Abstract
Objective
Femoral neck fractures (FNF) are known to have significant morbidity and mortality rates. Multiple chronic conditions (MCC) are defined as the presence of two or more chronic diseases that greatly affect the quality of life in older adults. The aim of this study is to explore the impact of MCC and Charlson comorbidity index (CCI) on surgical outcomes in patients with FNF.
Methods
Patients with FNF who underwent joint replacement surgery were selected for this study. Patients who had two or more diseases simultaneously were divided into two groups: the MCC group and the non-MCC (NMCC) group. The CCI was calculated to assess the severity of patients’ comorbidities in the MCC group. Baseline data, surgical details, and prognosis-related indicators were analyzed and compared between the two patient groups. Spearman correlation analysis was performed to assess the relationship between CCI and length of hospital stay, Harris score, skeletal muscle index (SMI), and age. Univariate and multivariate logistic regression analysis was conducted to identify the risk factors for mortality in FNF patients at 1 and 5 years after surgery.
Results
A total of 103 patients were included in the MCC group, while the NMCC group consisted of 40 patients. However, the patients in the MCC group were found to be older, had a higher incidence of sarcopenia, and lower SMI values (p < 0.001). Patients in the MCC group had longer hospitalization times, lower Harris scores, higher intensive care unit (ICU) admission rates, and higher complication rates (p = 0.045, p = 0.035, p = 0.019, p = 0.010). Spearman correlation analysis revealed that CCI was positively correlated with hospitalization and age (p < 0.001, p < 0.001), while it was negatively correlated with Harris score and SMI value (p < 0.001, p < 0.001). Univariate and multivariate logistic regression analysis demonstrated that MCC patients had higher 1-year and 5-year mortality rates. Hospitalization time was identified as a risk factor for death in FNF patients 1 year after joint replacement (p < 0.001), whereas CCI and age were identified as risk factors for death 5 years after surgery (p < 0.001, p < 0.001). Kaplan-Meier survival analysis results showed that the difference in death time between the two groups of patients with MCC and NMCC was statistically significant (p < 0.001). Cox proportional hazard model analysis showed that CCI, age and SMI were risk factors affecting patient death.
Conclusion
The surgical prognosis of patients with MCC, CCI and FNF is related. The higher the CCI, the worse the patient’s function and the higher the long-term risk of death.
Similar content being viewed by others
Introduction
Femoral neck fracture (FNF) is the most common type of hip fracture, often referred to as the ‘last fracture in life’. This fracture poses significant challenges to society and patients due to its high disability and mortality rates [1, 2]. Conservative treatment, which involves long-term immobilization, can lead to complications like deep vein thrombosis, pneumonia, and bedsores [3]. Hip fractures, including intertrochanteric and femoral neck fractures, are increasingly recognized as significant public health issues worldwide [4]. A report from China indicates that the overall mortality rate one year following an intertrochanteric fracture is 17.47%, while the mortality rate one year after a femoral neck fracture is 9.83% [5]. There is a higher risk of fracture displacement in later stages, prompting the need for surgical intervention in FNF patients. The surgical options of cortical bone and cancellous bone internal fixation are widely used in orthopedic surgeries, including joint and spinal injuries [6, 7]. In middle-aged and elderly patients, internal fixation surgery may compromise blood supply to the femoral neck, leading to avascular necrosis of the femoral head and nonunion. Therefore, joint replacement is often considered the preferred treatment option [8,9,10]. Whether through total hip arthroplasty (THA) or hemiarthroplasty (HA), patients are encouraged to engage in early postoperative exercises to facilitate rapid recovery [11].
The extension of people’s lifespan and the rise in elderly population due to economic development and improved medical standards have accelerated the aging process in recent years. The increasing prevalence of chronic diseases among the elderly poses a significant public health challenge. When an individual suffers from two or more chronic conditions, it is termed multiple chronic conditions (MCC), encompassing physical, psychological, or psychiatric ailments. The incidence of sarcopenia is notably higher among MCC patients [12]. The Charlson Comorbidity Index (CCI) is a widely utilized standardized tool for assessing comorbidities. It assigns weights based on the severity of each indicator and is employed to evaluate the comorbidity associated with both local and systemic diseases. A recent study demonstrated that the CCI score serves as an effective predictor of the return to pre-fracture activities of daily living one year following a fragility hip fracture [13]. Previous researchers have evaluated the relationship between various preoperative clinical scoring systems and mortality, finding that the CCI can predict mortality in patients following a hip fracture [14, 15]. A recent study further confirmed that high CCI scores is associated with lower survival rates in elderly patients with hip fractures [16]. Additionally, in total knee replacement surgery, CCI is commonly utilized as a surgical prognostic risk assessment tool [17].
