horizon, whereas others present the number of patients who experienced at least one event, and some studies present both. Studies also differ with respect to time horizon. In order to increase the flexibility of the model, we chose the Bayesian approach using the Markov Chain Monte Carlo (MCMC) estimation method implemented in JAGS using R.
We based our model on negative binomial distribution for the following reasons. Firstly, in the previous analysis we used the Poisson distribution as a natural choice to model count data that were available [9]. However, in the present paper we decided to account for the fact that episodes tend to be concentrated in a subgroup of patients; in most of the studies, we observed that fewer patients actually experienced any episode than suggested by a Poisson distribution for a given total number of observed episodes. This observation may stem from some individual predispositions (e.g., lifestyle, genetics, etc.) and would require the introduction of a zero-inflation mechanism, i.e., the fact that many patients will have no events but some will tend to have multiple events.
Secondly, we did not have access to individual patient data (i.e., we only observed the total number of episodes in a study); thus, we needed to work with a distribution that can be aggregated to describe the number of episodes in a cohort. We also needed to be able to account for varying time horizons under plausible assumptions. Thirdly, we wanted the results of the estimation to be easily usable in further modelling, and we did not want the SHE rates to vary over time, which would require knowing the longevity of the treatment, even assuming that the time to first SHE is distributed differently than the time to subsequent SHEs and would be a possible way of introducing the zero-inflation phenomenon.
The negative binomial distribution allows for a clustering effect and can be easily mathematically expressed for groups of patients observed for varying time horizons. We decided to use a mixed fixed-effect and random-effect approach by assuming that the parameter measuring the over-dispersion is fixed across studies for a given treatment regimen while the other parameter impacting the absolute rate was random. This approach allows us to account for heterogeneity between studies, whereas taking both parameters in the random-effect approach yielded unstable results. Non-informative prior distributions were taken.
The specification and JAGS codes are given in the supplementary material (Additional file 2).
As studies usually have only one arm, the SHE rate is estimated separately for the individual classes of drugs. Importantly, the aim of the present study was not to compare drugs, which requires two-arm studies, possibly with randomization, but rather to estimate the absolute rate of SHEs for all of the drug classes separately, accounting for the tendency to prescribe various drugs to patients with varying baseline risks of SHEs (e.g., lifestyle) in clinical practice.
The median values of posterior distributions were used as point estimates. The 2.5 and 97.5 percentiles were used as limits of 95 % confidence intervals (CIs). We used 10,000 iterations as a burn-in phase and then collected every fifth of 50,000 iterations. The estimated parameters of the model allow us to assess the SHE rate (i.e., average number of events per patient-year) and the probability of a given patient suffering at least one SHE over a year.
Data synthesis methods for the relative rates
Data used for the estimation of relative rates from identified studies were provided in terms of 2×2 tables including the number of SHEs and person-time calculated by multiplying a number of patients and a time horizon for each group. Data given in such a format can be aggregated using the Mantel-Haenszel method, which does not require calculating the value of individual outcomes.
In order to estimate relative rates based on RCTs we used the ‘metafor’ and ‘lme4’ libraries available in R software to estimate the incidence relative rate (IRR) when information on the number of episodes was available. We synthesized data using the rma.mh function available in the ‘metafor’ package. This function fits fixed-effects models to a 2×2 table and person-time data via the Mantel-Haenszel method, which is based on a weighted estimation approach. Zero events are not a problem in this method unless there are no events for one of the two groups. In such cases it is necessary to add a constant (e.g., 0.5) to the number of events [20].
Results
Systematic review of observational studies
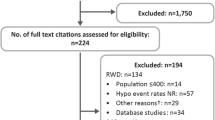
As mentioned previously, we conducted the systematic review in three stages. The basic literature search yielded 6214 records, and after duplicate removal 5220 articles were assessed by titles and abstracts. We reviewed 526 full texts, 101 of which [6, 21–120] met the inclusion criteria. A total of 55 individual trials were eligible for the analysis. The details of trial identification are given in Additional file 3, and the characteristics of included studies are given in Additional file 4.
For T1 DM we included 21 studies described in 33 articles. Among these studies, 14 provided data on SHEs in patients on insulin pumps (6714.61 patient-years total), 7 on BBA (9656.18 patient-years in total), and 6 on BBH (2881.57 patient-years total). As mentioned previously, we did not find any studies on treatment with biphasic insulin in T1 DM and we carried out a supplementary search for studies on pre-mixed insulin (details are given below). For T2 DM we included 35 studies described in 76 articles. Among these studies, 11 provided data on basal long-acting insulin analogue ± OADs (5347.87 patient-years total), 7 on basal human insulin ± OADs (2142.13 patient-years total), 6 on BBA ± OADs (1456.05 patient-years total), 3 on BBH ± OADs (227.46 patient-years total), 12 on biphasic insulin analogue ± OADs (48,168.49 patient-years in total), 6 on biphasic human insulin ± OADs (2265.87 patient-years total), and 6 on SU ± OADs but excluding insulin (1776.00 patient-years total).
The first update of the basic search resulted in 409 records, which were screened by title and abstract, and duplication removal resulted in 31 new full publications. A total of five articles [121–125] met the inclusion criteria, three of them describing new studies. All identified articles provided data for patients with T2 DM. Three publications presented data on biphasic insulin analogue ± OADs (2501.16 patient-years total), two on BOTA (8084.27 patient-years total), and one on biphasic human insulin ± OADs (505.84 patient-years total). The details of trial identification and the characteristics of included studies are given in Additional files 3 and 4, respectively.
The second update yielded 1727 records (1509 after duplication removal), which were assessed by titles and abstracts. We reviewed 318 full texts, from which 58 manuscripts describing 24 individual trials (18 new studies) [126–183] were eligible for the systematic review. The details of trial identification are given in Additional file 3. The characteristics of included studies are given in Additional file 4.
For T1 DM we included eight studies (seven new studies) described in eight articles, six of them provided data on SHEs in patients on insulin pumps (828.97 patient-years total) and one on BBA (99,804.50 patient-years total) and BBH (72,697.25 patient-years total). The results from one of the identified publications are not included in the systematic review because we previously identified and used another manuscript that presented results on the same patient population.
For T2 DM we included 18 studies (12 new studies) described in 53 manuscripts. Among these studies, six provided data on BOTA (1367.50 patient-years total), one on BOTH (332,525.00 patient-years total), four on BBA (106,938.75 patient-years total), one on biphasic insulin analogue ± OADs (124.20 patient-years total), one on biphasic human insulin ± OADs (119.25 patient-years total), and four on SU (1589.39 patient-years total). Results from the other publications were not used because they presented data on populations already assessed in articles identified in previous searches.
We assessed the quality of the studies using the Newcastle-Ottawa Scale (NOS) [16] for case–control and cohort studies. Other types of observation studies were assessed by focusing on four aspects: i) patient selection methods, ii) methods of recording outcomes regarding severe hypoglycaemia, iii) study size, and iv) study representativeness. Generally, the quality of the studies varied. Among the identified case–control studies, only one scored 5 out of 9 points; other studies were of lower quality: three scored 2 points, three scored 3 points, and two scored 4 points. Among cohort studies, three scored 8 of 9 possible points; other studies were of lower quality: one scored 5 points, eight scored 6 points, and six scored 7 points. The residual studies were of medium quality as assessed by descriptions with no scoring.
Additional systematic reviews
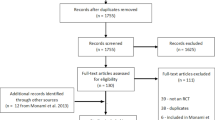
In order to estimate the expected annual number of SHEs related to OADs (mainly oral) in T2 DM we performed an additional systematic review. This search resulted in a total of 958 potentially relevant publications, which were assessed by titles and abstracts. After duplicate removal, 215 full publications were evaluated, from which 12 systematic reviews fulfilling predefined inclusion criteria were identified (Additional file 3). Of the included systematic reviews, a study conducted by Karagiannis et al. [184] was assessed to provide the most appropriate data on SHEs associated with various antidiabetic medications in T2 DM. We reviewed all studies included in Karagiannis et al. [184] in order to obtain the number of SHEs in each of them. We also verified the definition of severe hypoglycaemia; if it did not comply with the definition assumed for our systematic review, the results from the study were excluded. A total of 11 RCTs [185–195] were identified and included in the analysis.
Our main systematic review yielded no observational studies for biphasic therapies in T1 DM; therefore, we performed an additional systematic review. A total of 454 records were assessed by titles and abstracts, among which 24 full publications were evaluated and 12 met the inclusion criteria. An additional two publications were identified from the references (Additional file 3). A total of seven systematic reviews described five relevant RCTs (see Additional file 3) [196–209]. These studies were then used to assess the relative risk for biphasic therapies in T1 DM.
