Abstract
Background
The Tokyo Guidelines 2013 classifies acute cholecystitis (AC) into three grades and recommends appropriate therapy for each grade. For grade II AC, either early laparoscopic cholecystectomy (LC) or percutaneous transhepatic gallbladder drainage (PTGBD) should be performed. This study aimed to identify the risk factors for difficulty of LC for treating grade II AC.
Methods
Totally, 122 patients who underwent LC for grade II AC were enrolled and divided into difficult LC (DLC) and nondifficult LC (NDLC) groups. The DLC group included patients who experienced one of the following conditions: conversion from LC to open cholecystectomy, operating time ≥ 180 min, or blood loss ≥300 ml. Preoperative characteristics and postoperative outcomes were analyzed.
Results
In univariate analysis, risk factors included male sex, interval between symptom onset and admission, interval between symptom onset and LC, and anticoagulant therapy. The incidence of postoperative complications was higher in the DLC group than in the NDLC group (23.5% vs. 4.6%, p = 0.0016). According to receiver operating characteristic curves, the optimal cutoff value was calculated, and multivariate analysis showed that male sex [odds ratio (OR), 5.76; 95% confidence interval (CI), 1.979–19.51; p = 0.0009) and interval between symptom onset and LC of over 96 h (OR, 6.32; 95% CI, 2.126–20.15; p = 0.0009) were independent risk factors for difficulty of LC.
Conclusions
In patients with grade II AC, LC was technically difficult when performed over 96 h after symptom onset. Moreover, male sex was a risk factor. Therefore, PTGBD should be considered in these patients.
Similar content being viewed by others
Background
Acute cholecystitis (AC) is commonly observed, and laparoscopic cholecystectomy (LC) has become the standard treatment for this disease [1, 2]. The advantages of LC over open cholecystectomy include shorter hospital stay, reduced postoperative pain, and less mortality and morbidity [3,4,5]. Recently, the superiority of early LC over delayed LC was demonstrated in a randomized controlled trial [6,7,8,9,10,11]; however, surgeons often encounter difficulties in performing LC due to their inability to correctly identify the anatomy of Calot’s triangle as a result of severe inflammation. Therefore, in patients with severe AC, the rate of complications, such as bile leakage, common bile duct injury, and bowel injury, is high after LC [12, 13], suggesting the importance of evaluation of inflammation severity.
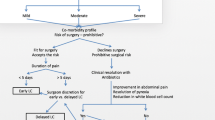
The Tokyo Guidelines 2007 was issued as the first international guideline for the diagnosis and treatment of AC; recently, it was revised to the Tokyo Guidelines 2013 (TG13) [14, 15]. The TG13 suggests criteria for the classification of AC based on clinical symptoms and physical examination, blood test, and imaging findings. According to the TG13, AC is classified into three grades: grade I (mild), grade II (moderate), and grade III (severe) (Table 1). The TG13 also recommends appropriate therapy depending on the grade of AC (Fig. 1). Patients with grade I AC are candidates for undergoing immediate LC, those with grade II AC can undergo either early LC or percutaneous transhepatic gallbladder drainage (PTGBD), and those with grade III AC are strongly recommended to undergo immediate PTGBD. For patients with grade II AC, either early LC or PTGBD should be performed when antibiotic therapy is not effective. The TG13 recommends performing early LC in medical centers where skilled surgeons can operate. Therefore, it is important to consider risk factors for difficulty of LC in patients with grade II AC. Although some studies have reported risk factors for difficulty of LC with a particular focus on conversion from LC to open cholecystectomy, their evaluations were not based on the grading of AC according to the TG13 [16,17,18,19,20,21,22].
This study aimed to assess risk factors affecting the technical difficulty of LC in patients with grade II AC.
Methods
The medical records of 240 patients who underwent LC for AC between 2010 and 2016 were retrospectively reviewed (Fig. 2). Totally, 55 patients who met one of the following conditions were excluded: tight adhesion due to a previous upper abdominal operation, LC following PTGBD, or AC with cholangitis caused by choledocholithiasis. At least one board-certified surgeon of the Japan Surgical Society participated in all operations.