Sarcopenia, characterized by declining skeletal muscle mass, strength, and function [18], leads to reduced body function and heightened risks of falls, fragility fractures, and disability [19]. FNF is common in elderly patients, particularly those with MCC and sarcopenia, elevating the risks associated with surgical anesthesia and mortality. Despite this, there remains a gap in understanding the impact of MCC on the efficacy and mortality of FNF joint replacement surgery in the elderly.
This study initially compared the differences in sarcopenia and skeletal muscle index (SMI) between the MCC group and NMCC group. Subsequently, the surgical outcomes were assessed for both groups, including hospitalization days, Harris score, intensive care unit (ICU) admission rate, and complication rate. Additionally, the correlation between the CCI and hospitalization duration, age, and Harris score was analyzed. Finally, the study investigated the mortality rates of the two patient groups and identified the risk factors influencing patient mortality.
Materials and methods
Patients and ethical considerations
This retrospective study was conducted at a single center and involved patients with FNF who underwent joint replacement surgery, including THA and HA, and were hospitalized in the Department of Orthopedics at Jiangning Hospital Affiliated to Nanjing Medical University between January 2017 and December 2018. Approval for this study was obtained from the Ethics Committee of Jiangning Hospital Affiliated to Nanjing Medical University. All procedures conducted in studies involving human participants adhered to the ethical standards set forth by the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its subsequent amendments.
Inclusion criteria
①FNF underwent joint replacement surgery; ②Age > 65 years old; ③Thorax computed tomography (CT) scan before surgery; ④First hip fracture and no lower limb dysfunction before surgery; ⑤Single fracture.
Exclusion criteria
①Age ≤ 65 years old; ②Hip joint developmental abnormalities; ③Receiving conservative treatment or internal fixation surgery; ④Pathological fracture; ⑤Lost to follow-up.
Diagnosis of MCC
MCC refers to having two or more diseases at the same time. The CCI score was utilized to assess the chronic comorbidities of patients. The CCI is derived from the patient’s clinical dataset and is determined based on hospital diagnosis codes. It comprises a total of six levels, with relevant weights ranging from 1 to 6, across 20 disease categories. The CCI score for each patient is calculated as the sum of all assigned weights. A score of 0 indicates the absence of any identified comorbidities. The diagnostic content and CCI score are shown in Table 1.
Diagnosis of sarcopenia
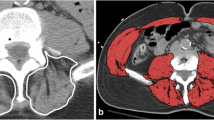
The diagnostic cutoff values for SMI as proposed by Nemec et al [20]. from Harvard University were utilized, with thresholds set at < 42.6 cm2/m2 for men and < 30.6 cm2/m2 for women. The SMI value was calculated by dividing the muscle area at the pedicle level of the patient’s 12th vertebral body on chest CT by the square of the height. The measured muscles included internal obliques, external obliques, rectus abdominis, erector spinae, latissimus dorsi, external intercostals, and intercostal muscles. Image analysis was performed using PACS 3.6 software.
Surgery process of FNF
The patient underwent surgical treatment under general anesthesia. They were positioned in the lateral decubitus position on the healthy side, and a lateral or posterolateral surgical approach was taken. The joint capsule was incised to expose the femoral neck and femoral head. Following the removal of the broken femoral head, osteotomy and correction of the femoral neck were conducted. Total hip replacement or artificial femoral head replacement was then carried out based on the condition and patient’s choice. After selecting the appropriate incremented prosthesis and implanting it, the surgical incision was closed. Postoperatively, low molecular weight heparin was administered for anticoagulation, while cephalosporin antibiotics were utilized to prevent infection. Additionally, lower limb muscle contraction exercises were initiated. Following the removal of the drainage tube on the second day post-operation, rehabilitation exercises commenced under the supervision of a rehabilitation physician.
