Abstract
Backgrounds
Increased risk of in-hospital mortality is critical to guide medical decisions and it played a central role in intensive care unit (ICU) with high risk of in-hospital mortality after primary percutaneous coronary intervention (PCI). At present,most predicting tools for in-hospital mortality after PCI were based on the results of coronary angiography, echocardiography, and laboratory results which are difficult to obtain at admission. The difficulty of using these tools limit their clinical application. This study aimed to develop a clinical prognostic nomogram to predict the in-hospital mortality of patients in ICU after PCI.
Methods
We extracted data from a public database named the Medical Information Mart for Intensive Care (MIMIC III). Adult patients with coronary artery stent insertion were included. They were divided into two groups according to the primary outcome (death in hospital or survive). All patients were randomly divided into training set and validation set randomly at a ratio of 6:4. Least absolute shrinkage and selection operator (LASSO) regression was performed in the training set to select optimal variables to predict the in-hospital mortality of patients in ICU after PCI. The multivariate logistical analysis was performed to develop a nomogram. Finally, the predictive efficiency of the nomogram was assessed by area under the receiver operating characteristic curve (AUROC),integrated discrimination improvement (IDI), and net reclassification improvement (NRI), and clinical net benefit was assessed by Decision curve analysis (DCA).
Results
A total of 2160 patients were recruited in this study. By using LASSO, 17 variables were finally included. We used multivariate logistic regression to construct a prediction model which was presented in the form of a nomogram. The calibration plot of the nomogram revealed good fit in the training set and validation set. Compared with the sequential organ failure assessment (SOFA) and scale for the assessment of positive symptoms II (SAPS II) scores, the nomogram exhibited better AUROC of 0.907 (95% confidence interval [CI] was 0.880-0.933, p < 0.001) and 0.901 (95% CI was 0.865-0.936, P < 0.001) in the training set and validation set, respectively. In addition, DCA of the nomogram showed that it could achieve good net benefit in the clinic.
Conclusions
A new nomogram was constructed, and it presented excellent performance in predicting in-hospital mortality of patients in ICU after PCI.
Similar content being viewed by others
Background
Coronary heart disease (CHD) has a very high morbidity and mortality rate worldwide [1,2,3]. Primary percutaneous coronary intervention (PCI) is one of the most important treatment strategies for coronary artery disease [4,5,6,7]. .However, many patients still have poor outcomes after PCI, including acute heart failure, stroke, and even death [8]. Early detection of high-risk patients is crucial and rapid intervention could impact the outcome. Therefore, we need to identify some indicators or models to assess the risk of death of patients after PCI. For clinicians, this model should be simple and accurate, as it is more suitable for clinical practice.
At present, there are many traditional tools, such as the Framingham risk score and Thrombolysis in Myocardial Infarction (TIMI) risk score, used to predict the prognosis of CHD patients [9, 10]. The significance of these evaluation tools is to enable clinicians to identify high-risk patients as soon as possible. Focusing on high-risk patients can make the work of clinicians more targeted. In acute myocardial infarction (AMI) patients, many new models could be used to predict the risk of death based on clinical indicators and coronary angiography [11,12,13]. With the development of machine learning, several studies have constructed nomograms for ST-segment elevation myocardial infarction patients to predict the risk of death [14, 15]. However, most of these studies focus on patients with acute coronary syndrome (ACS) and were based on the results of coronary angiography, echocardiography, and laboratory results, which are difficult to obtain at admission. The difficulty of using these models limits their clinical application. A study indicates that the SOFA and SAPS II can predictors of survival following acute myocardial infarction-related refractory cardiogenic shock, but did not perform well [16]. Although the SOFA and SAPS II scoring system are widely used in intensive care unit (ICU), both of them reflect the patient’s general condition and not specific to patients after PCI and has limitations in evaluating the risk of in-hospital death of patients after PCI.