SHE rates for treatments based on the main review
The information on the number of SHEs in individual studies is presented in Additional file 5.
The results of the data synthesis are presented in Table 1.
SHE rates for treatments based on supplementary reviews
Because the results indicated no significant difference between basal human insulin in a basal-bolus regimen and biphasic insulin analogues (IRRFE = 0.5000, 95 % CI 0.1250–1.992) and the confidence interval is very wide, we assumed that these two treatments are related to an identical SHE rate. The difference between biphasic human insulin and biphasic insulin analogue (IRRFE = 1.5015, 95 % CI 0.9571–2.3558) was also not significant, but the 95 % CI clearly moved towards values >1. The direction agreed with a general tendency of human insulin being related to a greater SHE rate in T2 DM; thus, we used the point estimate to correct the SHE rate compared to biphasic insulin analogue.
For OADs, i.e., metformin, DPP-4, GLP-1, and TZD, the risk of SHEs relative to SU were assessed using data from a previously identified systematic review [184] and referred the risk of SHEs for SU estimated in our systematic review of observational studies. The estimated relative rate for DPP-4 inhibitors and SU was 0.0783 (95 % CI 0.0284–0.2161). There was no significant difference in the risk rate between other OADs and GLP-1. Thus, we applied the relative rate to correct the SHE rate estimated for SU and use it for OADs.
The results are presented in Table 2. Because the average rates of SHE in this case are only based on indirect reasoning, we present no CI and assessed no probability of at least one event.
Discussion
Data
In this review, we attempted to assess the real-life risk of SHEs associated with various drug regimens. Data selection was constructed to best fit this goal. Because we wanted to assess risk in everyday clinical practice rather than an experimental setting, we decided to use observational studies and not RCTs. Moreover, many factors can change over time, such as clinical practice in treating DM, patient awareness, and lifestyle. Therefore, we decided to focus only on newer studies. As our systematic review was performed in three waves, our data selection encompassed the period starting 1 January 2002 and lasted no more than 13 years. We decided to disregard small studies, assuming that they would contribute little to the total information and that a smaller study size could potentially be associated with lower quality. Importantly, these decisions were made to fit the body of evidence to the goal of the study.
Not surprisingly, using observational studies resulted in significant heterogeneity, which we tried to reduce with a consistent definition of SHEs. We decided to use a definition that relates this event to resource consumption, as it makes the results of our study useful in subsequent economic evaluations. As much as the heterogeneity poses quantitative difficulties, it is unavoidable because the population of diabetic patients is heterogeneous when we account for treatment duration, compliance, and lifestyle, among other factors, which may result in very different risks of hypoglycaemia. In this sense it would be naïve to expect homogeneous results. This heterogeneity has been widely observed in published studies of different sizes and designs [3, 6–8]. In order to account for the heterogeneity we used a (partially) random-effect model, and the heterogeneity results in wide confidence intervals, which should simply be treated as an unavoidable price to pay.
We also tried to limit the impact of heterogeneity by splitting the drugs into treatment groups that seem to be clinically related to various SHE rates. The difficulty is that we can expect reverse feedback, i.e., patients with a high risk of hypoglycaemia may use drugs that cause hypoglycaemia less often, and the net effect may be weakened or even reversed.
We considered performing a meta-regression but ultimately decided that the number of studies is too small for most of the treatment regimens. Furthermore, our goal was not to understand other factors impacting the risk of SHEs, but to construct a set of parameters relating the risk to treatment groups used in clinical practice. Applying more complex models in subsequent studies aiming to assess the number/burden of SHEs in some populations would be more difficult because it would require knowing the values of other explanatory variables to input them in the model.
Some of the inclusion criteria help reduce heterogeneity. Using newer studies helps us focus on mechanisms that most likely prevail in present clinical practice. Using larger studies reduces randomness. Excluding studies comes at the cost of making the body of evidence small, but we ultimately decided that this compromise is worthwhile.
The included observational studies were generally case-control and cohort studies of medium and good quality, respectively, indirectly due to the use of the NOS [16], which can be applied only to these two types of studies. However, the majority of included studies could not be classified to either group and were assessed by descriptions with no scoring; the results suggest that they are also of medium quality. According to Shamliyan et al. [19] there are 97 tools (46 scales and 51 checklists) that can be used to evaluate observational studies, but transparent objective assessments of the quality of observational research are missing. We decided to use NOS because it is one of the two best and recommended tools to evaluate non-randomized interventional studies [17].
Ultimately, we had to use different types of studies to assess the risk of OADs in T2 DM and biphasic insulin in T1 DM. The former was part of the methodology assumed from the very beginning and resulted from our conviction that the risk associated with OADs is so small that any possible errors will be small in absolute terms; the latter resulted from the limited availability of observational data. Using data from RCTs violates our general methodology. Also, taking into account the lack of significance in both comparisons for biphasic insulin in T1 DM, other quantitative estimation approaches could be used. This part of our results should be treated with caution, but we still wanted to end up with a set of parameters.
Methodology
As mentioned above, the present research is an update of a previously published systematic review [9]. We think that the data synthesis was markedly improved in the present version. Using the binomial distribution allowed us to take into consideration that some patients may have greater risk of hypoglycaemia than others and that the events may cluster in some patients, but this distribution does not force this clustering. Estimation results confirm that this is the case, i.e., the estimated parameters show that this phenomenon occurs. For example, for insulin pumps in T1 DM, the average annual number of SHEs is 0.168. Assuming that a Poisson distribution governs the number of episodes in individual patients, we would conclude that 15.5 % have at least one event per year. Using the negative binomial distribution allows us to estimate this parameter separately, yielding 11.4 % instead.
Different approaches have been suggested and were tested by us. For example, a two-step model could be constructed in which a patient is randomly determined to not have any or to have at least one SHE, and then the actual number of SHEs is randomly determined. An alternative method is assuming that the time to the first SHE is distributed differently than the time to the subsequent SHEs. Such approaches would result in more complicated modelling, and it may be difficult to specify the model with no individual patient data and to subsequently use it because it would require knowing the treatment history and information on past SHEs.
Using the negative binomial distribution allows us to account for (but not enforce) the clustering effect and to estimate two parameters of interest: the annual number of events and the risk of experiencing at least one event. The former may be more useful in subsequent economic studies. The latter may be important when analysing the fear of hypoglycaemia attributed to individual treatment regimens.
Results
From a clinical perspective the results are consistent with the general overview of treatment regimens and associated risk of SHEs. We generally observed that the risk of SHEs is higher for T1 DM than T2 DM and mainly attributable to insulin injections; SU-based oral antidiabetic treatments were found to be related to the lowest risk of SHEs. Insulin analogues are related to a much smaller risk than human insulin, especially in basal-bolus therapy in T2 DM, and pre-mixes and BOTs seem to be related to a reduced risk compared to more intensive treatment with basal-bolus of human insulin (in T2 DM), but not for insulin analogues. The only one striking, but not unexpected, finding is the difference in SHE risk for insulin analogue and human insulin components of basal-bolus therapy in T2 DM. One could argue that we were not able to fully control for diabetes duration and other cofounders, which results in a much higher risk of SHEs attributable to human insulin. Nevertheless, the main and most valuable finding of our study is the quantitative estimation of consistently defined and reported SHE risk related to the most common treatment regimen rather than individual drugs.
Limitations
This study obviously has numerous limitations. We consider the heterogeneity of the studies to be the most important limitation. Even though heterogeneity was to be expected and is unavoidable, it results in wide confidence intervals; thus, the actual rates may be substantially different than our point estimates. For some treatment groups there were only a few studies, and they may mistakenly present the overall true picture. Nevertheless, the current results try to use the best currently available data.
It is important to correctly perceive the applicability of our results. We did not aim to compare therapies so as to draw interventional conclusions, i.e., so as to conclude about the effect of prescribing this drug instead of another on the risk of SHEs. Because we, in principle, did not use multi-arm studies or randomized trials, that kind of conclusions are not authorized. Thus, there are limitations with respect to the types of questions answered by our study.
Notably, we focussed only on one element related to the risk of hypoglycaemia, but there are many more. We do not claim that the therapy used is the most important determinant of SHE risk. However, this factor can be relatively well quantified and measured and subsequently used to try to estimate the number of SHEs more precisely using strata.