Evaluation index
Based on the MCC diagnosis, patients were categorized into MCC and NMCC (non-MCC) groups for comparison. The clinical data of patients were obtained from medical records and follow-up data, which included gender, age, length of hospitalization, Harris score, admission to the ICU, complications, number of satisfactions, and survival time. The Charlson Comorbidity Index (CCI) for each patient was then calculated according to Table 1. The study assessed various factors including length of stay, ICU admission rate, postoperative Harris score, SMI value, incidence of sarcopenia, complications, patient satisfaction, 1-year mortality, and 5-year mortality between the two groups. Additionally, the correlation between CCI score and FNF surgical efficacy in the MCC group was analyzed. The research process model diagram is shown in Fig. 1, and the flow chart is shown in Fig. 2.
Statistical analysis
Statistical analysis was conducted using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA). Measurement data were presented as mean ± standard (\(\bar{x} + s\)). Normality test p > 0.05, the data conforms to normal distribution. The t-test was utilized for group comparisons when variances were equal, while the rank sum test was employed for groups with unequal variances. Chi-square test was used for analyzing count data. Factors related to mortality were assessed through multivariate logistic regression analysis and Cox regression models. The Kaplan-Meier test was used to study the survival rate of postoperative patients. Univariate analysis was followed by multivariate analysis to adjust for other independent factors. The significance level for all tests was set at α = 0.05 (two-tailed).
Results
Comparison of general information between MCC and NMCC two groups
Based on the inclusion and exclusion criteria, a total of 103 individuals were included in the MCC group, while 40 individuals were included in the NMCC group. Within the MCC group, there were 49 males and 54 females, with an average age of (79.61 ± 7.52) years. Among them, 63 patients had sarcopenia, with an average SMI of (35.25 ± 10.72) cm2/m2 and an average CCI of (7.22 ± 2.37) points. In the NMCC group, there were 22 males and 18 females, with an average age of (74.68 ± 4.96) years. Ten patients in this group had sarcopenia, with an average SMI of (43.08 ± 8.31) cm2/m2. Differences in age, number of patients with sarcopenia, and SMI values between the two groups were statistically significant (p < 0.001, p < 0.001, p < 0.001). Patients in the MCC group tended to be older, with a higher incidence of sarcopenia and lower SMI values compared to the NMCC group (Table 2).
Comparison of surgical data between MCC and NMCC groups
The average hospitalization time for patients in the MCC group was (13.46 ± 3.29) days, with an average Harris score of (80.28 ± 4.93). Out of 103 patients, 28 were admitted to the ICU, resulting in an admission rate of 27.18%. Additionally, 34 patients experienced complications, leading to a complication rate of 33.01%. A majority of patients (97) expressed satisfaction with the surgical results, indicating a satisfaction rate of 94.17%. Over a 5-year period, there were 29 deaths in the first year, resulting in a survival rate of 71.84%, and 42 deaths by the end of the fifth year, with a survival rate of 59.22%. In comparison, patients in the NMCC group had an average hospitalization time of (12.33 ± 2.01) days and an average Harris score of (82.43 ± 6.46). Only 3 patients were admitted to the ICU, resulting in an admission rate of 7.50%, and 4 patients experienced complications, leading to a complication rate of 10%. The majority of patients (35) were satisfied with the surgical results, with a satisfaction rate of 87.50%. The 1-year mortality rate was 3, yielding a survival rate of 92.50%, and the 5-year mortality rate was 8, resulting in a survival rate of 80.00%. There were notable differences in hospitalization time, Harris score, ICU admission rate, complication incidence, 1-year mortality, and 5-year mortality, all with scientific significance (p = 0.045, p = 0.035, p = 0.019, p = 0.010, p = 0.015, p = 0.019). Patients in the MCC group experienced longer hospital stays, lower Harris scores, higher ICU admission rates, complication rates, and 1-year and 5-year mortalities compared to those in the NMCC group (Table 3).
Correlation analysis between CCI and various indicators in patients with MCC group
Spearman correlation analysis revealed significant associations between CCI and various factors including length of stay (r = 0.282, p < 0.001), Harris score (r = -0.424, p < 0.001), SMI (r = -0.718, p < 0.001), and patient age (r = 0.944, p < 0.001). Specifically, CCI exhibited a significant positive correlation with hospitalization and age, while showing a significant negative correlation with Harris score and SMI value (Table 4).