Simple clinical indicators such as vital signs, white blood cell (WBC), hemoglobin, platelets, potassium in serum, sodium in serum, prothrombin time (PT) and MB isoenzyme of creatine kinase (CKMB) are easy to obtain. They did not need complex and expensive equipment and these results are objective. We were committed to using simple clinical indicators to develop a model for predicting the risk of in-hospital death in patients in ICU after PCI. To the best of our knowledge, no previous study had investigated the ability to develop simple nomogram for prediction of in hospital mortality of patients in ICU after PCI.
Material and methods
Data source
All the patient data used in our study came from an online international database—Medical Information Mart for Intensive Care III (MIMIC III) (version 1.4). MIMIC III is a large, single-center database comprising information related to patients admitted to critical care units of Beth Israel Deaconess Medical Center. It included patients who were admitted to the ICU between 2001 and 2012. This database includes a total of 53,423 different admission data points. MIMIC III contains a wealth of clinical information, including vital signs, demographic information, laboratory results, treatment records and nursing records. The Institutional Review Boards at both Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center approved the use of the data for research. The database can be accessed by certified researchers, so no additional informed consent of the patient and ethical approval are required [17]. The certified researcher of this study is Miao Yuan (no. 7382002).
Study population and data extraction
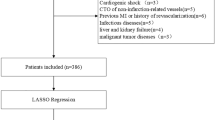
Adult patients with the diagnosis of insertion of coronary artery stents based on the International Classification of Diseases (ICD)-9 code were selected (ICD-9 codes were 3606 and 3607). We excluded patients who were younger than 18 years old. If a patient had information from more than one admission, we only considered the first ICU information.
We used Structured Query Language and PostgreSQL software (version 10.0) to obtain data from MIMIC III. These data included demographic characteristics (including age and sex), SOFA score, Elixhauser Comorbidity Index, SAPS II score, body mass index (BMI), mean value of vital signs during the first 24 hours of ICU stay (including heart rate, systolic pressure, diastolic pressure, and respiration rate), treatment (ventilation treatment type and the use of vasoactive drugs), laboratory results (including hemoglobin, platelets, sodium, potassium, PT, WBC, CKMB, anion gap, bicarbonate, chloride, troponin T (TnT), troponin I (TnI), low-density lipoprotein (LDL), triglyceride (TG), and lactate (Lac)), the type of coronary artery stent (drug eluting stent or non-drug eluting stent), with or without the diagnosis of AMI. We extracted vital signs and laboratory results within 24 hours after admission, and if patients had multiple results in the first 24 hours, we took their average.
Elixhauser Comorbidity Index is a method of categorizing comorbidities of patients based on the ICD diagnosis codes found in administrative data, such as hospital abstracts data. Each comorbidity category is dichotomous (the comorbidity either present or it is not). The Index can be used to predict hospital resource use and in-hospital mortality [18].
In our study, the endpoint was died in hospital. All variables with more than 20% missing values were deleted (including BMI, TnT, TnI, LDL, TG, and Lac). If the missing value of a continuous variable was less than 10%, the missing value was filled by multiple interpolation.
Statistical analysis
We used the Kolmogorov-Smirnov test to test the distribution of variables. Continuous normally distributed variables were represented by the mean ± standard deviation, while continuous nonnormal distribution were represented by the median (interquartile range). Categorical variables were expressed as numbers (percentages). If the variable conformed to normal distributions, the baseline characteristics among the two groups (survive group and died in hospital group) were compared using the unpaired student’s t test, and continuous variables with nonnormal distributions were compared by the Mann-Whitney U test. The differences of the ratio of survive between groups of categorical variables were evaluated by the chi-square test. When comparing the categorical variables between groups, if the theoretical frequency was less than 5, Fisher’s exact test was used for analysis.
We used without replacement sampling (the sample function in R) to randomly select 60% of all patients as training set and the last 40% as validation set. The data of the training set were used to build the prediction model, and the validation set was used to verify the model.