Relevance to previous research
We performed a simple search in the MEDLINE database to determine if there are other systematic reviews or meta-analyses that evaluate real-life risk of severe hypoglycaemia among patients with T1 and T2 DM for various therapies. We used the search terms “severe”, “major”, “serious”, “hypoglycaemia”, “diabetes”, “observational”, and “real life” to identify potentially relevant citations. We did not find other systematic reviews or meta-analyses that assessed average annual rates of SHEs associated with various insulin regimens and OADs based on observational studies.
A review by Bolen et al. [210] was closest to ours; the aim of their study was to summarize the English language literature on the benefits and harms of oral agents in adult patients with T2 DM. Bolen et al. identified two systematic reviews and 216 controlled trials and cohort studies. All systematic reviews and 167 trials evaluated adverse events, 67 % of which were RCTs and the rest observational. Bolen et al. used a random-effect model to estimate post-treatment differences in absolute risk for adverse events between individual drugs, drug groups, or therapies. Bolen et al. combined results for minor and major hypoglycaemia, whereas our aim was only to estimate the average annual rate of severe hypoglycaemia. The previous review also did not provide a definition of major hypoglycaemia; Bolen et al. [210] conducted a meta-analysis indicating that the use of second generation SU results in a higher frequency of minor and major hypoglycaemia episodes than therapy with metformin or TZD. This trend is in line with our analysis of data from RCTs included in Karagiannis et al., that SU drugs are associated with a higher risk of hypoglycaemia than OADs [184].
Another meta-analysis of observational studies was conducted by Goto et al. [211]. This review evaluated an association between severe hypoglycaemia and the risk of cardiovascular disease in patients with T2 DM based on cohort studies and RCTs, as long as an observational analysis of the analysed association was available. That meta-analysis included six studies: two secondary analyses of RCTs and four based on administrative databases. However, neither of those analyses fulfilled the inclusion criteria of our systematic review due to an inappropriate definition of severe hypoglycaemia. Goto et al. [211] used relative risk as a measure of effect to estimate the association between SHEs and cardiovascular disease. Their findings suggest that severe hypoglycaemia is associated with approximately twice the risk of cardiovascular disease and indicate that an evaluation and quantification of the risk of severe hypoglycaemia is needed.
Conclusions
Various drug regimens differ in terms of the risk of severe hypoglycaemia and our results are consistent with the general perception of a higher risk of hypoglycaemia being associated with T1 DM compared to T2 DM, insulin-based treatment versus oral antidiabetic drugs, and human insulin versus analogues. Observational studies seem to be well suited for assessing the real-life risk, but they increase the heterogeneity of data. A negative binomial distribution can be used to model various forms of data and allows us to account for clustering, which is an expected clinical phenomena confirmed by our results. The results of our studies can be used to provide parameters for cost-of-illness studies estimating the overall burden of hypoglycaemia.
Abbreviations
- BBA:
-
Basal-bolus insulin therapy with long-acting insulin analogue as the basal component
- BBH:
-
Basal-bolus insulin therapy with human insulin as the basal component
- BOT:
-
Basal therapy combined with oral antidiabetic medication
- BOTA:
-
Basal supported oral therapy with long-acting insulin analogue as the basal component
- BOTH:
-
Basal supported oral therapy with human insulin as the basal component
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- OAD:
-
Other antidiabetic drug
- NOS:
-
Newcastle-Ottawa scale
- RCT:
-
Randomized controlled trial
- SHE:
-
Severe hypoglycaemia event
- SU:
-
Sulfonylurea
- T1, T2 DM:
-
Type 1, Type 2 Diabetes mellitus
- TZD:
-
Thiazolidinediones
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;16:1047–53.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Diabetes Control and Complications Trial Research (DCCT) Group. The effect of intensive treatment in diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Whipple AO. The surgical therapy of hyperinsulinism. J Int Chir. 1938;3:237–76.
Workgroup on Hypoglycemia, Workgroup on Hypoglycemia, American Diabetes Association. Defending and reporting hypoglycaemia in diabetes. Diabets Care. 2005;28:1245–9.
UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes; effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–7.
Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1999;130:389–96.
MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med. 1993;10:238–45.
Jakubczyk M, Pawęska J, Niewada M, Rdzanek E, Czech M. Risk of severe hypoglycaemia for various treatment regimens – a systematic review and meta-analysis of observational studies. JHPOR. 2014; doi:10.7365/JHPOR.2014.2.9
Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097.
Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health. 2006;9:193–8.
Hammer M, Lammert M, Mejías SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12:281–90.
Lammert M, Hammer M, Frier BM. Management of severe hypoglycaemia: cultural similarities, differences and resource consumption in three European countries. J Med Econ. 2009;12:269–80.
Orozco-Beltrán D, Mezquita-Raya P, Ramírez De Arellano A, Galán M. Self-reported frequency and impact of hypoglycaemic events in Spain. Diabetes Ther. 2014;5:155–68.
Willis WD, Diago-Cabezudo JI, Madec-Hily A, Aslam A. Medical resource use, disturbance of daily life and burden of hypoglycaemia in insulin-treated patients with diabetes: results from a European online survey. Expert Rev Pharmacoecon Outcomes Res. 2013;13:123–30.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Mar 2015.
Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:1–173. iii-x.
Higgins JPT, Green S. Tools for assessing methodological quality or risk of bias in non-randomized studies. In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. http://handbook.cochrane.org/chapter_13/13_5_2_3_tools_for_assessing_methodological_quality_or_risk_of.htm. Accessed 10 Mar 2015.
Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63:1061–70.
Viechtbauer W. Package ‘metafor’. In: The Comprehensive R Archive Network. Package ‘metafor’. http://cran.r-project.org/web/packages/metafor/metafor.pdf; Accessed 10 Mar 2015.
Ali M, White J, Lee CH, Palmer JL, Smith-Palmer J, Fakhoury W, et al. Therapy conversion to biphasic insulin aspart 30 improves long-term outcomes and reduces the costs of type 2 diabetes in Saudi Arabia. J Med Econ. 2008;11:651–70.
Almustafa M, Yeo JP, Khutsoane D. Glycaemic control and hypoglycaemia in the PRESENT study. Diabetes Res Clin Pract. 2008;81 Suppl 1:S10–5.
Andayani TM, Ibrahim MIM, Asdie AH. The safety of triple therapy with oral antidiabetics versus insulin in type 2 diabetes. Asian J Pharm Clin Res. 2010;3:201–3.
Aung PP, Strachan MW, Frier BM, Butcher I, Deary IJ, Price JF. Severe hypoglycaemia and late-life cognitive ability in older people with Type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabet Med. 2012;29:328–36.
Berntorp K, Haglund M, Larsen S, Petruckevitch A, Landin-Olsson M. Initiation of biphasic insulin aspart 30/70 in subjects with type 2 diabetes mellitus in a largely primary care-based setting in Sweden. Prim Care Diabetes. 2011;5:89–94.
Biesenbach G, Bodlaj G, Pieringer H. Weight gain and metabolic control in newly insulin-treated patients with type 2 diabetes with different insulin regimens. Can J Diabetes. 2006;30:384–9.
Breum L, Almdal T, Eiken P, Lund P, Christiansen E. Initiating or switching to biphasic insulin aspart 30/70 therapy in subjects with type 2 diabetes mellitus. An observational study. Rev Diabet Stud. 2008;5:154–62.
Brod M, Valensi P, Shaban JA, Bushnell DM, Christensen TL. Patient treatment satisfaction after switching to NovoMix® 30 (BIAsp 30) in the IMPROVE study: an analysis of the influence of prior and current treatment factors. Qual Life Res. 2010;19:1285–93.
Bruttomesso D, Pianta A, Crazzolara D, Scaldaferri E, Lora L, Guarneri G, et al. Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med. 2002;19:628–34.
de Bock M, Gunn AJ, Holt JA, Derraik JG, Reed P, Cutfield W, et al. Impact of insulin pumps on glycaemic control in a pump-naive paediatric regional population. J Paediatr Child Health. 2012;48:247–52.
Dornhorst A, Lüddeke HJ, Honka M, Ackermann RW, Meriläinen M, Gallwitz B, et al. Safety and efficacy of insulin detemir basal-bolus therapy in type 1 diabetes patients: 14-Week data from the European cohort of the PREDICTIVE study. Curr Med Res Opin. 2008;24:369–76.
Dornhorst A, Lüddeke HJ, Koenen C, Meriläinen M, King A, Robinson A, et al. Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE®. Diabetes Obes Metab. 2008;10:75–81.
Dornhorst A, Lüddeke HJ, Sreenan S, Kozlovski P, Hansen JB, Looij BJ, et al. Insulin detemir improves glycaemic control without weight gain in insulin-naive patients with type 2 diabetes: Subgroup analysis from the PREDICTIVE® study. Int J Clin Pract. 2008;62:659–65.