Univariate and multivariate logistic regression analysis of one-year and five-year mortality rates in MCC group
Single-factor logistic regression analysis was conducted on six variables, including CCI, age, gender, length of stay, Harris score, and SMI. The findings indicated that CCI, age, length of stay, Harris score, and SMI were identified as risk factors for mortality in FNF patients one year post joint replacement (Table 5). Subsequently, a multivariate logistic regression analysis on these factors revealed that the length of hospitalization was a significant risk factor for mortality in FNF patients one year after joint replacement (Table 6). Furthermore, univariate logistic regression analysis demonstrated that CCI, age, length of stay, Harris score, and SMI were associated with increased mortality risk in FNF patients five years post joint replacement (Table 7). The subsequent multivariate logistic regression analysis on these variables indicated that CCI and age were risk factors for mortality in FNF patients five years after joint replacement, while Harris score acted as a protective factor (Table 8).
Kaplan-Meier survival analysis and Cox proportional hazard model analyzed the influencing factors of death in the two groups of patients
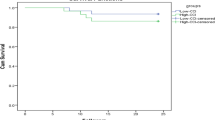
The log-rank test of the Kaplan-Meier curve indicated that the difference in five-year survival rates between the two groups of MCC and NMCC patients was statistically significant. The NMCC group exhibited a longer survival period, while chronic disease comorbidities were found to significantly reduce the survival time of patients (p = 0.016) (Fig. 3). Furthermore, the Cox proportional hazard model analysis identified CCI, age, and SMI as risk factors influencing patient mortality (Fig. 4, Table 9).
Discussion
Our results indicated that among patients with FNF, those with multiple chronic diseases were older and more likely to develop sarcopenia. The hospitalization days, risk of admission to the ICU, number of complications, and both one-year and five-year mortality rates were all higher in the MCC group compared to the NMCC group, while the Harris score was lower in the MCC group. The CCI in patients with FNF was significantly positively correlated with hospitalization duration and age, and significantly negatively correlated with the Harris score and SMI value. Multivariate logistic regression analysis revealed that CCI and age were risk factors for mortality in patients with FNF five years after joint replacement, whereas the Harris score served as a protective factor. Kaplan-Meier survival analysis demonstrated that the five-year survival rate of patients in the NMCC group was lower. Additionally, Cox proportional hazards model analysis identified CCI, age, and SMI as risk factors for patient mortality.
Chronic comorbidities, frailty, and sarcopenia are significant concerns in the elderly population in my country, impacting both physical and mental health. With age, organ function naturally declines, often leading to the development of chronic diseases. This complicates treatment and reduces clinical effectiveness, underscoring the importance of prevention and management of these conditions. Elderly individuals with chronic comorbidities are more likely to develop sarcopenia, which is closely linked to prognosis and mortality. The diagnosis of sarcopenia involves assessing skeletal muscle mass, muscle strength, and physical function, with grip strength being a key factor [21,22,23]. Various imaging techniques such as dual-energy X-ray (DXA), CT, and magnetic resonance imaging (MRI) are used for diagnosis, but global standards for sarcopenia are lacking. The CCI, established in 1987, is a tool used to assess disease severity and predict mortality [23]. While CCI has been shown to effectively predict mortality, its application in predicting fractures, particularly hip fractures, remains understudied.
Hip fractures, such as FNF and intertrochanteric fractures, are a significant global health concern. Projections suggest that by 2050, there will be 6.3 million hip fractures worldwide, impacting patient outcomes and mortality rates [24]. Statistics indicate that within one month of a hip fracture, the mortality rate is 13.3%, rising to 24.5% at one year and 34.5% at two years [2]. Additionally, one year post-fracture, 33% of patients are either dependent on others or residing in a nursing home [25]. Elderly individuals with hip fractures often present with multiple comorbidities, complicating treatment and leading to higher mortality and disability rates, as well as diminished quality of life and increased societal burden [26]. Various factors such as chronic diseases, advanced age, surgical interventions, and recovery time play crucial roles in determining prognosis and mortality rates [27]. Postoperative infection is a challenging complication associated with hip fracture surgery. Elderly patients with comorbidities undergoing joint replacement surgery are at high risk for complications [28]. A study investigating intramedullary nail infections following trochanteric fracture surgery revealed that patients experiencing treatment failure exhibited higher CCI scores [29]. Similarly, our study found that patients in the MCC group had a greater incidence of complications. Patients without comorbidities have an in-hospital mortality rate of 1–2%, which escalates with the presence of additional diseases [30]. Research has shown that a history of myocardial infarction and cardiac conditions like congestive heart failure can heighten the risk of postoperative mortality [31,32,33]. A meta-analysis conducted by Smith et al [34]. demonstrated that high CCI scores are correlated with elevated mortality rates. Both univariate and multivariate logistic regression analyses in a separate study identified CCI as a significant risk factor for in-hospital mortality [35]. Despite being recognized as a reliable predictor of mortality [23], the full extent of CCI’s impact on fractures remains to be thoroughly assessed.