We used the least absolute shrinkage and selection operator (LASSO) to select the optimal predictive risk factors for in-hospital mortality after PCI. LASSO implements the selection of variables by applying the regularization process. A penalty term is added to the coefficient of the model on the ordinary linear square, and a more refined model is obtained by constructing the penalty function [19].We assigned all covariable to different penalties. The penalty term λ of each covariable is optimized by 10 times cross-validation. The regression coefficient of the covariable with less correlation shrinks to zero by adding the penalty term λ. The covariates in which the penalty term λ is not zero are further incorporated into the construction of the prediction model.
The factors selected by LASSO regression were incorporated into the prediction model. The 95% confidence interval of the odds ratio (OR) value and the p value were used to evaluate the variables. The level of statistical significance was two-sided. We used multivariable logistic regression to construct a prediction model. The model was provided in the form of a nomogram.
The nomogram was compared with the SOFA and SAPS II. The sensitivity and specificity of the three models were used to generate a receiver operating characteristic (ROC) curve of each model, and the area under the receiver operating characteristic curve (AUROC) was calculated. The AUROC values and 95% confidence intervals were used to compare the different prediction models to confirm which model had better prediction ability. The comparison method was “Delong”. In addition to the AUROC, we used the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to compare the predictive value between the nomogram and the SOFA or SAPS II. The calibration of the nomogram was evaluated by plotting the calibration curve. Decision curve analysis (DCA) could be performed by quantifying the net benefit under different threshold probabilities. DCA was used to evaluate the net benefit of nomogram in predicting the in-hospital death of patients after PCI. In the DCA diagram, the probability of patient is in hospital death is Pi; when Pi reaches a certain threshold (Pt), it is positive and treatment taken. There will be benefits from treatment of patients, as well as harm from treatment of non-patients and loss (disadvantages) from patient non-treatment [20].
.Statistical analysis was performed using R version 4.0.3. We used the ‘glmnet’ R package to implement the LASSO regression model and the ‘rms’ package for the mortality risk prediction nomogram.
Results
Characteristics of the patients included in the study
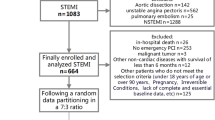
A total of 2160 patients were enrolled in the study according to the inclusion and exclusion criteria. The screening process is shown in Additional file 1. All patients were randomly divided into training set and validation set according to the ratio of 6:4. The training set contained 1296 patients, and the validation set contained 864 patients. All continuous variables didn’t conform to a normal distribution(p < 0.01) and were represented by the median (interquartile range). The demographic characteristics of all patients at baseline are shown in Table 1. The demographic characteristics of the training set and the validation set are shown in Additional file 2 and Additional file 3, respectively.
Univariate analysis
After PCI, the patients who died in the hospital were older than those who survived, with a median age of 76 years (66-83 years) for patients who died in the hospital and 69 years (58-79 years) for surviving patients. There were more males (63.6%) than females undergoing PCI(p < 0.001). However, among female patients, the risk of in-hospital mortality was 11.45% higher than that of male patients (7.42%). The SOFA score, SAPS II score, and Elixhauser Comorbidity Index were higher in patients who died in the hospital than in survivors (p < 0.001). In addition, patients who died in the hospital had lower systolic blood pressure and diastolic blood pressure and a higher heart rate and respiratory rate (p < 0.05). Patients who died in the hospital were more likely to have lower hemoglobin, platelets, sodium, and bicarbonate levels and higher potassium, PT, WBC, and anion gaps (p < 0.05). The levels of CKMB and chloride were not significantly different between the patients who died in the hospital and those who survived (p = 0.341, p = 0.934). The death rate was 12.44% in patients with non-drug-eluting stents and was higher than the 5.19% rate in patients with drug-eluting stents(p < 0.001). There is no significant difference of the in-hospital mortality between the AMI patients (8.62%) and non-AMI patients (9.18%) (p = 0.650).
