Abstract
Background
The long-term outcomes for patients after coronary artery bypass grafting (CABG) have been received more and more concern. The existing prediction models are mostly focused on in-hospital operative mortality after CABG, but there is still little research on long-term mortality prediction model for patients after CABG.
Objective
To develop and validate a novel nomogram for predicting 3-year mortality in critically ill patients after CABG.
Methods
Data for developing novel predictive model were extracted from Medical Information Mart for Intensive cart III (MIMIC-III), of which 2929 critically ill patients who underwent CABG at the first admission were enrolled.
Results
A novel prognostic nomogram for 3-year mortality was constructed with the seven independent prognostic factors, including age, congestive heart failure, white blood cell, creatinine, SpO2, anion gap, and continuous renal replacement treatment derived from the multivariable logistic regression. The nomogram indicated accurate discrimination in primary (AUC: 0.81) and validation cohort (AUC: 0.802), which were better than traditional severity scores. And good consistency between the predictive and observed outcome was showed by the calibration curve for 3-year mortality. The decision curve analysis also showed higher clinical net benefit than traditional severity scores.
Conclusion
The novel nomogram had well performance to predict 3-year mortality in critically ill patients after CABG. The prediction model provided valuable information for treatment strategy and postdischarge management, which may be helpful in improving the long-term prognosis in critically ill patients after CABG.
Similar content being viewed by others
Introduction
Coronary artery bypass grafting (CABG) is the most frequently performed operation in cardiac surgery [1], CABG has been the standard therapy for patients with left main or three-vessel coronary artery disease [2]. With the development of surgery technology and improvement of nursing quality, the operative mortality, operative complication, and in-hospital mortality have decreased significantly [1, 3, 4]. As many adverse clinical events occur after discharge, the long-term outcomes for patients after CABG have been received more and more concern [4, 5]. Accurate assessment of long-term mortality risk in patients after CABG is very important for clinicians to make individualized treatment and management strategy, this will bring more benefit for patients after CABG, thereby reducing its mortality.
In order to assess cardiac operative risk, a variety of risk score systems are widely used, in which European system for cardiac operative risk evaluation (EuroSCORE) and Society of Thoracic Surgeons (STS) are the most widely used [6, 7]. But long-term mortality prediction model for patients after CABG is still lack in clinic. It is really important to find a risk prediction model of long-term mortality, thereby improving long-term outcomes in patients after CABG. Nomogram is a more simple and convenient method for predicting clinical outcomes by giving a score to potential risk factors [8]. Recently, nomogram has been increasingly applied in evaluating prognosis of various diseases, such as tumor [9], myocardial infarction [10], acute renal failure [11], acute pancreatitis [12] and so on.
In the study, based on a publicly Medical Information Mart for Intensive cart III (MIMIC-III), we firstly identify the risk factors of 3-year mortality in critically ill patients after CABG, and then further develop a prognostic nomogram for predicting 3-year mortality in these patients. Finally, the accuracy of prognosis nomogram is verified by validation cohort.
Methods
Database
We developed the prediction model by extracting data from MIMIC-III, v1.4 [13], which is an openly available database contains information of 46520 critically ill patients who received treatment in intensive care unit (ICU) of Beth Israel Deaconess Medical Center from 2001 to 2012. After successful application (certification number: 37650993) and approved by the institutional review boards (IRB) of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC), we were granted access to the database and utilized the data. Because unidentified health data of patients was used, informed consent was waived by both IRB of MIT and BIDMC. And all procedures in our study were in accordance with the corresponding guidelines.
Participant selection and data extraction
We included critically ill patients who underwent CABG at this admission according to ICD-9 code. We excluded the patients as follows: (1) multiple admission; (2) inappropriate age (< 18 or > 89 years old); (3) length of stay in ICU < 24 h; (4) follow-up time < 3 years. The primary endpoint is 3-year mortality in this study.