Dornhorst A, Lüddeke HJ, Sreenan S, Koenen C, Hansen JB, Tsur A, et al. Safety and efficacy of insulin detemir in clinical practice: 14-Week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVETM European cohort. Int J Clin Pract. 2007;61:523–8.
Esteghamati A, Rajabian R, Amini M, Bahrami A, Khamseh ME, Afkhami-Ardekani M, et al. The safety and efficacy of biphasic insulin aspart 30 (BIAsp 30) in Iranians with type 2 diabetes: An open-label, non-randomised, multi-centre observational study - The Iran subgroup of the IMPROVE® study. Endokrynol Pol. 2010;61:364–70.
Fontaine P, Gin H, Pinget M, Thivolet C, Hanaire H, Robert JJ, et al. Effect of insulin detemir dose frequency on clinical outcomes in patients with diabetes in PREDICTIVE. Adv Ther. 2009;26:535–51.
Furlong NJ, McNulty SJ, O’Brien SV, Hardy KJ. Comparison of metformin versus sulphonylurea in combination with daily NPH insulin in patients with type 2 diabetes inadequately controlled on oral hypoglycaemic agents; median follow-up 29 months. Practical Diabetes Int. 2002;19:245–9.
Gao Y, Guo XH, Vaz JA. Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: Chinese PRESENT study. Diabetes Obes Metab. 2009;11:33–40.
Gao Y, Guo XH. Switching from human insulin to biphasic insulin aspart 30 treatment gets more patients with type 2 diabetes to reach target glycosylated hemoglobin <7%: The results from the China cohort of the PRESENT study. Chin Med J (Engl). 2010;123:1107–11.
Garber AJ, Wahlen J, Wahl T, Bressler P, Braceras R, Allen E, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8:58–66.
Garg SK, Gottlieb PA, Hisatomi ME, D’Souza A, Walker AJ, Izuora KE, et al. Improved glycemic control without an increase in severe hypoglycemic episodes in intensively treated patients with type 1 diabetes receiving morning, evening, or split dose insulin glargine. Diabetes Res Clin Pract. 2004;66:49–56.
Garg SK, Paul JM, Karsten JI, Menditto L, Gottlieb PA. Reduced severe hypoglycaemia with insulin glargine in intensively treated adults with type 1 diabetes. Diabetes Technol Ther. 2004;6:589–95.
Garg SK, Walker AJ, Hoff HK, D’Souza AO, Gottlieb PA, Chase HP. Glycemic parameters with multiple daily injections using insulin glargine versus insulin pump. Diabetes Technol Ther. 2004;6:9–15.
Giorda C, Boemi M, Borzì V, Chiaramonte F, Mattei P, Tribulato A. The IMPROVE study a multinational, multicentre, observational study in type 2 diabetes: results from the Italian cohort. Acta Biomed. 2010;81:115–24.
Gu Y, Hou X, Zhang L, Pan J, Cai Q, Bao Y, et al. The impact of initiating biphasic human insulin 30 therapy in type 2 diabetes patients after failure of oral antidiabetes drugs. Diabetes Technol Ther. 2012;14:244–50.
Güler S, Sharma SK, Almustafa M, Kim CH, Azar S, Danciulescu R, et al. Improved glycaemic control with biphasic insulin aspart 30 in type 2 diabetes patients failing oral antidiabetic Drugs: PRESENT study results. Arch Drug Inf. 2009;2:23–33.
Gumprecht J, Benroubi M, Borzi V, Kawamori R, Shaban J, Shah S, et al. Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix® 30) can improve glycaemic control in patients treated with basal insulins: A subgroup analysis of the IMPROVE observational study. Int J Clin Pract. 2009;63:966–72.
Gumprecht J, Zurawska G, Wolnik B, Dzida G. The IMPROVE study - A multinational, observational study in type 2 diabetes: Data from the Polish cohort. Endokrynol Pol. 2008;59:460–6.
Hanefeld M, Fleischmann H, Landgraf W, Pistrosch F. EARLY study: Early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes Stoffwech H. 2012;21:91–7.
Hartemann-Heurtier A, Sachon C, Masseboeuf N, Corset E, Grimaldi A. Functional intensified insulin therapy with short-acting insulin analog: effects on HbA1c and frequency of severe hypoglycaemia. An observational cohort study. Diabetes Metab. 2003;29:53–7.
Hassan MI, Aamir AH, Miyan Z, Siddiqui LA, Qureshi MS, Shaikh MZ. Safety and effectiveness of biphasic insulin aspart 30 (BIAsp 30) in people with type 2 diabetes mellitus in the Pakistani population: Results from the A1chieve study. J Pak Med Assoc. 2012;62:929–36.
Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin. 2009;25:2601–8.
Hermansen K, Lund P, Clemmensen K, Breum L, Kleis Moller M, Mette Rosenfalck A, et al. 3-Month results from Denmark within the globally prospective and observational study to evaluate insulin detemir treatment in type 1 and type 2 diabetes: The PREDICTIVE study. Rev Diabet Stud. 2007;4:89–97.
Herwig J, Scholl-Schilling G, Böhles H. Glycaemic control and hypoglycaemia in children, adolescents and young adults with unstable type 1 diabetes mellitus treated with insulin glargine or intermediate-acting insulin. J Pediatr Endocrinol Metab. 2007;20:517–25.
Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011;94:352–63.
Honkasalo M, Elonheimo O, Sane T. Many diabetic patients with recurrent severe hypoglycaemias hold a valid driving license. A community-based study in insulin-treated patients with diabetes. Traffic Inj Prev. 2010;11:258–62.
Honkasalo MT, Elonheimo OM, Sane T. Severe hypoglycaemia in drug-treated diabetic patients needs attention: a population-based study. Scand J Prim Health Care. 2011;29:165–70.
Ishii H, Iwase M, Seino H, Shuto Y, Atsumi Y. Assessment of quality of life in patients with type 2 diabetes mellitus before and after starting biphasic insulin aspart 30 (BIAsp 30) therapy: IMPROVE study in Japan. Curr Med Res Opin. 2011;27:643–50.
Iványi T, Fövényi J, Faludi P, Han J, Macconell L, Wille S, et al. Long-term effects of adding Exenatide to a regimen of metformin and/or sulfonylurea in type 2 diabetes: an uncontrolled, open-label trial in Hungary. Clin Ther. 2012;34:1301–13.
Jakisch BI, Wagner VM, Heidtmann B, Lepler R, Holterhus PM, Kapellen TM, et al. Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabet Med. 2008;25:80–5.
Jang HC, Guler S, Shestakova M. When glycaemic targets can no longer be achieved with basal insulin in type 2 diabetes, can simple intensification with a modern pre-mix insulin help? Results from a subanalysis of the PRESENT study. Int J Clin Pract. 2008;62:1013–8.
Jang HC, Lee SR, Vaz JA. Biphasic insulin aspart 30 in the treatment of elderly patients with type 2 diabetes: A subgroup analysis of the PRESENT Korea NovoMixstudy. Diabetes Obes Metab. 2009;11:20–6.
Kapellen TM, Heidtmann B, Bachmann J, Ziegler R, Grabert M, Holl RW. Indications for insulin pump therapy in different age groups - An analysis of 1567 children and adolescents. Diabet Med. 2007;24:836–42.
Kapellen TM, Wolf J, Rosenbauer J, Stachow R, Ziegler R, Szczepanski R, et al. Changes in the use of analogue insulins in 37 206 children and adolescents with type 1 diabetes in 275 German and Austrian centres during the last twelve years. Exp Clin Endocrinol Diabetes. 2009;117:329–35.
Katz ML, Volkening LK, Anderson BJ, Laffel LM. Contemporary rates of severe hypoglycaemia in youth with Type1 diabetes: Variability by insulin regimen. Diabet Med. 2012;29:926–32.
Kawamori R, Eliaschewitz FG, Takayama H, Hayashida CY. Efficacy of insulin glargine and glimepiride in controlling blood glucose of ethnic Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;79:97–102.
Kawamori R, Valensi P. IMPROVE observational study of biphasic insulin aspart 30/70 in patients with Type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2010;5:507–16.
Keen AJ, Duncan E, McKillop-Smith A, Evans ND, Gold AE. Dose Adjustment for Normal Eating (DAFNE) in routine clinical practice: who benefits? Diabet Med. 2012;29:670–6.
Khader S, Abdelfattah W, Almansari A, Elnnagar NK. Safety and efficacy of switching to biphasic insulin aspart 30/70 (BIAsp 30) under the routine diabetic care in patients with type 2 diabetes: The IMPROVE observational study in the Gulf region. Int J Diabetes Mellit. 2010;2:110–3.