Under different types of mechanical ventilation treatment, patients using noninvasive mechanical ventilation (NIMV) had the highest mortality rate (71.13%), followed by those using invasive mechanical ventilation (IMV) (15.39%), oxygen therapy (6.38%) and none (1.51%). The difference between the four treatments was significant (p < 0.001). Patients who used multiple vasoactive drugs at the same time had a higher risk of death in the hospital (p < 0.001).
Selection of the risk predictors
17 risk predictors were screened by LASSO regression, including the type of coronary stent, age, Elixhauser Comorbidity Index, ventilation treatment type, use of vasoactive drugs, heart rate, systolic pressure, diastolic pressure, respiratory rate, bicarbonate, chloride, hemoglobin, platelets, PT, WBC, CKMB, and anion gap were selected as risk predictors of in-hospital mortality in patients after PCI (Fig. 1a, b).
Use of the LASSO regression to select the variables in the training set. a We use the method of 10 times cross-validation to select the optimal value of λ. The value of parameter λ is determined by the mean square error. We chose the λ value with the lowest mean square error. The λ value was 0.004. b Twenty-two variables were screened by LASSO regression, among which the optimal λ resulted in 17 nonzero coefficients
Development of a prediction nomogram
The results of the multivariable logistic regression analysis among the risk predictors are given in Table 2. Based on the multivariate logistic regression analysis, the predicting equation was generated with the calculated coefficients: logit(1/1 − P) = 1.617 − 0.685*the type of stent+ 0.031*age+ 0.023*Elixhauser comorbidity index+ 1.706*ventilation+ 0.217*vasoactive drug+ 0.021*heart rate− 0.007*systolic blood pressure− 0.024*diastolic blood pressure+ 0.048*respiratory rate− 0.114*bicarbonate− 0.061*chloride− 0.099*hemoglobin− 0.002*platelet+ 0.024*PT + 0.025*WBC + 0.050*anion gap-0.001*CKMB. A nomogram representing the predicting model was established according to the variables and their corresponding regression coefficients (Fig. 2).
Nomogram for predicting in-hospital mortality in patients with PCI. Each variable has a coordinate axis, and by drawing a vertical line through the corresponding point on this coordinate axis, the point of intersection with the topmost scoring axis is the score of the variable. Adding all the variable scores together gives the in-hospital mortality probability corresponding to the total score at the bottom of the nomogram. In the type of stent option, “0” represents a non-drug-eluting stent and “1” represents a drug-eluting stent. In the ventilation treatment type options, “0” represents none, “1” represents oxygen therapy, “2” represents noninvasive mechanical ventilation, and “3” represents invasive mechanical ventilation. In the vasoactive drug option, “0” represents none, “1” represents vasopressin, “2” represents dobutamine, “3” represents epinephrine, “4” represents phenylephrine, “5” represents dopamine, “6” represents norepinephrine, “7” represents the use of any two vasoactive drugs, “8” represents the use of any three vasoactive drugs, and “9” represents the use of any four or more vasoactive drugs
Verification of the predictive value of the nomogram
AUROC, IDI, and NRI to compare the nomogram with the SOFA and SASP II for predicting the in-hospital risk of mortality in critical ill patients after PCI are shown in Table 3. Comparing the nomogram with SOFA in the training set and validation set, both IDI and NRI higher than 0 indicated that the prediction efficiency of the nomogram was higher than that of SOFA (p < 0.001). The same results were obtained by comparing the nomogram with SAPS II (p < 0.001). In Fig. 3, the results showed that regardless of the training set or validation set, the AUROC of the nomogram was significantly higher than that of SOFA and SAPS II (p < 0.001). This means that the nomogram performed better than SOFA and SAPS II in predicting the risk of in-hospital mortality of critical ill patients after PCI.
