Abstract
Background
Eosinophilic solid and cystic renal cell carcinoma (ESC-RCC) is a novel subtype of renal cell carcinoma characterized by its relatively low incidence and indolent behavior. We report a rare case of ESC-RCC concurrent with clear cell renal cell carcinoma (ccRCC) in a single kidney.
Case presentation
A 48-year-old male, was found to have a mixed echogenic mass in the left kidney during a physical examination. He has no history of hematuria and flank pain. An abdominal CT scan revealed a 3.0 * 1.9 * 2.5 cm3 mass with unclearly bordered at the lower pole of the left kidney. Abdominal MRI showed two nodules of different sizes in the left kidney, suggesting the possibility of a tumor. The patient underwent a subtotal nephrectomy, and the postoperative pathological results indicated ESC-RCC combined with ccRCC. The patient recovered well without tumor recurrence during the 12-month follow-up.
Conclusion
We reported a case of renal composite tumors, comprising the rare ESC-RCC and the more common ccRCC. Imaging combined with postoperative pathological examination is crucial for the definitive diagnosis of these rare tumors.
Similar content being viewed by others
Introduction
Eosinophilic solid and cystic renal cell carcinoma (ESC-RCC) is a novel subtype of RCC characterized by solid and cystic structures and eosinophilic tumor cells. It was first reported in 2016 and was officially listed as a new subtype of renal cell carcinoma in the 2022 World Health Organization (WHO) classification of renal tumors. ESC-RCC accounts for approximately 0.2% of all renal tumors and only less 70 cases were reported worldwide [1].
Renal composite tumors are defined as the presence of two distinct pathological components originating from the kidney, with a significant confluent zone and separated by normal renal parenchyma [2]. Renal composite tumor is a rare clinical phenomenon, with most clinical reports being of composite clear cell renal cell carcinoma (ccRCC) and papillary RCC, the two most common types of kidney tumors [3]. Since ccRCC has been separately classified as a distinct subtype of RCC, the composite ESC-RCC and ccRCC has not been reported yet. This study first reports a rare case of ESC-RCC co-occuring with ccRCC.
Case presentation
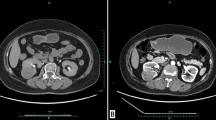
A 48-year-old male patient was found to have an abnormal mixed echo in the lower pole of the left kidney during a physical examination ultrasound (Fig. 1a-b). The patient exhibited no symptoms of hematuria, urinary frequency, or urgency, and no other relevant urinary symptoms were noted. He was generally healthy with no significant medical history and family history. The patient was admitted for further examination. The CT scan revealed two iso-dense nodules with irregular shapes and indistinct borders in the lower pole of the left kidney. Lesion 1, located in the anterior aspect of the left kidney, demonstrated significant enhancement in the arterial phase and washout in the delayed phase; Lesion 2, situated in the posterior aspect, exhibited mild and homogeneous enhancement in both the arterial and delayed phases (Fig. 1c-d). MRI imaging revealed two nodules in the lower pole of the left kidney, with inconsistent signal characteristics. Lesion 1, located anteriorly, displayed a slightly elevated T2 signal, while Lesion 2, situated posteriorly, showed hyperintensity. In-phase and Opposite-phase imaging indicated no evidence of fat components. Diffusion-weighted imaging revealed slightly high signal intensity in Lesion 1 and equal signal intensity in Lesion 2 (Fig. 1e-h). Based on the results of the imaging examination, a renal tumor is highly suspected. The patient then underwent a retroperitoneoscopic partial nephrectomy of the left kidney, the tumor and surrounding renal tissue were completely removed. The surgery removed a tumor with an overall volume of approximately 3.5 *3.0 *3.0 cm2, consisting of two masses of different colors and sizes, with indistinct boundaries between them at the connecting point (Fig. 2a). Morphological examination and immunohistochemical (IHC) analysis were conducted separately on the two lesions. Lesion 1, the size is approximately 1.2 * 0.5*0.2 cm3. H&E staining analysis reveals optically clear cytoplasm, prominent cell membranes, and a vascular network of tumor cells (Fig. 2b). IHC indicates the tumor cells were nuclear positive for CA9, CD10, vimentin, and EMA, which suggest the pathological diagnosis of ccRCC (Fig. 2c-f). Lesion 2 measures approximately 2.5*2.0 *1.3 cm3. H&E staining reveals that the tumor contains cystic areas, lined by eosinophilic cells with abundant cytoplasm (Fig. 3a). IHC indicated that the tumor cells were positive for CK20, CD10 and PAX8, but negative for CK7 and CA9, suggesting the diagnosis of ESC-RCC (Fig. 3b-f). Accordingly, the final diagnosis was renal composite ESC-RCC and ccRCC. The postoperative TNM staging of the patient was T1aN0M0, staging I, low risk (University of California, Los Angles Integrated Staging System). The patient recovered well after surgery, with no tumor recurrence or progression observed during the one-year follow-up.