Khutsoane D, Sharma SK, Almustafa M, Jang HC, Azar ST, Danciulescu R, et al. Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: experience from the PRESENT study. Diabetes Obes Metab. 2008;10:212–22.
Kristensen PL, Hansen LS, Jespersen MJ, Pedersen-Bjergaard U, Beck-Nielsen H, Christiansen JS, et al. Insulin analogues and severe hypoglycaemia in type 1 diabetes. Diabetes Res Clin Pract. 2012;96:17–23.
Kurtoglu S, Atabek ME, Dizdarer C, Pirgon O, Isguven P, Emek S. Insulin detemir improves glycemic control and reduces hypoglycaemia in children with type 1 diabetes: Findings from the Turkish cohort of the PREDICTIVE® observational study. Pediatr Diabetes. 2009;10:401–7.
Leckie AM, Graham MK, Grant JB, Ritchie PJ, Frier BM. Frequency, severity, and morbidity of hypoglycaemia occurring in the workplace in people with insulin-treated diabetes. Diabetes Care. 2005;28:1333–8.
Leinung M, Thompson S, Nardacci E. Benefits of continuous glucose monitor use in clinical practice. Endocr Pract. 2010;16:371–5.
Levit S, Toledano Y, Wainstein J. Improved glycaemic control with reduced hypoglycaemic episodes and without weight gain using long-term modern pre-mix insulins in type 2 diabetes. Int J Clin Pract. 2011;65:165–71.
Ligthelm RJ. Self-titration of biphasic insulin aspart 30/70 improves glycaemic control and allows easy intensification in a Dutch clinical practice. Prim Care Diabetes. 2009;3:97–102.
Lüddeke HJ, Sreenan S, Aczel S, Maxeiner S, Yenigun M, Kozlovski P, et al. PREDICTIVE- a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab. 2007;9:428–34.
Mäkelä JK, Schmüser C, Askonen K, Saukkonen T. Starting or switching to biphasic insulin aspart 30 (BIAsp 30) in type 2 diabetes: a multicenter, observational, primary care study conducted in Finland. Diabetes Res Clin Pract. 2012;95:10–8.
Marre M, Pinget M, Gin H, Thivolet C, Hanaire H, Robert JJ, et al. Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain: 52-week data from the PREDICTIVE study in a cohort of French patients with type 1 or type 2 diabetes. Diabetes Metab. 2009;35:469–75.
Meneghini LF, Dornhorst A, Sreenan S. Once-daily insulin detemir in a cohort of insulin-naive patients with type 2 diabetes: a sub-analysis from the PREDICTIVE study. Curr Med Res Opin. 2009;25:1029–35.
Meneghini LF, Rosenberg KH, Koenen C, Merilainen MJ, Lüddeke HJ. Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab. 2007;9:418–27.
Müller-Godeffroy E, Treichel S, Wagner VM. Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus --a large-scale multicentre pilot study. Diabet Med. 2009;26:493–501.
Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics. 2006;117:2126–31.
Nobels F, D’Hooge D, Crenier L. Switching to biphasic insulin aspart 30/50/70 from biphasic human insulin 30/50 in patients with type 2 diabetes in normal clinical practice: Observational study results. Curr Med Res Opin. 2012;28:1017–26.
Oishi M, Abe N, Yokoyama H, Kuribayashi N, Tomonaga O, Matoba K, et al. Observational 6-month open-label study of Japanese type 2 diabetes patients switching from NPH insulin to insulin detemir in basal-bolus regimen: 23rd article of the Japan diabetes clinical data management study group (JDDM23). J Int Med Res. 2012;40:787–97.
Oyer DS, Shepherd MD, Coulter FC, Bhargava A, Deluzio AJ, Chu PL, et al. Efficacy and tolerability of self-titrated biphasic insulin Aspart 70/30 in patients aged >65 years with type 2 diabetes: an exploratory post Hoc subanalysis of the INITIATEplus trial. Clin Ther. 2011;33:874–83.
Peczyńska J, Urban M, Głowińska B, Florys B. Decreased consciousness of hypoglycaemia and the incidence of severe hypoglycaemia in children and adolescents with diabetes type 1. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2002;8:77–82.
Perriello G, Caputo S, De Pergola G, Di Carlo A, Grassi G, Lapolla A, et al. Improved glycemic control with weight loss and a low risk of hypoglycaemia with insulin detemir: insights from the Italian cohort of the PREDICTIVE study after 6-month observation in type 2 diabetic subjects. Expert Opin Pharmacother. 2011;12:2449–55.
Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wändell P. Self-reported experience of hypoglycaemia among adults with type 2 diabetes mellitus (Exhype). Diabetes Res Clin Pract. 2011;92:19–25.
Preumont V, Buysschaert M, De Beukelaer S, Mathieu C. Insulin detemir in routine clinical practice: A 26-week follow-up in type 1 diabetic patients from the Belgian PREDICTIVE cohort. Acta Clin Belg. 2009;64:49–55.
Reda E, Von Reitzenstein A, Dunn P. Metabolic control with insulin pump therapy: the Waikato experience. N Z Med J. 2007;120:U2401.
Rudolph JW, Hirsch IB. Assessment of therapy with continuous subcutaneous insulin infusion in an academic diabetes clinic. Endocr Pract. 2002;8:401–5.
Scaramuzza AE, Iafusco D, Rabbone I, Bonfanti R, Lombardo F, Schiaffini R, et al. Use of integrated real-time continuous glucose monitoring/insulin pump system in children and adolescents with type 1 diabetes: a 3-year follow-up study. Diabetes Technol Ther. 2011;13:99–103.
Scheidegger U, Allemann S, Scheidegger K, Diem P. Continuous subcutaneous insulin infusion therapy: effects on quality of life. Swiss Med Wkly. 2007;137:476–82.
Shah S, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, et al. Safety and effectiveness of biphasic insulin aspart 30/70 (NovoMix® 30) when switching from human premix insulin in patients with type 2 diabetes: Subgroup analysis from the 6-month IMPROVE observational study. Int J Clin Pract. 2009;63:574–82.
Shah S, Das AK, Kumar A, Unnikrishnan AG, Kalra S, Baruah MP, et al. Baseline characteristics of the Indian cohort from the IMPROVE study: a multinational, observational study of biphasic insulin aspart 30 treatment for type 2 diabetes. Adv Ther. 2009;26:325–35.
Shah S, Zilov A, Malek R, Soewondo P, Bech O, Litwak L. Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: Results from the A1chieve observational study. Diabetes Res Clin Pract. 2011;94:364–70.
Shah SN, Litwak L, Haddad J, Chakkarwar PN, Hajjaji I. The A1chieve study: a 60 000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract. 2010;88 Suppl 1:S11–6.
Sharma SK, Al-Mustafa M, Oh SJ, Azar ST, Shestakova M, Guler S, et al. Biphasic insulin aspart 30 treatment in patients with type 2 diabetes poorly controlled on prior diabetes treatment: Results from the PRESENT study. Curr Med Res Opin. 2008;24:645–52.
Sharma SK, Joshi SR, Kumar A, Unnikrishnan AG, Hoskote SS, Moharana AK, et al. Efficacy, safety and acceptability of biphasic insulin aspart 30 in Indian patients with type 2 diabetes: results from the PRESENT study. J Assoc Physicians India. 2008;56:859–63.
Shestakova M, Bech OM, Momani MS. Study design and baseline characteristics of patients in the PRESENT study. Diabetes Res Clin Pract. 2008;81 Suppl 1:S3–9.
Shestakova M, Sharma SK, Almustafa M, Min KW, Ayad N, Azar ST, et al. Transferring type 2 diabetes patients with uncontrolled glycaemia from biphasic human insulin to biphasic insulin aspart 30: experiences from the PRESENT study. Curr Med Res Opin. 2007;23:3209–14.
Sreenan S, Virkamaki A, Zhang K, Hansen JB. Switching from NPH insulin to once-daily insulin detemir in basal-bolus-treated patients with diabetes mellitus: Data from the European cohort of the PREDICTIVE® study. Int J Clin Pract. 2008;62:1971–80.
Strojek K, Tarasiuk A, Bijos P, Czech A. Gensulin M30 in patients with type 2 diabetes and secondary failure to oral antidiabetic drugs. the Progens-first-step study: A multicentre observational study in the outpatient setting. Diabet Dośw i Klin. 2008;8:179–84.
Sudhakaran C, Fathima M, Anjana RM, Unnikrishnan RI, Mohan V. Effectiveness of exenatide in Asian Indians in a clinical care setting. Diabetes Technol Ther. 2010;12:613–8.