In training set and validation set the calibration curve the nomogram demonstrated a high degree of fit (Fig. 4a, b). The clinical use of the nomogram in clinical application was compared with SOFA and SAPS II by DCA. In the training set and validation set, treatment directed by this nomogram gained more net benefit than SOFA and SAPS II when the threshold probability (Pt) > 0.1 and Pt < 0.8 (Fig. 5).
The calibration curve of the nomogram. a Training set, b validation set. The x-axis represents the in-hospital mortality risk predicted by the nomogram. The y-axis represents the actual in-hospital mortality rate. The diagonal dotted line represents the ideal prediction model. The solid line represents the prediction performance of the nomogram. The solid line is closer to the diagonal line, which shows that the nomogram has a higher degree of fit
Decision curve analysis (DCA) of the training set and validation set. a Training set, b validation set. The y-axis measures the net benefit. The different colored lines represent the different predictive models of in-hospital death risk. The black solid line represents the assumption that all patients died in the hospital. The gray solid line represents the assumption that no patients died in the hospital. The decision curve shows that using this nomogram to predict in-hospital mortality adds more benefit than the SOFA and SAPS II scores (0.1 < Pt < 0.8)
Discussion
Nomogram is a graphic representation that consists of several lines. It is a pictorial representation of a complex mathematical formula. The numerical probability of individual clinical events can be generated by combining different prognostic variables and decisive variables [21]. Nomogram is widely used to predict prognoses in many diseases [22,23,24]. Nomogram has several advantages. First, it is user-friendly and could increase the accuracy of prediction. Second, it could help clinicians make better decisions. Recently, machine learning analysis was used to create a nomogram for predicting the prognosis of different patient cohorts [25, 26]. The clinical value of these predictive models lies in the identification of high-risk patients on one hand and the improvement in patient management through personalized medicine on the other. Most of them include multiple laboratory results, the results of coronary angiography, and echocardiogram parameters as predictive factors. The complexity of the factors limits their clinical application. This research has overcome these shortcomings. In our study, we attempted to establish a nomogram for predicting the in-hospital mortality of patients after PCI. It worth noting that the model in this study has a high AUROC and DCA in both the training and validation sets. In general, accurate prognostic assessment will help physicians intervene in a timely manner that balances risks and benefits.
Our study found that among patients after PCI, the risk of in-hospital mortality was higher in females than in males. This was consistent with previous studies [27,28,29,30]. One possible reason is that the symptoms of CHD in females are atypical, which delays the time to medical contact for female patients. A recent study showed that female patients are more likely to be complicated with coronary microvascular dysfunction, which may also be a reason for the higher risk of death [31].
Patients in ICU after PCI with lower systolic blood pressure, diastolic blood pressure and higher heart rate had a higher risk of in-hospital mortality. We suggested that the possible reason was that these patients were in shock or in a preshock state, and timely correction of shock or preshock might improve the prognosis of these patients. Patients in ICU who died in the hospital after PCI were more likely to be complicated with homeostasis disorders, including low bicarbonate, high potassium, and high anion gap. This suggests that metabolic acidosis was often present in patients with a high risk of death. Previous study has also shown an increased risk of death in critically ill patients with metabolic acidosis [32].
As indicated in some studies, higher WBCs and PT and lower hemoglobin and platelet counts have been investigated as risk factors for in-hospital mortality in patients after PCI. Indeed, as suggested by Liu et al. [33], we concluded that the elevation of WBCs on admission correlates with the in-hospital mortality of patients after PCI. Lower platelet counts, hemoglobin and higher PT may be related to bleeding after PCI. A previous study found that any amount of bleeding caused by PCI, including mild bleeding, was associated with worse outcomes [34]. However, due to the lack of data, we could not determine the real relationship between a higher PT, low platelet count, hemoglobin, and high risk of in-hospital death. More clinical research is needed in the future. CKMB can be used to estimate the extent of myocardial injury and is associated with the prognosis of CHD [35,36,37]. In our study, although there was no difference in CKMB levels between patients who died in the hospital and those who survived, it could still be observed that patients who died in the hospital had higher CKMB levels than those who survived. This difference might be because we included the value of CKMB at admission of patients after PCI. Not all patients undergo PCI because of AMI, some of them are stable angina, silent ischemia or after thrombolysis. They may in different stages of disease when they were admitted to the hospital. The CKMB at admission does not necessarily correspond to the peak of the CKMB or the severity of the disease.