Imaging findings of composite renal tumor. a-b: Ultrasound images demonstrated a solid echoic nodule (lesion 1) at the lower pole of the left kidney (fine arrow), with CDFI indicating rich blood vessels. Another cystic echoic nodule (lesion 2) is also seen (coarse arrow). c-d: Enhancement CT showed that the anterior lesion (lesion 1, fine arrow) exhibited significant enhancement during the arterial phase and washout during the delayed phase. The posterior lesion (lesion 2, coarse arrow) exhibited mild and homogeneous enhancement in both the arterial and delayed phases. e-h: MRI images revealed that Lesion 1 (fine arrow) displayed slight hyperintensity on T2WI, while Lesion 2 (coarse arrow) showed heterogeneous hyperintensity. Both lesions appeared hypointense on T1WI and in/out phase
The gross specimen and the pathological findings of ccRCC. a: The surgical excision of the gross specimen reveals two masses of different colors and sizes. Lesion 1 appears solid (fine arrow), while Lesion 2 exhibits multiple cystic areas (coarse arrow). The boundary between the two masses is very clear. b: H&E staining analysis reveals optically clear cytoplasm, prominent cell membranes, and a vascular network of tumor cells at 100× magnification. c-f: IHC indicates the tumor cells were positive for CA9 (c), CD10 (d), vimentin (e), and EMA (f) at the magnification of ×200, suggesting the pathological diagnosis of ccRCC
Pathological examination of ESC-RCC. a: H&E staining reveals that the tumor contains cystic areas, lined by eosinophilic cells with abundant cytoplasm. b-f: IHC indicates that the tumor cells were positive for CK20 (b), PAX8 (c) and CD10 (d), but negative for CA9 (e) and CK7 (f), suggesting the diagnosis of ESC-RCC
Discussion
ESC-RCC is an under-recognized, newly identified RCC subtype with unique clinical manifestations and molecular immune phenotypes. Before 2010, ESC-RCC was initially defined as RCC associated with tuberous sclerosis complex (TSC) because the majority of ESC-RCC cases had been identified in adult women with TSC [4]. However, since 2016, sporadic cases of ESC-RCC have been reported in patients without TSC [5]. Subsequent studies have shown that most cases of ESC-RCC do not occur concurrently with TSC; only approximately 10% of patients have both conditions simultaneously [6]. The tumor was previously classified as “unclassified renal tumors with oncocytic or eosinophilic granular cell morphology” or “unclassified renal cell carcinoma” until it was recognized as a rare novel type of RCC in 2022 [7, 8].
ESC-RCC tends to occur in middle-aged women, but it can also affect people of all ages and men. The male-to-female ratio of the tumor is 1:1.2–1.7, and the age range of onset is between 14 and 79 years old [9]. ESC progresses indolently, and many patients do not exhibit obvious clinical symptoms, often being discovered incidentally. Nearly 89% of patients receive a diagnosis in the early stages (stage T1) [5, 10]; however, scattered reports also exist of cases with distant metastases [7]. The incidence of ESC-RCC, renal collision, and composite tumors is relatively low. To the best of our knowledge, this is the first reported case of a composite ESC-RCC and ccRCC to date.
ESC-RCC is typically well-defined, unifocal and solitary tumor with small size. Grossly, the tumor appears yellow, gray, or brownish in color, is well delineated and encapsulated, with numerous cystic and solid areas internally [11]. Microscopically, the cystic wall is lined with hobnail-like tumor cell, while the solid areas are densely populated with tumor cells featuring eosinophilic cytoplasm; foam cells and lymphocytes are present in the stroma [12].