Sudhakaran C, Kishore U, Anjana RM, Unnikrishnan R, Mohan V. Effectiveness of sitagliptin in asian Indian patients with type 2 diabetes-an Indian tertiary diabetes care center experience. Diabetes Technol Ther. 2011;13:27–32.
Suzuki D, Toyoda M, Kondo M, Miyatake H, Tanaka E, Sato H, et al. Efficacy of long-acting insulin analog insulin glargine at high dosage for basal-bolus insulin therapy in patients with type 2 diabetes. Tokai J Exp Clin Med. 2012;37:35–40.
Temizel M, Mert M, Bozbey C, Arman Y, Cevizci E, Altintaş N, et al. Evaluation of the weight-increasing effects of biphasic analog and regular NPH insulin mixtures in patients with Type 2 diabetes mellitus. J Diabetes. 2010;2:250–5.
Tsai ST, Pathan F, Ji L, Yeung VT, Chadha M, Suastika K, et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes. 2011;3:208–16.
Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, et al. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: Safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. Int J Clin Pract. 2009;63:522–31.
Valensi P, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, et al. The IMPROVE study-a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008;62:1809–19.
Vergès B, Brun JM, Tawil C, Alexandre B, Kerlan V. Strategies for insulin initiation: insights from the French LIGHT observational study. Diabetes Metab Res Rev. 2012;28:97–105.
Vexiau P, Mavros P, Krishnarajah G, Lyu R, Yin D. Hypoglycaemia in patients with type 2 diabetes treated with a combination of metformin and sulphonylurea therapy in France. Diabetes Obes Metab. 2008;10 Suppl 1:16–24.
Wenying Y, Benroubi M, Borzi V, Gumprecht J, Kawamori R, Shaban J, et al. Improved glycaemic control with BIAsp 30 in insulin-naive type 2 diabetes patients inadequately controlled on oral antidiabetics: subgroup analysis from the IMPROVE study. Curr Med Res Opin. 2009;25:2643–54.
Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care. 2006;29:2355–60.
Yang W, Gao Y, Liu G, Chen L, Fu Z, Zou D, et al. Biphasic insulin aspart 30 as insulin initiation or replacement therapy: the China cohort of the IMPROVE study. Curr Med Res Opin. 2010;26:101–7.
Yang W, Lv X, Li Q, Jia W, Tian H. A prospective study to optimize insulin treatment by switching to insulin glargine in type 2 diabetic patients previously uncontrolled on pre-mix insulin: the optimization study. Curr Med Res Opin. 2012;28:533–41.
Yenigun M, Honka M. Switching patients from insulin glargine-based basal-bolus regimens to a once daily insulin detemir-based basal-bolus regimen: results from a subgroup of the PREDICTIVE study. Int J Clin Pract. 2009;63:425–32.
Zick R, Petersen B, Richter M, Haug C. Comparison of continuous blood glucose measurement with conventional documentation of hypoglycaemia in patients with Type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9:483–92.
Zjačic-Rotkvić V, Cigrovski-Berković M, Grulović N, Baršić B. Efficacy and safety of a basal-bolus regimen with insulin glargine in patients with type 2 diabetes after failing premix insulin therapy: A multicenter postmarketing study. Diabetol Croat. 2012;41:41–8.
El Naggar NK, Soewondo P, Khamseh ME, Chen JW, Haddad J. Switching from biphasic human insulin 30 to biphasic insulin aspart 30 in type 2 diabetes is associated with improved glycaemic control and a positive safety profile: results from the A1chieve study. Diabetes Res Clin Pract. 2012;98:408–13.
El-Naggar N, Almansari A, Khudada K, Salman S, Mariswamy N, Abdelfattah W, et al. The A1 chieve study - an observational non-interventional study of patients with type 2 diabetes mellitus initiating or switched to insulin analogue therapy: subgroup analysis of the Gulf population. Int J Clin Pract. 2013;67:128–38.
Gómez-Peralta F, Carramiñana-Barrera F, Félix-Redondo FJ, Fraile-Gómez J. Glycaemic control in patients with type 2 diabetes switching from premixed insulin to long-acting basal insulin analogue plus oral antidiabetic drugs: an observational study. Int J Clin Pract. 2012;66:959–68.
Pīrāgs V, El Damassy H, Dąbrowski M, Gönen MS, Račická E, Martinka E, et al. Low risk of severe hypoglycaemia in patients with type 2 diabetes mellitus starting insulin therapy with premixed insulin analogues BID in outpatient settings. Int J Clin Pract. 2012;66:1033–41.
Khunti K, Caputo S, Damci T, Dzida GJ, Ji Q, Kaiser M, et al. The safety and efficacy of adding once-daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012;14:1129–36.
Hendrieckx C, Halliday JA, Bowden JP, Colman PG, Cohen N, Jenkins A, et al. Severe hypoglycaemia and its association with psychological well-being in Australian adults with type 1 diabetes attending specialist tertiary clinics. Diabetes Res Clin Pract. 2014;103:430–6.
Nørgaard K, Scaramuzza A, Bratina N, Lalić NM, Jarosz-Chobot P, Kocsis G, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15:273–80.
Laubner K, Molz K, Kerner W, Karges W, Lang W, Dapp A, et al. Daily insulin doses and injection frequencies of neutral protamine hagedorn (NPH) insulin, insulin detemir and insulin glargine in type 1 and type 2 diabetes: a multicenter analysis of 51 964 patients from the German/Austrian DPV-wiss database. Diabetes Metab Res Rev. 2014;30:395–404.
Levy-Shraga Y, Lerner-Geva L, Modan-Moses D, Graph-Barel C, Mazor-Aronovitch K, Boyko V, et al. Benefits of continuous subcutaneous insulin infusion (CSII) therapy in preschool children. Exp Clin Endocrinol Diabetes. 2013;121:225–9.
Maiorino MI, Bellastella G, Petrizzo M, Improta MR, Brancario C, Castaldo F, et al. Treatment satisfaction and glycemic control in young Type 1 diabetic patients in transition from pediatric health care: CSII versus MDI. Endocrine. 2014;46:256–62.
Maltoni G, Zucchini S, Scipione M, Rollo A, Balsamo C, Bertolini C, et al. Severe hypoglycemic episodes: a persistent threat for children with Type 1 diabetes mellitus and their families. J Endocrinol Invest. 2013;36:617–21.
Ziegler R, Tubili C, Chico A, Guerci B, Lundberg E, Borchert M, et al. ProAct study: new features of insulin pumps improve diabetes management and glycemic control in patients after transition of continuous subcutaneous insulin infusion systems. Diabetes Technol Ther. 2013;15:738–43.
Carlsson A, Forsander G, Ludvigsson J, Larsen S, Ortqvist E. A multicenter observational safety study in Swedish children and adolescents using insulin detemir for the treatment of type 1 diabetes. Pediatr Diabetes. 2013;14:358–65.
Buturovic BA, Lekic A, Grulovic N. Improved glycaemic control with insulin glargine as part of a basal-bolus regimen in T2DM patients inadequately controlled on premixed therapy. Med Arch. 2013;67:342–5.
Echtay A, Tsur A, Hasan MI, Abu-Hijleh MO, Al Khatib N, Andari E, et al. Clinical experience with insulin detemir in patients with type 2 diabetes from the near East countries. Diabetes Ther. 2013;4:399–408.
Guo XH, Lv XF, Han P, Zhang XZ, Yang HZ, Duan WR, et al. Efficacy and safety of glimepiride as initial treatment in Chinese patients with Type 2 diabetes mellitus. Curr Med Res Opin. 2013;29:169–74.
Klen J, Dolžan V, Janež A. CYP2C9, KCNJ11 and ABCC8 polymorphisms and the response to sulphonylurea treatment in type 2 diabetes patients. Eur J Clin Pharmacol. 2014;70:421–8.
Klen J, Goričar K, Janež A, Dolžan V. The role of genetic factors and kidney and liver function in glycemic control in type 2 diabetes patients on long-term metformin and sulphonylurea cotreatment. Biomed Res Int. 2014;2014:934729.
Kulzer B, Seitz L, Kern W. Real-world patient-reported rates of non-severe hypoglycaemic events in Germany. Exp Clin Endocrinol Diabetes. 2014;122:167–72.
Nakashima E, Kuribayashi N, Ishida K, Taketsuna M, Takeuchi M, Imaoka T. Efficacy and safety of stepwise introduction of insulin lispro mix 50 in Japanese patients with type 2 diabetes inadequately controlled by oral therapy. Endocr J. 2013;60:763–72.