All relevant clinical data was extracted within the first 24 h after ICU admission using Structured Query Language (SQL). For the model development, we retrospectively collected the following data: (1) Demographic data: age and gender; (2) Comorbidities: diabetes, previous myocardial infarction, previous stroke, congestive heart failure (CHF) and renal failure; (3) 24 h vital signs: systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), respiratory rate and mean blood pressure (MBP); (4) Laboratory parameters: hemoglobin, white blood cell (WBC), lactate, anion gap, phosphoric acid, activated partial thromboplastin time (APTT), blood urea nitrogen (BUN), alanine aminotransferase (ALT), glutamic oxalacetic transaminase (AST), phosphoric acid (PA), creatinine, platelet, potassium, and sodium, PCO2, PO2, SpO2, and pH; (5) Management in hospital: mechanical ventilation, continuous renal replacement treatment (CRRT), and vasopressor use; (6) Scoring systems: the Oxford Acute Severity of Illness Score (OASIS), the Sequential Organ Failure Assessment (SOFA), and the Simplified Acute Physiology Score II (SAPS II), which were calculated within the first 24 h after ICU admission.
Missing data management
For the model development, we excluded lactate, AST, ALT and PA because of the portion of the missing value > 20% (Additional file 1). For other variables with missing value < 20%, missing values of variables were filled by a multiple imputation method, which could reduce the bias caused by missing values [14].
Statistical analysis
Continuous data were reported as median and inter quartile range and compared by Kruskal–Wallis H test. Categorical data were presented as count and percentage and compared using Pearson’s χ2 test or Fisher’s exact test as appropriate. The objective of this study was to develop an easy-use prediction model for 3-year mortality in critically ill patients. For the model development, the univariate and multivariable logistic regression were implemented to screen independent predictors in the primary cohort. The variables with P < 0.05 in univariate logistic regression were selected into the following analysis. For identifying the final prediction model, a backward step-selection method with the Akaike information criterion (AIC) was used to select predictors in multivariable logistic regression. The “mice” package was used to multiple imputation for variables with less than 20% missing values [14, 15]. The “rms” package was applied for plotting nomogram and calibration curve. The Receiver Operating Characteristic (ROC) curve was performed to assess discrimination ability of the nomogram for the 3-year mortality using “pROC” package [16]. DeLong’s non-parametric approach was implemented to compare differences of the area under the curve (AUC) between model and other traditional scoring systems [17]. Calibration slope and the Brier value were performed to evaluate the calibration of the model [18]. Bootstrapping with 1000 resamples was used for calibration analysis. The decision curve analysis (DCA) was used to evaluate the clinical practicability of the nomogram by quantifying the standardized net benefits at various threshold probabilities using the “rmda” package [19]. A two-sided P value of < 0.05 was considered statistically significant. We performed all statistical analyzes using R software (version 4.0.3).
Results
Baseline characteristics of the primary and validation cohort
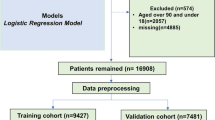
A total of 2929 patients who underwent CABG were included into the final cohort after the screening by the inclusion and exclusion criteria (Fig. 1). We assigned 2050 patients (265 deaths, 3-year mortality rate 12.9%) to the primary cohort and 879 patients (113 deaths, 3-year mortality rate 12.9%) to the validation cohort. All baseline characteristics of the primary and validation cohort are shown in Table 1. There were no obviously statistical differences between the primary and validation cohort. For the following model development, we excluded lactate, AST, ALT, and PA because of the portion of the missing value > 20% (Additional file 1).