In this study, the 21 items of CCI were weighted differently. There was no statistically significant gender difference between the MCC group and the NMCC group, reducing bias in SMI-related comparisons. Patients in the MCC group were older, had higher rates of sarcopenia, ICU admission, and complications, lower SMI values and Harris scores, and longer hospital stays. Correlation analysis revealed that CCI was positively correlated with hospitalization and age, and negatively correlated with Harris score and SMI value. A study investigating bipolar hemiarthroplasty for unstable intertrochanteric fractures in the elderly identified the Charlson Comorbidity Index (CCI) as an independent predictor of 1-year mortality in univariate regression analyses. The sensitivity of the CCI in predicting 1-year mortality was found to be 80% [36]. Among patients with hip fractures and acute kidney injury (AKI), a lower CCI score was significantly associated with higher mortality rates in those experiencing severe AKI. Notably, in patients with a CCI as low as 3, the detrimental impact of severe AKI was more pronounced. Conversely, higher CCI scores (≥ 8) did not demonstrate a significant effect on mortality [37]. Previous research by Simo S. A et al [38]. demonstrated a significant correlation between CCI score and mortality in hip fracture patients over a 10-year follow-up period. Patients with a CCI score ≥ 4 had a 3.1–8.5 times higher risk of death during follow-up compared to those with a lower CCI score. Univariate and multivariate logistic regression analysis identified hospitalization time as a risk factor for death within 1 year post joint replacement in FNF patients. Prolonged hospitalization may be due to poor physical condition or ICU admission, increasing the risk of death within 1 year. CCI and age were identified as risk factors for death 5 years after joint replacement in FNF patients, while Harris score was a protective factor. Therefore, higher number of chronic diseases and older age in patients are associated with increased long-term mortality risk. These findings align with previous research, suggesting CCI as a potential predictor for hip fracture healing and mortality [39,40,41]. However, some scholars have expressed differing opinions. Eveline de Haan et al [42]. verified the predictive capabilities of the CCI for 30-day and 1-year mortality in patients undergoing hip fracture surgery. They found that the area under the curve was relatively low for both timeframes, regardless of whether the original or adjusted CCI was used. Consequently, the CCI is not recommended for predicting 30-day and 1-year mortality in this patient population. We believe that this contradictory result may stem from variations in the types of patients included in the studies and the statistical methods employed. Nevertheless, the CCI remains a valuable tool for predicting surgical efficacy and survival in patients with FNF.
Chronic disease management focuses on prevention and control rather than cure. Many chronic diseases can be prevented and controlled, highlighting the need for grassroots medical and health institutions to prioritize chronic disease management. Patients should be educated on chronic diseases, encouraged to adopt healthy habits, engage in physical exercise, participate in communication, and prioritize mental well-being. Hospitals and doctors should establish specialized outpatient clinics for chronic comorbidities, maintain health records, monitor relevant patient groups, use therapeutic drugs judiciously, advocate for comprehensive treatment, and provide enhanced care for elderly patients with multiple chronic conditions.
However, this study has limitations including its retrospective nature, inability to intervene on certain variables, lack of detailed assessment on indicators related to patient frailty, and exclusion of surgery-related indicators. Some patients had comorbidities in addition to those classified by the CCI, but these were not included in this study. This exclusion may introduce bias into the results, indicating that more detailed research is necessary in the future.
Conclusions
In conclusion, the study found that the surgical prognosis of patients with multiple chronic conditions, CCI, and FNF are interconnected. Patients with a high CCI exhibit a greater incidence of sarcopenia, a higher proportion of ICU requirements and complications, poor recovery of limb function, and an increased risk of long-term mortality. This underscores the necessity for clinicians to consider the management of multiple chronic conditions in conjunction with fracture treatment.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Robert SS. Gender and race/ethnicity differences in hip fracture incidence, morbidity, mortality, and function. Clin Orthop Relat Res. 2010;469(7).
Fangke H, Chengying J, Jing S, Peifu T, Yan W. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury. 2011;43(6).
Lucas EN, Edward JF, Kevin PB, Charles D, Lucille A, Christopher SH. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1).