As there was no quantitative evaluation for difficulty of cholecystectomy, we used objective and subjective criteria [16, 20]. Difficult LC was defined as the presence of one of the following conditions: conversion from laparoscopic cholecystectomy to open cholecystectomy, operating time ≥ 180 min, or blood loss ≥300 ml. The criteria for conversion was as follows: other organ injury, refractory bleeding, and difficulty identifying important structures inside Calot’s triangle. The decision for conversion was made by each surgeon. The remaining 122 patients with grade II AC were divided into two groups according to the aforementioned criteria: the difficult LC (DLC; n = 34) and nondifficult LC(NDLC; n = 88) groups. Preoperative characteristics and postoperative outcomes were further analyzed. This study protocol was reviewed and approved by the Ethics Committee of the South Miyagi Medical Center.
Data are shown as mean ± standard error of the mean. Categorical variables were analyzed by the chi-square test, whereas continuous data were analyzed by either the two-tailed Student’s t-test or Wilcoxon test, based on the results of the Shapiro–Wilk test. Multivariate analysis was performed with logistic regression analysis. Statistical significance was defined as p < 0.05. The optimal cutoff value was calculated using the receiver operating characteristic (ROC) curve and was defined as the number that indicated the highest sum of the sensitivity and specificity on the ROC curve. Statistical analysis was performed with JMP Pro 11 (SAS Institute, Cary, NC, USA). Detailed data of patient characteristics are shown in Additional file 1.
Results
Among the enrolled patients, AC was classified as grade I in 60 patients (32.4%), as grade II in 122 (65.9%), and as grade III in 3 (1.7%). Among 122 patients with grade II AC, 33 were classified into the DLC group. There were 18, 15, and 16 patients whose operating time was >180 min, whose intraoperative blood loss was >300 ml, and whose procedure was changed to open cholecystectomy, respectively. Four patients fulfilled all three abovementioned criteria. The most common reason for conversion was the inadequate exposure of Calot’s triangle due to severe adhesion and inflammation. Additionally, accidental cystic duct injury in three patients contributed to conversion. In one patient, the cystic duct was injured during exposure of Calot’s triangle. In the other two patients, the dissection of necrotic and fragile cystic ducts using a linear stapler resulted in bile leakage from the stump of the cystic duct. No patient had gallbladder cancer.
Detailed patient characteristics are shown in Table 2. The ratio of male-to-female patients was significantly higher in the DLC group (82.4% vs. 58.0%; p = 0.011). Intervals between symptom onset and LC (126 ± 9.04 vs. 70.3 ± 5.62 h; p < 0.0001) and between symptom onset and admission (65.6 ± 7.07 vs. 32.9 ± 4.40 h; p = 0.0001) were significantly longer in the DLC group than in the NDLC group. The percentage of patients receiving anticoagulant therapy was higher in the DLC group than in the NDLC group (29.4% vs. 13.6%, p = 0.042). All other parameters did not differ between the groups.
Table 3 shows laboratory data and imaging findings. Laboratory data, including inflammation-associated parameters, such as white blood cell count and C-reactive protein levels, did not differ between the groups. No difference was observed between the groups in terms of the thickness of the gallbladder wall and existence of a gallbladder stone and/or sludge (Table 3).
Perioperative characteristics are shown in Table 4. Operating time and blood loss were greater and the rate of subtotal cholecystectomy and/or mucoclasis was significantly higher in the DLC group than in the NDLC group. The percentage of patients with necrosis and/or abscess did not differ between the groups.
Perioperative outcomes are shown in Table 5. The DLC group had a significantly higher incidence of postoperative complications than the NDLC group (23.5% vs. 4.6%; p = 0.0016). In particular, bile leakage and surgical site infection developed more frequently in the DLC group than in the NDLC group (11.8% vs. 1.1%; p = 0.0079 for bile leakage and 5.9% vs. 0%; p = 0.0218 for surgical site infection). The percentage of patients with Clavien–Dindo classification ≥II was significantly higher in the DLC group than in the NDLC group (17.7% vs. 4.5%; p = 0.018); however, the number of patients with Clavien–Dindo classification ≥IIIa did not differ between the groups. The duration of postoperative hospital stay was longer in the DLC group than in the NDLC group (9.26 ± 0.60 days vs. 5.89 ± 0.37 days; p < 0.0001).