Model development in the primary cohort
Baseline demographics, comorbidities, vital signs, laboratory parameters, and in-hospital management for the prediction of 3-year mortality were examined by the univariate logistic regression (Additional file 2). The age, gender, CHF, renal failure, mean DBP, hemoglobin, WBC, APTT, creatinine, BUN, SpO2, anion gap, sodium, and CRRT were potential prognostic factors of 3-year mortality (P < 0.05) (Table 2). All the above predictors were entered into the multivariable logistic regression. The age, CHF, WBC, creatinine, SpO2, anion gap, and CRRT were selected as the independent predictors for 3-year mortality in the final prediction model (P < 0.05 of each predictor) (Table 2). The VIF was calculated and there was no significant multicollinearity in the model (VIF < 2). Furthermore, the correlation between continuous variables and outcome was visualized by loess curves in Additional file 3. A prognostic nomogram for 3-year mortality was plotted with the seven prognostic factors derived from the the multivariable logistic regression (Fig. 2).
Each prognostic factor was assigned various weighted score in the nomogram. The values of age, WBC, creatinine, SpO2, and anion gap ranged from 25 to 90, 0 to 55, 0 to 16, 45 to 100, and 4 to 30, respectively. The highest total score was 240 points, and the scale of the 3-year mortality probability ranged from 0.1 to 0.9. If a patient who underwent CABG had an age of 68 years old, HF, a WBC value of 7.3*109/L, a creatinine value of 7.6 U/L, a SpO2 value of 78%, an AG value of 20 mmol/L, and without underwent CRRT, the 3-year mortality probability was 74.3%.
Model performance
The ROC curves indicated that the nomogram (AUC: 0.810) had the good predictive capacity, which was greater than SAPSII (AUC: 0.690), SOFA (AUC: 0.639), and OASIS (AUC: 0.601) in the primary cohort (All P value < 0.001) (Fig. 3A). Meanwhile, the similar predictive performance (AUC: 0.802) was found in the validation cohort, which were greater than traditional scoring systems (SAPSII, AUC: 0.685; SOFA, AUC: 0.583; OASIS, AUC: 0.583; All P value < 0.001) (Fig. 3B). The calibration plot indicated the nomogram had adequate fit for 3-year mortality in primary (Brier score: 0.090, calibration slope: 1.000) and validation cohort (Brier score: 0.090, calibration slope: 0.943), respectively (Fig. 4A, B). The decision curve analysis (DCA) showed that this nomogram had a large threshold probability range than the SAPSII, SOFA, and OASIS. And, at the same threshold probability, this nomogram showed higher net benefit than SAPSII-, SOFA-, and OASIS-assisted decisions in primary and validation cohort, respectively (Fig. 5A, B).
Discussion
The present study developed and internally validated a novel prognosis nomogram model based on MIMIC-III database to predict 3-year mortality in critically ill patients after CABG. Seven risk factors for 3-year mortality of these patients were identified by logistic regression method, including age, CHF, WBC, Creatinine, SpO2, anion gap and CRRT. The prediction ability of the novel prognosis nomogram was evaluated by AUC, calibration curve analysis and decision curve analysis in development and validation cohort, and the results found the novel prognosis nomogram model had fine stability and precise prediction ability, which was significantly superior to SOFA, OASIS and SAPSII.
A large difference is observed in severity, progress and prognosis of disease on patients after CABG. Many predictive score systems for the risk of cardiac operative have been created over decades, such as EuroSCORE [6], STS [7] and EuroSCORE II [20]. These predictive score systems not only need more information, but also is complicated and difficult to acquired rapidly. Meanwhile, these score systems are not designed for predicting long-term mortality risk in patients after CABG. The population of MIMIC III database was from critically ill patients in ICU. It is widely known that the severity score systems including SOFA, OASIS, SAPSII are typically used for risk stratification of critically ill patients, and have good prediction ability for predicting the outcome of patients with critically ill [21,22,23]. In the study, the prediction ability of novel nomogram was better than the severity score systems (SOFA, OASIS, SAPSII) for predicting 3-years mortality in critically ill patients after CABG. The novel nomogram model contained only seven accessible factors but had better prediction ability and calibration in the present study, so the novel nomogram may be worth generalizing extensively in clinical application.