Imaging examinations, including MRI, hold diagnostic value for ESC-RCC. Yi et al. summarized the MRI characteristics and classified ESC-RCC into three types: (a) Type I shows a characteristic “lotus root-like” appearance; (b) Type II displays a “honeycomb-like” appearance with thick-walled cystic tumors and nodules on the walls; (c) Type III presents as a solid tumor without cystic, necrotic, or hemorrhagic areas [13]. Our case is consistent with the characteristics of type 2 ESC-RCC. Undoubtedly, pathology is the gold standard for diagnosing ESC-RCC. A typical immunophenotype for ESC-RCC is characterized by focal or diffuse CK20 positivity paired with CK7 negativity, distinguishing it from other eosinophilic tumors of the kidney [11]. Since mutations in TSC, including TSC1 and TSC2, are present in 85% of ESC-RCC cases, these mutations can be important molecular characteristics for ESC-RCC [11]. It is important to emphasize that TSC mutations cannot be used as a diagnostic criterion for ESC-RCC. Some patients exhibit copy number gains or losses and loss of heterozygosity in other chromosomes, which are related to the regulation of mTOR signaling pathway [11]. Mutations in TSC1 or TSC2 genes result in a loss of function of the hamartin/tuberin complex, leading to the constitutive activation of mTORC1. This indicates that mTOR inhibitors may be used to treat TSC-related tumors by inhibiting mTORC1, especially for ESC-RCC with TSC mutation. The limitation of this study is that we did not perform genetic testing; therefore, we do not know whether the patient has any relevant genetic mutations. The appearance of renal composite tumors is atypical, and they often share common driver genetic mutations. Nearly 95% of ccRCCs are associated with 3p deletions and somatic inactivating mutations of the von Hippel-Lindau gene [14]. However, solitary ESC-RCCs often exhibit TSC mutations. Our case may help promote in-depth research on the pathogenic genes and metastatic behavior of this type of malignancy.
The treatment principles for ESC-RCC refer to the standards for renal cancer treatment [15]. For localized kidney cancer, surgery is the mainstay treatment. Patients who cannot tolerate surgery may be closely observed or undergo ablation or stereotactic radiotherapy. For locally advanced kidney cancer, surgery or systemic drug therapy can be chosen based on the patient’s condition. For patients with newly diagnosed metastatic kidney cancer, systemic drug therapy, including targeted therapy and immunotherapy, can be chosen. According to the National Comprehensive Cancer Network Guidelines for Kidney Cancer, the preferred systemic treatment for non- ccRCC is tyrosine kinase inhibitors (TKIs) [16]. However, some studies indicated that for patients with distant metastasis, the efficacy of mTOR inhibitors is superior to that of TKI [17]. Other recommended regimens include TKI plus mTOR inhibitor, PD-1 inhibitors alone, or in combination with TKIs. However, there are few reports in the literature regarding its efficacy in ESC-RCC.
Due to the indolent biological characteristics of ESC-RCC, 90% of patients are diagnosed at either stage T1 or T2 [18]. These patients can be cured through partial nephrectomy. Approximately 5-10% of patients with ESC-RCC experience distant metastasis, commonly occurring in the lungs, liver, and bones [11, 13]. Existing literature reports that only one reported case of death among patients with distant metastasis [1, 13]. Therefore, the overall prognosis for ESC-RCC is generally favorable.
In conclusion, we reported a rare case of composite ESC-RCC and ccRCC who recovered well after partial nephrectomy. We detailed the imaging characteristics of this newly reported renal composite tumor and summarized the diagnosis and treatment of ESC-RCC. Our case not only enriches the data bank of renal collision composite tumors, but also deepens our understanding of this emerging type of RCC.
Data availability
The clinical data supporting the conclusions of this manuscript will be made available by the authors.
References
Pathak NJ, Singh AG, Jain PS, Soni SM, Ganpule AP, Sabnis RB, Desai MR. Eosinophilic solid and cystic renal cell carcinoma: a single Indian tertiary center experience of three cases of a newly described entity. Afr J Urol. 2022;28(1):48.
Sung CT, Shetty A, Menias CO, Houshyar R, Chatterjee S, Lee TK, Tung P, Helmy M, Lall C. Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol (New York). 2017;42(12):2909–26.
Lall C, Houshyar R, Landman J, Verma S, Goyenechea M, Bhargava P, Pulford C, Okhunov Z, Siaghani PJ, Menias C. Renal collision and composite tumors: imaging and pathophysiology. Urology. 2015;86(6):1159–64.