Our results showed that the risk of in-hospital mortality was higher in patients with non-drug-eluting stents than in patients with drug-eluting stents. Previous studies have demonstrated that drug-eluting stents perform better than non-drug-eluting stents in the prevention of neointimal proliferation, restenosis, and associated clinical events [38,39,40].
We found that the risk of in-hospital death of patients who received IMV during hospital stay was higher than that of patients who received oxygen therapy or no oxygen treatment, which was consistent with our previous perception that patients with more severe illnesses are more likely to require mechanical ventilation [41]. However, we found that patients who received NIMV during hospital stay had a higher risk of in-hospital death than those who received IMV. In general, we think that patients with severe illness need to receive IMV rather than NIMV, which is somewhat contradictory to our previous perception. We speculate that the number of patients with IMV was small, would lead to bias.
There are some points that should be considered when using nomograms. Most of the laboratory results we used were within 24 hours after admission, so we need to obtain the relevant laboratory results as soon as possible after admission. Because most patients have multiple vital sign measurements after admission, we selected the average value of multiple vital sign measurements, which also needs to be considered.
There are several limitations of this study. First, the data for this study were extracted from MIMIC III, which is the database for a single center. Second, this study was a retrospective observational study, how the models identified in this study can be used for individualized evaluation and treatment of patients remains to be explored. Because of the unidentified confounding factors, the reliability of the results is lower than that of randomized, controlled clinical studies. Third, this study used data from the public database, and some of the data were not recorded. Even though we used multiple interpolation to fill in the missing data and appropriate calculation was based on this corrected data, there were still some differences from the original data. These differences might produce bias. Finally, the sample size of this study is still insufficient, and further studies may be needed.
Conclusion
A nomogram was developed, and the AUROC of this nomogram was 0.907, which is excellent in predicting in-hospital mortality in critical ill patients after PCI. The nomogram revealed that it could augment net benefits in a wide range of threshold (0.1 < Pt < 0.8) in the prediction of the in-hospital mortality of patients after PCI.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CHD:
-
Coronary heart disease
- PCI:
-
Primary percutaneous coronary intervention
- TIMI:
-
Thrombolysis in myocardial infarction
- AMI:
-
Acute myocardial infarction
- MIMIC III:
-
Medical Information Mart for Intensive Care III
- ICD-9:
-
International Classification of Diseases-9
- SOFA:
-
Sequential Organ Failure Assessment
- SAPS II:
-
Scale for the Assessment of Positive Symptoms II
- BMI:
-
Body mass index
- ICU:
-
Intensive care unit
- PT:
-
Prothrombin time
- WBC:
-
White blood cell count
- CKMB:
-
MB isoenzyme of creatine kinase
- TnI:
-
Troponin I
- TnT:
-
Troponin T
- LDL:
-
l ow-density lipoprotein
- TG:
-
Triglyceride
- Lac:
-
Lactate
- LASSO:
-
Least absolute shrinkage and selection operator
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- AUROC:
-
Area under the receiver operating characteristic curve
- NRI:
-
Net reclassification improvement
- IDI:
-
Integrated discrimination improvement
- DCA:
-
Decision curve analysis
- NIMV:
-
Noninvasive mechanical ventilation
- IMV:
-
Invasive mechanical ventilation
References
Collaborators GB. Burden of disease in Brazil, 1990-2016: a systematic subnational analysis for the global burden of disease study 2016. Lancet (London, England). 2018;392(10149):760–75.