Recent studies on prediction model for prognosis of patients after CABG has been developed. The research developed that a model of predicting hospital readmission in patients after CABG, 30-days all cause readmission can be predicted by the model [24]. Some studies developed predictive model of renal disease among patients after CABG. A nomogram model based on 7 predictors provided reliable prediction ability of acute kidney injury in heart failure patients after CABG [25]. The ACHE score was end-stage renal disease prediction model follow CABG with a long-term follow-up, which had advantages in simplicity and preciseness [26]. In order to improve the nursing quality and make individualized clinical decision, several models of predicting ICU length of stay after CABG have been developed [27, 28]. A study built up machine learning models to predict 30-day mortality and three complications in critically ill patients after open-heart surgery (including CABG) from MIMIC III database [29]. The machine learning model predicted the short-term outcome by window 10 software with more than 30 risk factors. However, our nomogram model directly predicted 3-year mortality in critically ill patients after CABG by seven risk factors. The machine learning model and our nomogram model were from MIMIC III database, but their predictors and predicting outcome were different. Previous study established long-term survival prediction model after CABG in 31–90 days, 91–365 days, 1–3 years and > 3 years, respectively, these four times intervals model shared thirteen common risk factors [30]. However, our nomogram model based on critically ill patients to predict 3-year mortality after CABG, which was different from that long-term survival prediction model. The long-term survival prediction model at 4 distinct time intervals suggested the effect of thirteen risk factors on mortality after CABG may be different at different points in time. But the long-term mortality risk should be rapidly assessed in order to early risk stratification in clinical practice, which may provide clinician important clues for individualized treatment strategies to improve prognosis in critically ill patients after CABG. In fact, our novel nomogram model has the advantages of convenience, exactness and high efficiency, can be a satisfactory model for predicting 3-year mortality in critically ill patients after CABG.
The predictors of novel nomogram model are common and easily accessible clinical parameters, and associated with prognosis after CABG. Age and CHF were acknowledged as the risk factors influencing the prognosis of patients after CABG [6, 7, 20, 31]. WBC play an essential role in cardiovascular disease, some studies have found elevated WBC was associated with cardiovascular complications and mortality in patients after CABG [29, 32]. Creatinine increased during perioperative stage served as independent risk factor for mortality in patients after CABG, and some risk assessment models after cardiac surgery included creatinine [6, 20, 33, 34]. SpO2 as an earlier warning index of hypoxemia is very important prognostic factor for critically ill patients with cardiovascular disease [35, 36]. Recent studies have found anion gap is correlated with cardiovascular disease and a useful indicator in assessing risk stratification in critically ill patients [37,38,39]. CRRT was commonly used in critically ill patients in ICU, critically ill patients requiring CRRT had a greater risk of hospital and post-discharge mortality in ICU [40, 41]. Above all, the seven risk factors of our novel nomogram are generally available and widely supported in clinical application.
There still exist some limitations in the present study. The study was based on MIMIC III database of critically ill patients in a single center, and internal verification was carried out to validate the performance of nomogram model, it may be not suitable to generalize the nomogram model based on critically ill patients after CABG to all patients after CABG, and could not be considered as a preoperative assessment of the patients. But the nomogram model will be more helpful for the ICU team to assess the prognosis for critically ill patients after CABG. With the development of medical technology and the improvement of the quality of nursing, the mortality of patients after CABG has decreased in recent years, but all patients underwent CABG from 2001 to 2012 in the study, some new potential confounding factors may affect the performance of prognosis nomogram. In addition, some patients after CABG were not enrolled due to incomplete data and some importance data was missing that might affect the mortality of patients after CABG in the study, which may cause bias of the results.
In conclusion, the novel developed nomogram showed well performance as a prediction model of 3-years mortality in critically ill patients after CABG, and consisted of simple seven clinical variables, which might be widely applied in risk stratification of long-term mortality for these patients in ICU. The novel nomogram gave valuable information to help clinician for decision making in treatment and management of critically ill patients after CABG, and these patients would benefit from strengthen postdischarge management and close follow-up.
Availability of data and material
All data presented in the study are available in the MIMIC III database.