Schreiner A, Daneshmand S, Bayne A, Countryman G, Corless CL, Troxell ML. Distinctive morphology of renal cell carcinomas in tuberous sclerosis. Int J Surg Pathol. 2010;18(5):409–18.
Trpkov K, Hes O, Bonert M, Lopez JI, Bonsib SM, Nesi G, Comperat E, Sibony M, Berney DM, Martinek P, et al. Eosinophilic, solid, and cystic renal cell carcinoma: clinicopathologic study of 16 unique, sporadic neoplasms occurring in women. Am J Surg Pathol. 2016;40(1):60–71.
Parilla M, Kadri S, Patil SA, Ritterhouse L, Segal J, Henriksen KJ, Antic T. Are sporadic eosinophilic solid and cystic renal cell Carcinomas characterized by somatic tuberous sclerosis gene mutations? Am J Surg Pathol. 2018;42(7):911–7.
Trpkov K, Hes O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology. 2019;74(1):31–59.
Lobo J, Ohashi R, Amin MB, Berney DM, Compérat EM, Cree IA, Gill AJ, Hartmann A, Menon S, Netto GJ, et al. WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology. 2022;81(4):426–38.
Yin J, Zenezan D, Doan KD, Nobee A, Wei S, Mollaee M, Proca DM. Radiologic and clinicopathologic features of eosinophilic solid and cystic renal cell carcinoma: report of two cases and review of literature. Int J Clin Exp Pathol. 2023;16(10):303–8.
Kamboj M, Gupta G, Pasricha S, Rawal S, Sharma A, Durga G, Mehta A. Eosinophilic solid and cystic renal cell carcinoma: a rare under-recognized indolent entity. Indian J Pathol Microbiol. 2021;64(4):799–801.
Siadat F, Trpkov K. ESC, ALK, HOT and LOT: three letter acronyms of emerging renal entities knocking on the Door of the WHO classification. Cancers. 2020;12(1).
He X, Chen Y, Tang H, Xu Y, Zhu X, Wang C, Chen Q, Guo D. Eosinophilic solid and cystic renal cell carcinoma with TSC2 mutation: a case report and literature review. Diagn Pathol. 2023;18(1):53.
Yi M, Wang S, Wang P, Wang Z, Lu J, Liu Y. Eosinophilic solid and cystic renal cell carcinoma: a review of literature focused on radiological findings and differential diagnosis. Abdom Radiol (New York). 2023;48(1):350–7.
Sükösd F, Kuroda N, Beothe T, Kaur AP, Kovacs G. Deletion of chromosome 3p14.2-p25 involving the VHL and FHIT genes in conventional renal cell carcinoma. Cancer Res. 2003;63(2):455–7.
Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, Derweesh IH, et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Cancer Network: JNCCN. 2022;20(1):71–90.
Motzer RJ, Jonasch E, Agarwal N, Alva A, Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, et al. NCCN Guidelines® insights: kidney Cancer, Version 2.2024. J Natl Compr Cancer Network: JNCCN. 2024;22(1):4–16.
Palsgrove DN, Li Y, Pratilas CA, Lin MT, Pallavajjalla A, Gocke C, De Marzo AM, Matoso A, Netto GJ, Epstein JI, et al. Eosinophilic solid and cystic (ESC) renal cell Carcinomas Harbor TSC mutations: Molecular Analysis supports an Expanding Clinicopathologic Spectrum. Am J Surg Pathol. 2018;42(9):1166–81.
Yunker A, Holder L, Nething J. Newly Described Eosinophilic, Solid and Cystic Renal Cell Carcinoma: A Case Report and Review of the Literature. 2020.
Funding
This study was supported by Innovative Research Program of Xiangyang No.1 People’s Hospital (Grants number: XYY2023SD06 and XYY2023QB07).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.Z.; data curation and writing, writing—review and editing, X.Z, L.L, M.Y, L.W and D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics and Scientific Committee of Hubei University of Medicine with approval number XYY2021002.
Consent for publication
Consent was signed by the patient for all the images, other personal and clinical details. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Li, L., Wang, L. et al. Composite eosinophilic solid and cystic renal cell carcinoma and clear cell renal cell carcinoma: a rare case report and literature review. BMC Urol 24, 160 (2024). https://doi.org/10.1186/s12894-024-01542-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-024-01542-4