Table 6 shows the results of multivariate analysis for risk factors for difficult of LC. ROC curve analysis yielded a value of 96 h as the optimal cutoff interval between symptom onset and LC, whereas a value of 72 h was the optimal cutoff interval between symptom onset and admission. With these cutoff values, multivariate analysis was performed. Interval between the symptom onset and LC over 96 h [odds ratio (OR), 6.32; 95% confidence interval (CI), 2.126–20.15; p = 0.0009) and male sex (OR, 5.76; 95% CI, 1.979–19.51, p = 0.0009) were independent risk factors for difficulty of LC.
Discussion
Recently, early LC has become a standard treatment for AC [23, 24]; however, surgeons often experience difficulty during LC for AC as a result of severe local inflammation, which can increase the rate of postoperative complications, such as bile leakage, common bile duct injury, and bowel injury [12]. The TG13 has indicated severity criteria for AC and has recommended its appropriate management [15]. For grade II AC, however, surgeons can select either early LC or PTGBD. The TG13 recommends early LC by a well-experienced surgeon; therefore, it is important to consider risk factors for difficulty of LC while determining the appropriate treatment. Although some studies have reported risk factors for difficulty, they did not evaluate them according to each grade of AC as indicated by the TG13. Therefore, risk factors for difficulty of LC in grade II AC remain unclear.
In this retrospective study, we assessed risk factors for difficulty of LC in 122 patients with grade II AC. The conversion rate was 13.1%. Because previous studies had a conversion rate ranging from 12.9% to 24% [16, 19], the conversion rate in our study was acceptable. Although some investigators defined difficult LC according to the conversion rate, the decision for conversion depends on the skill of the surgeon and is very subjective. Thus, the conversion rate differs among surgeons. Operating time and blood loss are well-accepted parameters that affect postoperative complications [20]. Therefore, operating time and blood loss were also included in the definition. In the DLC group, the incidences of postoperative complications and postoperative hospital stay were significantly greater than those in the NDLC group. Moreover, the rate of subtotal cholecystectomy and/or mucoclasis was significantly higher in the DLC group. Accordingly, we considered that the definition for technical difficulty of LC was appropriate.
Male sex, interval between symptom onset and LC, interval between symptom onset and admission, and anticoagulant therapy were considered as candidates for risk factors in univariate analysis; however, multivariate analysis revealed that male sex and interval between symptom onset and LC were independent risk factors. In some patients receiving anticoagulant therapy, the timing of LC was delayed so that patients could recover from the effect of drugs. Hence, there was a confounding bias between the risk factors anticoagulation therapy and interval between symptom onset and LC. Alternatively, we evaluated the effect of antibiotics on the difficulty of LC in patients with grade II AC. Initially, we hypothesized that the early administration of antibiotics makes it easy to complete LC; however, we could not prove this hypothesis in our study. Therefore, an early decision should be made for LC regardless of antibiotic therapy.
Regarding postoperative complications, bile leakage occurred significantly more often in the DLC group than in the NDLC group. Fortunately, there was no major bile duct injury requiring bile duct reconstruction in our study; however, this result indicated that minor bile duct injury during operation occurred more often in the DLC group due to tight adhesion around the gallbladder neck. There were more patients with Clavien–Dindo classification ≥II in the DLC group than in the NDLC group, and they had a prolonged postoperative hospital stay. Meanwhile, the numbers of patients with Clavien–Dindo classification ≥IIIa did not differ between the groups.
Interval between symptom onset and LC has been reported as a risk factor for conversion, and the critical point ranged from 48 h to 96 h [16, 18, 25]. In our study, the cutoff value was 96 h, which was similar to previous data. The edematous phase, which is the phase before proceeding to tight adhesion, predominates in this period [25]; therefore, the TG13 recommends early LC.
Ambe et al. [26] suggested that male sex was a risk factor for severe gallbladder inflammation in AC and for complications in patients with AC undergoing LC [22]. Yajima et al. [27] also reported that male sex was a risk factor for conversion from laparoscopic cholecystectomy to open cholecystectomy. Male sex was also an independent risk factor for difficult LC in our study. Ambe et al. discussed that male patients gave poor attention to their health compared to female patients, and consequently, their visit to the hospital was delayed. Therefore, male patients have a long interval between symptom onset and LC. In our study, however, no significant difference was observed in the interval between symptom onset and LC. There may be other reasons for this finding. Although previous studies also reported that older age, high white blood cell count, and high C-reactive protein levels are essential risk factors [16, 17, 19, 20], there was no difference in these variables in our study.