References
ElBardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81.
Serruys PW, Morice M-C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72.
Hawkes AL, Nowak M, Bidstrup B, Speare R. Outcomes of coronary artery bypass graft surgery. Vasc Health Risk Manag. 2006;2:477–84.
Baillot RG, Joanisse DR, Stevens LM, Doyle DP, Dionne B, Lellouche F. Recent evolution in demographic and clinical characteristics and in-hospital morbidity in patients undergoing coronary surgery. Can J Surg. 2009;52:394–400.
Shahian DM, O’Brien SM, Sheng S, Grover FL, Mayer JE, Jacobs JP, et al. Predictors of long-term survival after coronary artery bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study). Circulation. 2012;125:1491–500.
Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13.
Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1–coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2-22.
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–80.
Gilbride L, Siker M, Bovi J, Gore E, Schultz C, Hall WA. Current predictive indices and nomograms to enable personalization of radiation therapy for patients with secondary malignant neoplasms of the central nervous system: a review. Neurosurgery. 2018;82:595–603.
Guo Q, Wu M, Li H, Ouyang H, Sun R, Wang J, et al. Development and validation of a prognostic nomogram for myocardial infarction patients in intensive care units: a retrospective cohort study. BMJ Open. 2020;10:e040291.
Xu J, Weng J, Yang J, Shi X, Hou R, Zhou X, et al. Development and validation of a nomogram to predict the mortality risk in elderly patients with ARF. PeerJ. 2021;9:e11016.
Jiang X, Su Z, Wang Y, Deng Y, Zhao W, Jiang K, et al. Prognostic nomogram for acute pancreatitis patients: an analysis of publicly electronic healthcare records in intensive care unit. J Crit Care. 2019;50:213–20.
Johnson AEW, Pollard TJ, Shen L, Lehman L-WH, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77.
Van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Sun D, Zhu Y, Zhao H, Bian T, Li T, Liu K, et al. Loss of ARID1A expression promotes lung adenocarcinoma metastasis and predicts a poor prognosis. Cell Oncol (Dordr). 2021;44:1–16.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74.
Sullivan PG, Wallach JD, Ioannidis JPA. Meta-analysis comparing established risk prediction models (EuroSCORE II, STS Score, and ACEF Score) for perioperative mortality during cardiac surgery. Am J Cardiol. 2016;118:1574–82.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
Carvounis CP, Feinfeld DA. A simple estimate of the effect of the serum albumin level on the anion Gap. Am J Nephrol. 2000;20:369–72.
Jia L, Hao L, Li X, Jia R, Zhang H-L. Comparing the predictive values of five scales for 4-year all-cause mortality in critically ill elderly patients with sepsis. Ann Palliat Med. 2021;10:2387–97.
Benuzillo J, Caine W, Evans RS, Roberts C, Lappe D, Doty J. Predicting readmission risk shortly after admission for CABG surgery. J Card Surg. 2018;33:163–70.
Lin H, Hou J, Tang H, Chen K, Sun H, Zheng Z, et al. A novel nomogram to predict perioperative acute kidney injury following isolated coronary artery bypass grafting surgery with impaired left ventricular ejection fraction. BMC Cardiovasc Disord. 2020;20:517.
Lee Y, Park J, Jang M-J, Moon HR, Kim DK, Oh K-H, et al. Development of model to predict end-stage renal disease after coronary artery bypass grafting: The ACHE score. Medicine (Baltimore). 2019;98:e15789.
Atashi A, Verburg IW, Karim H, Miri M, Abu-Hanna A, de Jonge E, et al. Models to predict length of stay in the Intensive Care Unit after coronary artery bypass grafting: a systematic review. J Cardiovasc Surg (Torino). 2018;59:471–82.
Rotar EP, Beller JP, Smolkin ME, Chancellor WZ, Ailawadi G, Yarboro LT, et al. Prediction of prolonged intensive care unit length of stay following cardiac surgery. Semin Thorac Cardiovasc Surg. 2021. https://doi.org/10.1053/j.semtcvs.2021.02.021.