Nomura S, Sakamoto H, Glenn S, Tsugawa Y, Abe S, Rahman M, et al. Population health and regional variations of disease burden in Japan, 1990-2015: a systematic subnational analysis for the global burden of disease study 2015. Lancet (London, England). 2017;390(10101):1521–38.
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387(10015):251–72.
Widimsky P, Wijns W, Fajadet J, Belder MD, Knot J, Aaberge L, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;8:943–57.
Terkelsen JC. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304(7):763–71.
Wiviott SD. Optimal medical therapy with or without PCI for stable coronary artery disease. Current Cardiovascular Risk Reports. 2007;1(4):277–8.
Fox KA, Anderson FA, Dabbous OH, Steg PG, Lopez-Sendon J, Van de Werf F, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The global registry of acute coronary events (GRACE). Heart. 2007;93(2):177–82.
Lim SY, Jeong MH, Bae EH, Kim W, Kim JH, Hong YJ, et al. Predictive factors of major adverse cardiac events in acute myocardial infarction patients complicated by cardiogenic shock undergoing primary percutaneous coronary intervention. Circ J. 2005;69(2):154–8.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743.
Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, Lemos JD, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–7.
Weintraub WS, Grau-Sepulveda MV, Weiss JM, Delong ER, Peterson ED, O’Brien SM, et al. Prediction of long-term mortality after percutaneous coronary intervention in older adults: results from the National Cardiovascular Data Registry. Circulation. 2012;125(12):1501.
Khera R, Haimovich J, Hurley N, McNamara R, Spertus J, Desai N, et al. Use of machine learning models to predict death after acute myocardial infarction. JAMA Cardiol. 2021;6(6):633–41.
Valgimigli M, Campo G, Malagutti P, Anselmi M, Bolognese L, Ribichini F, et al. Prediction of 1Year clinical outcomes using the SYNTAX score in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Jacc Cardiovasc Interv. 2011;4(1):66–75.
Wang Q, Qian W, Sun Z, Zhu W, Liu Y, Chen X, et al. Nomograms based on pre-operative parametric for prediction of short-term mortality in acute myocardial infarction patients treated invasively. Aging. 2020;13(2):2184–97.
Yuyang X, Xianghua Y, Wang Y, Wenlu Q. Zhang: development and validation of risk nomogram model predicting coronary microvascular obstruction in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous catheterization. Med Sci Monit. 2019;25:5864–77.
Kim H, Park K, Ha S, Lee S, Choi H, Kim S, et al. Predictors of survival following veno-arterial extracorporeal membrane oxygenation in patients with acute myocardial infarction-related refractory cardiogenic shock: clinical and coronary angiographic factors. J Thorac Dis. 2020;12(5):2507–16.
Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Tibshirani RJ. Regression shrinkage and selection via the LASSO. J R Stat Soc Ser B: Methodological. 1996;73(1):273–82.
Vickers A, Cronin A, Elkin E, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53.
Balachandran V, Gonen M, Smith J, DeMatteo R. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80.
Liang W, Li Z, Jiang G, Wang Q, He J. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–9.
Tevis SE, Weber SM, Kent CK, Kennedy GD. Nomogram to predict postoperative readmission in patients who undergo general surgery. JAMA Surg. 2015;150(6):505–10.
Jie P, Jing Z, Zhang Q, Xu Y, Jie Z, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121–7.
Al'Aref SJ, Singh G, Rosendael A, Kolli KK, Minutello RM. Determinants of in-ospital mortality after percutaneous coronary intervention: a machine learning approach. J Am Heart Assoc. 2019;8(5):e011160.
Kong S, Chen C, Zheng G, Yao H, Zhou H. A nomogram based on lactate to predict major adverse cardiovascular events for acute coronary syndrome after percutaneous coronary intervention; 2021.
Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130(9):757–67.