Our study had two limitations. First, our study was retrospective and had a relatively small sample size. Second, the decision for conversion was made by each surgeon according to subjective criteria. These two limitations can be confounding factors for perioperative outcomes, including operating time, blood loss, and conversion rate.
Conclusions
LC was technically difficult when performed later than 96 h after symptom onset in patients with grade II AC. Moreover, male sex was a risk factor. Therefore, PTGBD should be considered in these patients.
Abbreviations
- AC:
-
Acute cholecystitis
- CI:
-
Confidence interval
- DLC:
-
Difficult laparoscopic cholecystectomy
- LC:
-
Laparoscopic cholecystectomy
- NDLC:
-
Nondifficult laparoscopic cholecystectomy
- OR:
-
Odds ratio
- PTGBD:
-
Percutaneous transhepatic gallbladder drainage
- ROC:
-
Receiver operating characteristic
- TG13:
-
Tokyo Guidelines 2013
References
Yamashita Y, Takada T, Strasberg SM, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Gomi H, Dervenis C, Windsor JA, Kim SW, de Santibanes E, Padbury R, Chen XP, Chan AC, Fan ST, Jagannath P, Mayumi T, Yoshida M, Miura F, Tsuyuguchi T, Itoi T, Supe AN. Tokyo guideline revision committee. TG13 surgical management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:89–96.
Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005;92:44–9.
Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–96.
Purkayastha S, Tilney HS, Georgiou P, Athanasiou T, Tekkis PP, Darzi AW. Laparoscopic cholecystectomy versus mini-laparotomy cholecystectomy: a meta-analysis of randomised control trials. Surg Endosc. 2007;21:1294–300.
Graves HA Jr, Ballinger JF, Anderson WJ. Appraisal of laparoscopic cholecystectomy. Ann Surg. 1991;213:655–62.
Lo CM, Liu CL, Fan ST, Lai EC, Wong J. Prospective randomized study of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1998;227:461–7.
Kolla SB, Aggarwal S, Kumar A, Kumar R, Chumber S, Parshad R, Seenu V. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a prospective randomized trial. Surg Endosc. 2004;18:1323–7.
Gutt CN, Encke J, Koninger J, Harnoss JC, Weigand K, Kipfmüller K, Schunter O, Götze T, Golling MT, Menges M, Klar E, Feilhauer K, Zoller WG, Ridwelski K, Ackmann S, Baron A, Schön MR, Seitz HK, Daniel D, Stremmel W, Büchler MW. Acute cholecystitis: early versus delayed cholecystectomy, a multicenter randomized trial (ACDC study, NCT00447304). Ann Surg. 2013;258:385–93.
Lai PB, Kwong KH, Leung KL, Kwok SP, Chan AC, Chung SC, Lau WY. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 1998;85:764–7.
Kwon YJ, Ahn BK, Park HK, Lee KS, Lee KG. What is the optimal time for laparoscopic cholecystectomy in gallbladder empyema? Surg Endosc. 2013;27:3776–80.
Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford). 2015;17:857–62.
Lee SW, Yang SS, Chang CS, Yeh HJ. Impact of the Tokyo guidelines on the management of patients with acute calculous cholecystitis. J Gastroenterol Hepatol. 2009;24:1857–61.
Gomi H, Solomkin JS, Takada T, Strasberg SM, Pitt HA, Yoshida M, Kusachi S, Mayumi T, Miura F, Kiriyama S, Yokoe M, Kimura Y, Higuchi R, Windsor JA, Dervenis C, Liau KH, Kim MH. Tokyo guideline revision committee. TG13 antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:60–70.
Sekimoto M, Takada T, Kawarada Y, Nimura Y, Yoshida M, Mayumi T, Miura F, Wada K, Hirota M, Yamashita Y, Strasberg S, Pitt HA, Belghiti J, de Santibanes E, Gadacz TR, Hilvano SC, Kim SW, Liau KH, Fan ST, Belli G, Sachakul V. Need for criteria for the diagnosis and severity assessment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepato-Biliary-Pancreat Surg 2007;14:11-4.
Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Kiriyama S, Kimura Y, Tsuyuguchi T, Itoi T, Yoshida M, Miura F, Yamashita Y, Okamoto K, Gabata T, Hata J, Higuchi R, Windsor JA, Bornman PC, Fan ST, Singh H, de Santibanes E, Kusachi S, Murata A, Chen XP, Jagannath P, Lee S, Padbury R, Chen MF. Tokyo guidelines revision committee. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo guidelines. J Hepatobiliary Pancreat Sci. 2012;19:578–85.