Youliang H, Ruideng W, Zhengyang C, Fang Z, Hongquan J, Yun T et al. One-year mortality risk in older individuals with femoral intertrochanteric fracture: a tertiary center in China. BMC Geriatr. 2024;24(1).
Zhiyong C, Hui F, Xiangyu M, Siying Z, Zhaorui L, Kaifeng Y et al. Age-specific 1-year mortality rates after hip fracture based on the populations in mainland China between the years 2000 and 2018: a systematic analysis. Arch Osteoporos. 2019;14(1).
Chemmami A, Aour B, Zahaf S, Dahmane M, Mehdi I, Boutchicha D. Biomechanical comparison of three total artificial discs: sb-charite iii®, prodiscl® and maverick® reinforced by a posterior fixation system in the spinal column: a threedimensional finite element analysis. Struct Integr Life. 2021;21(1):65–83.
Zahaf S, Kebdani S. Biomechanical Study between the rigid and dynamic fixation systems of the spinal column analyzed by the Finite element Method. Nano Biomed Eng. 2017;9(2).
Ron BE, Basel K, Eyal Y, Ezequiel P, Omer M, David S et al. Better Short-Term Outcomes after Total Hip Arthroplasty Compared to Hemiarthroplasty in active older patients with displaced intracapsular femoral Neck fracture. Isr Med Assoc J. 2023;25(12).
Kylie TC, Megan D, Brandon L, Maddison M, Ryan D, William M et al. Risk factors for postoperative delirium in orthopaedic hip surgery patients: a database review. BMC Musculoskelet Disord. 2024;25(1).
Zahaf S, Dahmane M, Belaziz A, Bouri I, Afane N, Failure analysis of semi-elliptical, crack behavior in the cement mantle of a total hip prosthesis. Mater Phys Mech. 2022;48(2).
Jan V, Milan V, Geert V, Filip V. Postoperative radiograph of the hip arthroplasty: what the radiologist should know. Insights Imaging. 2015;6(6).
Ying H, Mengru Z, Lei Z, Jingzheng S, Yuan Y, Fuyou L et al. Dietary inflammatory potential is Associated with Sarcopenia among chronic kidney Disease Population. Front Nutr. 2022;9(0).
Nitchanant K, Pichitchai A, Jiraporn K, Phichayut P, Aasis U. Prognostic factors for functional recovery at 1-Year following fragility hip fractures. Clin Orthop Surg. 2024;16(1).
Debbie N-A, Anne Sofie L, Jes Bruun L, Benn Rønnow D, Susanne vdM, Mathias M, et al. Metaanalysis of risk factors for mortality in patients with hip fracture. Dan Med J. 2013;60:8.
Akash KG, Sandeep P, Devendra C, Tanvir S, Rajendra KK, Ashish B. Pre-hospital delays represent unnoticed intervals that affect mortality rates in geriatric hip fractures: a prospective cohort study. Cureus. 2023;15(9).
Suo-Hsien W, Chia-Wei C, Shion-Wei C, Ting-Shuo H, Rueyshyang S, Ngi-Chiong L et al. Surgical intervention may provides better outcomes for hip fracture in nonagenarian patients: a retrospective observational study. Heliyon. 2024;10(3).
Travis MK, Aaron S, Lindsey NP, Ahmed AM, Ali S, Chance CM. Comparing Common Risk Assessment Tools to predict outcomes in total knee arthroplasty. J Arthroplasty. 2024;(0).
Su Mi L, Mi Yeun H, Su Hyun K, Ran Hui C, Seock Hui K, Jun Chul K et al. Indoxyl sulfate might play a role in Sarcopenia, while myostatin is an Indicator of muscle Mass in patients with chronic kidney disease: analysis from the RECOVERY study. Toxins (Basel). 2022;14(10).
Heloísa T, Gabrielle K, Thaísa HJ, Ricardo RP, Carolina AM, Victória ZCB. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10(0).
Ursula N, Benedikt H, Claire S, Louis C, Ronald LE. Diagnosing Sarcopenia on thoracic computed tomography: quantitative Assessment of skeletal muscle Mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. 2017;24(9).
Seung Hoo L, Hyun Sik G. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and osteoporosis. J Bone Metab. 2020;27(2).
Vanesa D-Y, Ester M, Dolores S-R, Anna G-S, Xavier D, Eva MP et al. Sarcopenia according to the revised European Consensus on Definition and diagnosis (EWGSOP2) Criteria predicts hospitalizations and long-term mortality in Rehabilitation patients with stable chronic obstructive Pulmonary Disease. J Am Med Dir Assoc. 2019;20(8).