Pinnow EE, Gruberg L, Apple S, Suddath W, Pichard AD, Satler LF, et al. Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2010;59(3):338–43.
Bipin PS, Subash SGP, Toralben P, Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174(11:3):1822.
Nanna MG, Hajduk AM, Krumholz HM, Murphy TE, Dodson JA. Sex-based differences in presentation, treatment, and complications among older adults hospitalized for acute myocardial infarction: the SILVER-AMI study. Circ Cardiovasc Qual Outcomes. 2019;12(10):e005691.
Smith LR, Salifu MO, Mcfarlane IM. Non-obstructive coronary artery disease in women: current evidence and future directions. Int J Clin Res Trials. 2020;5(2):152.
Jung B, Rimmele T, Le Goff C, Chanques G, Corne P, Jonquet O, et al. Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit Care. 2011;15(5):1–9.
Liu J, Shao-Nan LI, Guang-Lian LI, Pan YZ. Predictive value of WBC count on in-hospital MACE in patients underwent primary PCI. Pract Clin Med. 2007;8(4):1–3.
Vavalle JP, Rao SV. Impact of bleeding complications on outcomes after percutaneous coronary interventions. Interv Cardiol. 2016;1(1):51–62.
Pettersson T, Ohlsson O, Tryding N. Increased CKMB (mass concentration) in patients without traditional evidence of acute myocardial infarction. A risk indicator of coronary death. Eur Heart J. 1992;13(10):1387–92.
Hedström E, Åström-Olsson K, Öhlin H, Frogner F, Carlsson M, Billgren T, et al. Peak CKMB and cTnT accurately estimates myocardial infarct size after reperfusion. Scand Cardiovasc J. 2007;41(1):44–50.
Ma Y, Li L, Shang XM, Tan Z, Geng XB, Zhao BQ, et al. Analysis of factors related to short-term prognosis in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Exp Ther Med. 2013;5(4):1206–10.
Morice MC, Serruys PW, Sousa EJ. A randomized comparison of a sirolimus eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773–80.
Colombo A. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108(7):788–94.
Thuesen L, Kelbaek H, Kløvgaard L, Helqvist S, Jørgensen E, Aljabbari S, et al. Comparison of sirolimus-eluting and bare metal stents in coronary bifurcation lesions: subgroup analysis of the stenting coronary arteries in non-stress/Benestent disease trial (SCANDSTENT). Am Heart J. 2006;152(6):0–1145.
Rocha B, Cunha G, Maltes S, Moura A, Coelho F, Torres J, et al. Cardiovascular disease in an intensive care unit: patterns of an often fatal omen. Eur Heart J. 2021;42(Supplement_1):ehab724–1520.
Acknowledgements
Not applicable.
Conflict of interest
None.
Funding
This work was supported by the Natural Science Foundation of China (81570382).
Author information
Authors and Affiliations
Contributions
Ms. Miao Yuan was responsible for querying and analyzing the data and was a major contributor in writing the manuscript. Dr. DengFeng Gao was responsible for interpreted the patient data. Dr. Fuxian Ren responsible for the point to point response and the statistics. Mr. BinChen Ren was responsible for the revision of the manuscript. Ms. Yu Wang responsible for drawing and modification of figures and tables. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards (IRBs) at both Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center approved the use of the data for research. The database can be accessed by certified researchers, so no additional informed consent of the patient and ethic approvement are required. The certified researcher of this study is Miao Yuan (no. 7382002). All methods of this article were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Flowchart of data extraction.
Additional file 2.
Baseline characteristics of training set.
Additional file 3.
Baseline characteristics of validation set.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, M., Ren, B.C., Wang, Y. et al. Development of a novel tool: a nomogram for predicting in-hospital mortality of patients in intensive care unit after percutaneous coronary intervention. BMC Anesthesiol 23, 5 (2023). https://doi.org/10.1186/s12871-022-01923-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01923-y