Kalra S, Deepak MC, Narang P, Singh V, Uvaraj MG, Agrawal N. Usage pattern, glycemic improvement, hypoglycemia, and body mass index changes with sulfonylureas in real-life clinical practice: results from OBSTACLE Hypoglycemia Study. Diabetes Technol Ther. 2013;15:129–35.
Kalra S, Deepak MC, Narang P, Singh V, Maheshwari A. Correlation between measures of hypoglycaemia and glycemic improvement in sulfonylurea treated patients with type 2 diabetes in India: Results from the OBSTACLE hypoglycaemia study. J Postgrad Med. 2014;60:151–5.
Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med. 2014;31:92–101.
Panelo AA, Dorado ED, Lelis M, Javelona JQ. A post-marketing surveillance study report to evaluate the safety and efficacy of a fixed-dose combination of glimepiride and metformin in the treatment of type 2 diabetes mellitus. Philipp J Intern Med. 2013;51:3.
Lieverse AG, Rodriguez M, Czupryniak L, Landgraf W, Loizeau V, Pilorget V, et al. Glycaemic control of type 2 diabetics with insulin glargine in everyday practice. Diabetes Stoffwech H. 2013;22:141–7.
Damci T, Emral R, Svendsen AL, Balkir T, Vora J. Lower risk of hypoglycaemia and greater odds for weight loss with initiation of insulin detemir compared with insulin glargine in Turkish patients with type 2 diabetes mellitus: local results of a multinational observational study. BMC Endocr Disord. 2014;14:61.
Tentolouris N, Kyriazopoulou V, Makrigiannis D, Baroutsou B. Intensification of insulin therapy in patients with type 2 diabetes: a retrospective, non- interventional cohort study of patients treated with insulin glargine or biphasic human insulin in daily clinical practice. Diabetol Metab Syndr. 2013;5:4.
Banerjee S, Maji D, Baruah M. Addition of insulin aspart with basal insulin is associated with improved glycemic control in Indian patients with uncontrolled type 2 diabetes mellitus: the A1chieve observational study. J Assoc Physicians India. 2013;61 Suppl 1:24–7.
Bebakar WM, Lim-Abrahan MA, Jain AB, Seah D, Soewondo P. Safety and effectiveness of insulin aspart in type 2 diabetic patients: results from the ASEAN cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S17–23.
Belhadj M, Dahaoui A, Jamoussi H, Farouqi A. Exploring insulin analogue safety and effectiveness in a Maghrebian cohort with type 2 diabetes: results from the A1chieve study. Diabetes Res Clin Pract. 2013;101 Suppl 1:S4–14.
Chen L, Xing X, Lei M, Liu J, Shi Y, Li P, et al. Biphasic insulin aspart 30 improved glycemic control in Chinese patients with type 2 diabetes poorly controlled on oral glucose-lowering drugs: a subgroup analysis of the A1chieve study. Chin Med J (Engl). 2014;127:208–12.
Chraibi A, Ajdi F, Belkhadir J, El Ansari N, Marouan F, Farouqi A. Safety and effectiveness of insulin analogues in Moroccan patients with type 2 diabetes: a sub-analysis of the A1chieve study. Diabetes Res Clin Pract. 2013;101 Suppl 1:S27–36.
Dieuzeide G, Chuang LM, Almaghamsi A, Zilov A, Chen JW, Lavalle-González FJ. Safety and effectiveness of biphasic insulin aspart 30 in people with type 2 diabetes switching from basal-bolus insulin regimens in the A1chieve study. Prim Care Diabetes. 2014;8:111–7.
Haddad J, Khoshniatnikoo M, Benabbas Y, Guler S, Prusty V, Soewondo P. Safety and effectiveness of switching from a basal-only to biphasic insulin Aspart 30 insulin regimen. Diabetes Ther. 2013;4:309–19.
Hassan MI, Aamir AH, Miyan Z, Siddiqui LA, Mahmood S, Vohra EA. Switch from biphasic human insulin 30 to biphasic insulin aspart 30 in Pakistani subjects. J Pak Med Assoc. 2013;63:1290–4.
Home PD, Latif ZA, González-Gálvez G, Prusty V, Hussein Z. The effectiveness and safety of beginning insulin aspart together with basal insulin in people with type 2 diabetes in non-Western nations: results from the A1chieve observational study. Diabetes Res Clin Pract. 2013;101:326–32.
Home PD, Shen C, Hasan MI, Latif ZA, Chen JW, González GG. Predictive and explanatory factors of change in HbA1c in a 24-week observational study of 66,726 people with type 2 diabetes starting insulin analogs. Diabetes Care. 2014;37:1237–45.
Hussein Z, Lim-Abrahan MA, Jain AB, Goh SY, Soewondo P. Switching from biphasic human insulin to biphasic insulin aspart 30 in type 2 diabetes: results from the ASEAN subgroup of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S24–9.
Khamseh ME, Haddad J, Yang W, Zilov A, Bech OM, Hasan MI. Safety and effectiveness of biphasic insulin aspart 30 in different age-groups: a1chieve sub-analysis. Diabetes Ther. 2013;4:347–61.
Kumar A, Sharma SK, Rajput R, Unnikrishnan AG. Initiating therapy or switching to biphasic insulin aspart improves glycaemic control in type 2 diabetes: an Indian experience from the A1chieve study. J Assoc Physicians India. 2013;61 Suppl 1:16–20.
Latif ZA, Hussein Z, Litwak L, El Naggar N, Chen JW, Soewondo P. Safety and effectiveness of insulin aspart in Basal-bolus regimens regardless of age: a1chieve study results. Diabetes Ther. 2013;4:103–18.
Latif ZA, Pathan MF, Siddiqui MN, Sobhan MJ, Rahman MM, Ashrafuzzaman SM. Safety and effectiveness of biphasic insulin aspart 30 in a Bangladeshi subgroup of type 2 diabetic patients switched from biphasic human insulin 30: a sub-analysis of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S30–4.
Lezzar A, Ayad F, Dahaoui A, Salah-Mansour A, Berrouiguet AY. Initiating or switching to biphasic insulin aspart 30 in type 2 diabetes patients from Algeria: a sub-analysis of the A1chieve study. Diabetes Res Clin Pract. 2013;101 Suppl 1:S37–44.
Lim-Abrahan MA, Jain AB, Bebakar WM, Seah D, Soewondo P. Safety and effectiveness of biphasic insulin aspart 30 in type 2 diabetes: results from the ASEAN cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S3–9.
Lim-Abrahan MA, Yu-Gan S, Jain AB, Sobrepena LM, Racho VA. Safety and effectiveness of biphasic insulin aspart 30 in type 2 diabetes patients switched from biphasic human insulin 30: results from the Filipino cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S35–40.
Malek R, Arbouche Z, Dahaoui A, Bachaoui M. Safety and effectiveness of insulin analogues in type 2 diabetic patients from Algeria: a sub-analysis of the A1chieve study. Diabetes Res Clin Pract. 2013;101 Suppl 1:S15–26.
Malek R, Gonzalez-Galvez G, El Naggar N, Shah S, Prusty V, Litwak L. Safety and effectiveness of insulin detemir in different age-groups in the a1chieve study. Diabetes Ther. 2013;4:77–90.
Malek R, Arbouche Z, Bachaoui M, Zinai S, Dahaoui A, Senoussaoui S, et al. Criteria influencing the choice of starting insulin regimen in patients with type 2 diabetes in routine clinical practice: baseline data from the Algerian cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;101 Suppl 1:S45–9.
Randeree H, Liebl A, Hajjaji I, Khamseh M, Zajdenverg L, Chen JW, et al. Safety and effectiveness of bolus insulin aspart in people with type 2 diabetes: a1chieve sub-analysis. Diabetes Ther. 2013;4:153–66.
Rao PV, Bhattacharyya A, Sahay RK. Initiation of insulin aspart to Indian subjects on OADs show significant improvement in glycaemic outcomes: the A1chieve observational study. J Assoc Physicians India. 2013;61 Suppl 1:21–3.
Shah S, Yang W, Hasan MI, Malek R, Molskov Bech O, Home P. Biphasic insulin aspart 30 in insulin-naive people with type 2 diabetes in non-western nations: results from a regional comparative multinational observational study (A(1)chieve). Diabetes Technol Ther. 2013;15:954–63.
Soewondo P, Pramono RB, Langi YA, Soetedjo NN, Kshanti IA. Clinical experience with BIAsp 30: results from the Indonesian cohort of the international A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S54–9.