K L A MECPP, C R. M. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5).
Carmen AB, Marcelo C-P, David MC, Allison BR. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14).
Cynthia LL, Anna NAT, Sherine EG, Jeanine ER, Joseph L. M. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50(10).
Jenson CSM, Ian DC, Lyn MM. Evidence-based guidelines for the management of hip fractures in older persons: an update. Med J Aust. 2010;192(1).
C TWL. F, F L. Assessment of postoperative short-term and long-term mortality risk in Chinese geriatric patients for hip fracture using the Charlson comorbidity score. Hong Kong Med J. 2015;22(1).
Flaviu M. Bone cement implantation syndrome: a rare disaster following cemented hip arthroplasties-clinical considerations supported by Case studies. J Pers Med. 2023;13(9).
Bernadette P, Marco AVG, Antonio BG, Álvaro AR, Jaime E, Joaquín GC. Risk factors for therapeutic failure and one-year mortality in patients with Intramedullary nail-Associated infection after Trochanteric and Subtrochanteric hip fracture repair. Antibiot (Basel). 2024;13(5).
Valentin N, John K, Michiel GH, David CR. Charlson comorbidity indices and in-hospital deaths in patients with hip fractures. Clin Orthop Relat Res. 2012;471(5).
Pia Nimann K, Susanne vdM, Pia E, Bo A. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39(2).
A N A T, D J G, D C R, E S F. L J 3rd M. excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11).
L PV. R, L M. Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos Int. 2007;18(12).
Toby S, Kelum P, Martin B, Alice O, Phyo Kyaw M. Pre-operative indicators for mortality following hip fracture surgery: a systematic review and meta-analysis. Age Ageing. 2014;43(4).
Liang EW, John Carson C, Tet Sen A. H, Joyce Suang Bee K. Comorbidity as the dominant predictor of mortality after hip fracture surgeries. Osteoporos Int. 2019;30(12).
Germán G, Cesar Angel P, Leonel PA, Glenda E, Hernan DS. Bipolar hemiarthroplasty in unstable intertrochanteric fractures in elderly patients. The predictive value of the Charlson Comorbidity Index in 1-year mortality. J Clin Orthop Trauma. 2022;25(0).
Saulo Lacerda BdS, Maria Luiza Medeiros F, Tiago Lins Oliveira G, Alexandre Braga L. Comorbidities, acute kidney injury and long-term mortality in elderly patients hospitalized because of hip fracture: a moderation analysis. Aging Clin Exp Res. 2024;36(1).
Simo SAM, Susanna S, Heikki K. Charlson comorbidity index predicts the 10-year survivorship of the operatively treated hip fracture patients. Eur J Orthop Surg Traumatol. 2022;33(4).
Barbara T, Lara AH, Jacqueline CTC. New ICD-10 version of the multipurpose Australian Comorbidity Scoring System outperformed Charlson and Elixhauser comorbidities in an older population. J Clin Epidemiol. 2016;79(0).
David M, James M, Antonella D, Andrew J, Daniel P, Cheryl Z et al. Coding algorithms for defining Charlson and Elixhauser co-morbidities in Read-coded databases. BMC Med Res Methodol. 2019;19(1).
Tom K, Benjamin B, Mathias B, Sebastian P, Christopher B, Matthias K et al. Predictors of long-term survival after hip fractures?-5-year results of a prospective study in Germany. Arch Osteoporos. 2019;14(1).
Eveline dH B, vO, Veronique A J I M vR T, Martijn K, Louis dJ, Gert RR. Validation of the Charlson Comorbidity Index for the prediction of 30-day and 1-year mortality among patients who underwent hip fracture surgery. Perioper Med (Lond). 2024;13(1).
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Pingping Wang was responsible for the conception and design of the article, statistical analysis and chart drawing, writing and revision of the article. Shenghua Guo was responsible for data collection and data extraction, and quality control and review of the article.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Jiangning Hospital Affiliated to Nanjing Medical University.
Human ethics and consent to participate declarations
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, P., Guo, S. Correlation between Charlson comorbidity index and surgical prognosis in elderly patients with femoral neck fractures: a retrospective study. BMC Musculoskelet Disord 25, 678 (2024). https://doi.org/10.1186/s12891-024-07814-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07814-2