Asai K, Watanabe M, Kusachi S, Matsukiyo H, Saito T, Kodama H, Kiribayashi T, Enomoto T, Nakamura Y, Okamoto Y, Saida Y, Nagao J. Risk factors for conversion of laparoscopic cholecystectomy to open surgery associated with the severity characteristics according to the Tokyo guidelines. Surg Today. 2014;44:2300–4.
Halachmi S, DiCastro N, Matter I, Cohen A, Sabo E, Mogilner JG, Abrahamson J, Eldar S. Laparoscopic cholecystectomy for acute cholecystitis: how do fever and leucocytosis relate to conversion and complications? Eur J Surg. 2000;166:136–40.
Hadad SM, Vaidya JS, Baker L, Koh HC, Heron TP, Hussain K, Thompson AM. Delay from symptom onset increases the conversion rate in laparoscopic cholecystectomy for acute cholecystitis. World J Surg. 2007;31:1298–301. discussion 1302-3
Wevers KP, van Westreenen HL, Patijn GA. Laparoscopic cholecystectomy in acute cholecystitis: C-reactive protein level combined with age predicts conversion. Surg Laparosc Endosc Percutan Tech. 2013;23:163–6.
Hayama S, Ohtaka K, Shoji Y, Ichimura T, Fujita M, Senmaru N, Hirano S. Risk factors for difficult laparoscopic cholecystectomy in acute cholecystitis. JSLS. 2016;20:e2016.00065.
Sakpal SV, Bindra SS, Chamberlain RS. Laparoscopic cholecystectomy conversion rates two decades later. JSLS. 2010;14:476–83.
Ambe PC, Kohler LI. The male gender an independent risk factor for complication in patients undergoing laparoscopic cholecystectomy for acute cholecystitis? Int Surg. 2015;100:854–9.
Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–50.
Borzellino G, Sauerland S, Minicozzi AM, Verlato G, Di Pietrantonj C, de Manzoni G, Cordiano C. Laparoscopic cholecystectomy for severe acute cholecystitis. A meta-analysis of results. Surg Endosc. 2008;22:8–15.
Brodsky A, Matter I, Sabo E, Cohen A, Abrahamson J, Eldar S. Laparoscopic cholecystectomy for acute cholecystitis: can the need for conversion and the probability of complications be predicted? A prospective study. Surg Endosc. 2000;14:755–60.
Ambe PC, Weber SA, Wassenberg DI. Gallbladder inflammation more severe in male patients presenting with acute cholecystitis? BMC Surg. 2015;15:48.
Yajima H, Kanai H, Son K, Yoshida K, Yanaga K. Reasons and risk factors for intraoperative conversion from laparoscopic to open cholecystectomy. Surg Today. 2014;44:80–3.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Not applicable
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KI wrote this paper and contributed to the conception of the study, critically revised the work for important intellectual content, and approved the version to be published. KI also agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. TU contributed to the conception of the study, critically revised the work for important intellectual content, and approved the version to be published. TU also agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. DD contributed to the design of the work, drafted the work, and approved the version to be published. DD also agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. KS contributed to the design of the work and drafted the work. KS also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. SG contributed to the design of the work and drafted the work. SG also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. MT contributed to the design of the work and drafted the work. MT also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. TM contributed to the conception and critically revised the work. NT contributed to the conception and critically revised the work for important intellectual content. NT also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. CS contributed to the conception and critically revised the work for important intellectual content. CS also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy of any part of the work were appropriately investigated and resolved. HN contributed to the conception and critically revised the work for important intellectual content. HN also approved the version to be published and agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was reviewed and approved by the Ethics Committee of the South Miyagi Medical Center on March 31, 2017. Registration number: 16–17.
Informed consent was waived by the Ethics Committee of the South Miyagi Medical Center.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Detailed data of patient characteristics. 1: yes, 0: no (XLSX 61 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Inoue, K., Ueno, T., Douchi, D. et al. Risk factors for difficulty of laparoscopic cholecystectomy in grade II acute cholecystitis according to the Tokyo guidelines 2013. BMC Surg 17, 114 (2017). https://doi.org/10.1186/s12893-017-0319-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-017-0319-6