Zhong Z, Yuan X, Liu S, Yang Y, Liu F. Machine learning prediction models for prognosis of critically ill patients after open-heart surgery. Sci Rep. 2021;11:3384.
Karim MN, Reid CM, Huq M, Brilleman SL, Cochrane A, Tran L, et al. Predicting long-term survival after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2018;26:257–63.
Petrie MC, Jhund PS, She L, Adlbrecht C, Doenst T, Panza JA, et al. Ten-year outcomes after coronary artery bypass grafting according to age in patients with heart failure and left ventricular systolic dysfunction: an analysis of the extended follow-up of the STICH Trial (surgical treatment for ischemic heart failure). Circulation. 2016;134:1314–24.
Aizenshtein A, Kachel E, Liza GR, Hijazi B, Blum A. Effects of preoperative WBC count on post-CABG surgery clinical outcome. South Med J. 2020;113:305–10.
Miceli A, Bruno VD, Capoun R, Romeo F, Angelini GD, Caputo M. Occult renal dysfunction: a mortality and morbidity risk factor in coronary artery bypass grafting surgery. J Thorac Cardiovasc Surg. 2011;141:771–6.
Oliveira MABD, Santos CAD, Brandi AC, Dotta AH, Botelho PHH, Godoy MFD, et al. Effect of preoperative creatinine levels on mortality after coronary artery bypass grafting surgery: an observational study. Braz J Cardiovasc Surg. 2019;34:149–55.
James SK, Erlinge D, Herlitz J, Alfredsson J, Koul S, Fröbert O, et al. Effect of oxygen therapy on cardiovascular outcomes in relation to baseline oxygen saturation. JACC Cardiovasc Interv. 2020;13:502–13.
Yu Y, Wang J, Wang Q, Wang J, Min J, Wang S, et al. Admission oxygen saturation and all-cause in-hospital mortality in acute myocardial infarction patients: data from the MIMIC-III database. Ann Transl Med. 2020;8:1371.
Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–16.
Yang S-W, Zhou Y-J, Zhao Y-X, Liu Y-Y, Tian X-F, Wang Z-J, et al. The serum anion gap is associated with disease severity and all-cause mortality in coronary artery disease. J Geriatr Cardiol. 2017;14:392–400.
McDonald CI, Brodie D, Schmidt M, Hay K, Shekar K. Elevated venous to arterial carbon dioxide gap and anion gap are associated with poor outcome in cardiogenic shock requiring extracorporeal membrane oxygenation support. ASAIO J. 2021;67:263–9.
Nash DM, Przech S, Wald R, O’Reilly D. Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care. 2017;41:138–44.
Keleshian V, Kashani KB, Kompotiatis P, Barsness GW, Jentzer JC. Short, and long-term mortality among cardiac intensive care unit patients started on continuous renal replacement therapy. J Crit Care. 2020;55:64–72.
Acknowledgements
The authors would like to thank the developers and maintainers associated with the MIMIC-III database.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZH and SY participated in the design of the study, acquired data, performed the statistical analysis and drafted the manuscript. TW participated in its design and helped to draft the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We were granted access to the database and utilized the data after completing and passing Examination for Protecting Human Research Participants and applied for data access (ID: 37650993). The project was approved by the institutional review boards (IRB) of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC). As this study used the anonymous data relating to patients, the requirement for signed informed consent was waived by both IRB of MIT and BIDMC.
Consent for publication
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The histogram and pattern of missing data.
Additional file 2.
Univariate analyses for the relationship between the candidate risk factors and 3-year mortality in the primary cohort.
Additional file 3.
Loess curves for the correlation between continuous variables and 3-year mortality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Tian, W. & Sun, Y. A novel nomogram for predicting 3-year mortality in critically ill patients after coronary artery bypass grafting. BMC Surg 21, 407 (2021). https://doi.org/10.1186/s12893-021-01408-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-021-01408-8