Soewondo P, Mohamed M, Jain AB, Sy RA, Khoo CM. Safety and effectiveness of insulin detemir in type 2 diabetes: results from the ASEAN cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S10–6.
Soewondo P, Lindarto D, Wibisono S, Renaldi O, Dalem-Pemayun TG. Clinical safety and effectiveness of biphasic insulin aspart 30 in type 2 diabetes patients switched from biphasic human insulin 30: results from the Indonesian cohort of the A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S41–6.
Soewondo P, Kshanti IA, Pramono RB, Langi YA, Dalem-Pemayun TG. Clinical experience with insulin detemir: results from the Indonesian cohort of the international A1chieve study. Diabetes Res Clin Pract. 2013;100 Suppl 1:S47–53.
Zilov A, El Naggar N, Shah S, Shen C, Haddad J. Insulin detemir in the management of type 2 diabetes in non-Western countries: safety and effectiveness data from the A1chieve observational study. Diabetes Res Clin Pract. 2013;101:317–25.
Valensi P, Shaban J, Benroubi M, Kawamori R, Borzì V, Shah S, et al. Predictors of achieving HbA(1c) <7% and no hypoglycaemia 6 months after initiation of biphasic insulin aspart 30 in patients with type 2 diabetes in the IMPROVE study. Curr Med Res Opin. 2013;29:601–9.
Caputo S, Andersen H, Kaiser M, Karnieli E, Meneghini LF, Svendsen AL. Effect of baseline glycosylated hemoglobin A1c on glycemic control and diabetes management following initiation of once-daily insulin detemir in real-life clinical practice. Endocr Pract. 2013;19:462–70.
Karnieli E, Baeres FM, Dzida G, Ji Q, Ligthelm R, Ross S, et al. Observational study of once-daily insulin detemir in people with type 2 diabetes aged 75 years or older: a sub-analysis of data from the Study of Once daily LeVEmir (SOLVE). Drugs Aging. 2013;30:167–75.
Ross S, Dzida G, Ji Q, Kaiser M, Ligthelm R, Meneghini L, et al. Safety of once-daily insulin detemir in patients with type 2 diabetes treated with oral hypoglycemic agents in routine clinical practice. J Diabetes. 2014;6:243–50.
Vora J, Caputo S, Damci T, Orozco-Beltran D, Pan C, Svendsen AL, et al. Effect of once-daily insulin detemir on oral antidiabetic drug (OAD) use in patients with type 2 diabetes. J Clin Pharm Ther. 2014;39:136–43.
Gönen MS, Yürümez A, Hersek Ö, Altunoğlu E, Rakıcı H, Scism-Bacon J, et al. The risk of severe hypoglycaemia in patients with type 2 diabetes mellitus starting insulin therapy with premixed insulin analogues taken twice daily: an observational study in Turkish patients. Turk Jem. 2013;17:83–8.
Walz L, Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wändell P. Impact of symptomatic hypoglycemia on medication adherence, patient satisfaction with treatment, and glycemic control in patients with type 2 diabetes. Patient Prefer Adherence. 2014;30(8):593–601.
Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369.
Berlie H, Hurren KM, Pinelli NR. Glucagon-like peptide-1 receptor agonists as add-on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2012;5:165–74.
Black C, Donnelly P, McIntyre L, Royle PL, Shepherd JP, Thomas S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;2:CD004654.
Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389–94.
Monami M, Iacomelli I, Marchionni N, Mannucci E. Dipeptydil peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2010;20:224–35.
Norris SL, Lee N, Thakurta S, Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26:837–46.
Pinelli NR, Cha R, Brown MB, Jaber LA. Addition of thiazolidinedione or exenatide to oral agents in type 2 diabetes: a meta-analysis. Ann Pharmacother. 2008;42:1541–51.
Pinelli NR, Hurren KM. Efficacy and safety of long-acting glucagon-like peptide-1 receptor agonists compared with exenatide twice daily and sitagliptin in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Pharmacother. 2011;45:850–60.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2:CD006739.
Shyangdan DS, Royle PL, Clar C, Sharma P, Waugh NR. Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Endocr Disord. 2010;10:20.
Wang Y, Li L, Yang M, Liu H, Boden G, Yang G. Glucagon-like peptide-1 receptor agonists versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta-analysis of clinical trials. Diabetes Obes Metab. 2011;13:972–81.
Boehm BO, Home PD, Behrend C, Kamp NM, Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med. 2002;19:393–9.
Chapman TM, Noble S, Goa KL. Insulin aspart: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2002;62:1945–81.
Chapman TM, Noble S, Goa KL. Spotlight on insulin aspart in type 1 and 2 diabetes mellitus. Treat Endocrinol. 2003;2:71–6.
Chen JW, Lauritzen T, Bojesen A, Christiansen JS. Multiple mealtime administration of biphasic insulin aspart 30 versus traditional basal-bolus human insulin treatment in patients with type 1 diabetes. Diabetes Obes Metab. 2006;8:682–9.
Ceriello A, Cremasco F, Romoli E, Rossi A, Gentilella R. Insulin lispro protamine suspension in the treatment of patients with type 1 and type 2 diabetes mellitus: a systematic review of published data. Expert Opin Pharmacother. 2012;13:255–81.
Clements MR, Tits J, Kinsley BT, Råstam J, Friberg HH, Ligthelm RJ. Improved glycaemic control of thrice-daily biphasic insulin aspart compared with twice-daily biphasic human insulin; a randomized, open-label trial in patients with type 1 or type 2 diabetes. Diabetes Obes Metab. 2008;10:229–37.
Davidson J, Vexiau P, Cucinotta D, Vaz J, Kawamori R. Biphasic insulin aspart 30: literature review of adverse events associated with treatment. Clin Ther. 2005;27(Suppl B):S75–88.
Mortensen H, Kocova M, Teng LY, Keiding J, Bruckner I, Philotheou A. Biphasic insulin aspart vs. human insulin in adolescents with type 1 diabetes on multiple daily insulin injections. Pediatr Diabetes. 2006;7:4–10.
Lemoine P. Biphasic Insulin Aspart 30 Treatment Option in Children and Adolescents. US Endocrinology,2006;2:DOI:10.17925/USE.2006.00.02.1e
Raja-Khan N, Warehime SS, Gabbay RA. Review of biphasic insulin aspart in the treatment of type 1 and 2 diabetes. Vasc Health Risk Manag. 2007;3:919–35.
Roach P, Bai S, Charbonnel B, Consoli A, Taboga C, Tiengo A, et al. Effects of multiple daily injection therapy with Humalog mixtures versus separately injected insulin lispro and NPH insulin in adults with type I diabetes mellitus. Clin Ther. 2004;26:502–10.
Rys P, Pankiewicz O, Łach K, Kwaskowski A, Skrzekowska-Baran I, Malecki MT. Efficacy and safety comparison of rapid-acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes Metab. 2011;37:190–200.
Valensi P. Biphasic insulin aspart 30/70 (BIAsp 30) in the treatment of type 1 and type 2 diabetes. Diabetes Metab Syndr Obes. 2009;2:61–71.
Velásquez-Mieyer PA, Neira CP. Biphasic insulin aspart 30 for the treatment of type 1 diabetes mellitus. Expert Opin Pharmacother. 2008;9:2377–82.
Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–99.
Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533.
Acknowledgments
The authors would like to acknowledge Jelka Zaletel, Tomáš Doležal, Bence Nagy, Tereza Šarić, Karel Rychna, Irina Ryzhenkova, Sanda Sandalj, and Zsofia Tarjanyi for their helpful comments. We would also like to thank Novo Nordisk for sponsoring this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The project was supported by an unrestricted scientific grant from Novo Nordisk. The author(s) declare that they have no competing interests. There is no specific organization that may in any way gain or lose financially from the publication of this manuscript.
Authors’ contributions
MJ, JP, MN, ER, and MC are the authors of the general approach. MJ, JP, ER, OAS, and MN participated in the systematic review. MJ designed the statistical approach. All authors participated in preparing, reading, and approving the final manuscript.
Additional files
Additional file 1:
Search strategies. (PDF 220 kb)
Additional file 2:
Model specification and JAGS codes. (PDF 343 kb)
Additional file 3:
Study selection process. (PDF 318 kb)
Additional file 4:
Studies characteristics. (PDF 332 kb)
Additional file 5:
Individual studies data. (PDF 466 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Czech, M., Rdzanek, E., Pawęska, J. et al. Drug-related risk of severe hypoglycaemia in observational studies: a systematic review and meta-analysis. BMC Endocr Disord 15, 57 (2015). https://doi.org/10.1186/s12902-015-0052-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-015-0052-z