Abstract
Background
Musculoskeletal conditions and physical frailty have overlapping constructs. We aimed to quantify individual contributions of musculoskeletal factors to frailty.
Methods
Participants included 347 men and 360 women aged ≥60 yr (median ages; 70.8 (66.1–78.6) and 71.0 (65.2–77.5), respectively) from the Geelong Osteoporosis Study. Frailty was defined as ≥3, pre-frail 1–2, and robust 0, of the following; unintentional weight loss, weakness, low physical activity, exhaustion, and slowness. Measures were made of femoral neck BMD, appendicular lean mass index (ALMI, kg/m2) and whole-body fat mass index (FMI, kg/m2) by DXA (Lunar), SOS, BUA and SI at the calcaneus (Lunar Achilles Insight) and handgrip strength by dynamometers. Binary and ordinal logistic regression models and AUROC curves were used to quantify the contribution of musculoskeletal parameters to frailty. Potential confounders included anthropometry, smoking, alcohol, prior fracture, FMI, SES and comorbidities.
Results
Overall, 54(15.6%) men and 62(17.2%) women were frail. In adjusted-binary logistic models, SI, ALMI and HGS were associated with frailty in men (OR = 0.73, 95%CI 0.53–1.01; OR=0.48, 0.34–0.68; and OR = 0.11, 0.06–0.22; respectively). Muscle measures (ALMI and HGS) contributed more to this association than did bone (SI) (AUROCs 0.77, 0.85 vs 0.71, respectively). In women, only HGS was associated with frailty in adjusted models (OR = 0.30 95%CI 0.20–0.45, AUROC = 0.83). In adjusted ordinal models, similar results were observed in men; for women, HGS and ALMI were associated with frailty (ordered OR = 0.30 95%CI 0.20–0.45; OR = 0.56, 0.40–0.80, respectively).
Conclusion
Muscle deficits appeared to contribute more than bone deficits to frailty. This may have implications for identifying potential musculoskeletal targets for preventing or managing the progression of frailty.
Similar content being viewed by others
Background
With the progressive age, the prevalence of frailty increases [1,2,3]. Frailty, a clinical syndrome that affects multiple physiological systems, is associated with a diminished functional reserve and an increased vulnerability to adverse events such as falls and fractures [4], as well as minor stressors such as colds [5, 6]. There are a variety of definitions for frailty depending on the assessment tool used [5]. However, the two models that dominate the literature and are validated in large populations are the Fried frailty phenotype and the frailty index of accumulation of deficits [6, 7]. The former focuses on the physical phenotype of frailty and considers the deterioration of physical performance and robustness. In contrast, the frailty index of deficit accumulation includes biomedical and psychosocial factors to identify frailty on a cumulative scale [7].
With ageing, there is a reduction of bone mass, muscle mass and strength, which may result in osteoporosis, sarcopenia or a combination of the two, known as osteosarcopenia [8,9,10]. Research has shown that musculoskeletal conditions are a major cause of functional impairment and disability [11, 12] and that frailty is associated with lower bone mineral density (BMD), muscle or lean mass and handgrip strength (HGS) [6, 13,14,15]. However, to our knowledge, to date, no study has quantified the contribution of musculoskeletal components to frailty. This is pertinent to the understanding the condition and highlighting potential targets for interventions. Thus, the aim of this study was to investigate the association between musculoskeletal factors and frailty, defined using the Fried frailty phenotype, and quantify their contributions to frailty.
Methods
This cross-sectional study included men and women enrolled in Geelong Osteoporosis Study (GOS). GOS is an ongoing population-based cohort study involving more than 3200 randomly selected adults (approximately 98% Caucasian) from the Barwon Statistical Division in south-eastern Australia. Full details of the study have been published elsewhere [16]. For these analyses, we utilised cross-sectional data from the 15-yr follow-up assessment phase for both men (2016–2019) and women (2011–2014). Men and women aged ≥60 yr who provided sufficient data for addressing Fried frailty criteria were included in this study (n = 360 and n = 347 respectively) (Fig. 1a and b). There were no exclusions for comorbidities or behaviours.
The study was approved by the Human Research Ethics Committee at Barwon Health. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from all participants in the study.
Visit assessment
Participants completed questionnaires that documented physical activity [17] dietary information [18], lifestyle factors and health behaviours, sociodemographic factors, medications, comorbidities and post-baseline fractures that were radiologically confirmed.
Frailty assessment
Frailty was identified using a modified Fried frailty phenotype [6], which categorised individuals into frail, pre-frail or robust groups, based on the responses to five criteria including unintentional weight loss, weakness, low physical activity, exhaustion and slowness. Weakness was determined using handgrip strength (HGS) measured with a hand-held Jamar dynamometer (Sammons Preston, Bolingbrook, IL, UK) for women [19] and Vernier dynamometer (Venier Software and Technology, Beaverton, USA) for men. Vernier values were converted to Jamar equivalents using an equation previously described [20]. HGS values below cut-points equivalent to the lowest 20% stratified by sex and body mass index (BMI) were considered as weakness [6]. The HGS procedure was demonstrated to participants before the trials. For women, there was no interval between trials, and for men, there was a 3 s interval between the trials; for both sexes the mean of the maximum reading from each hand was used in analyses. Slowness was measured using the Timed Up & Go (TUG) test that measures functional mobility including balance and muscle performance [21]. The time was recorded for the participant to stand from a chair (standard height without armrests), walk 3 m, turn around and return to sit back in the chair. A score ≥ 10s was considered as slow [22]. Unintentional weight loss, exhaustion and low physical activity were self-reported. Low physical activity was ascertained using a single question that asked about the current mobility status of participations. Low physical activity was indicated when participants selected one of the following responses: “limited”, “inactive”, “chair” or “bedridden”, or “bedfast”. Participants with at least three of the five items in the modified Fried tool were categorised as frail, 1–2 items as pre-frail, and zero items as robust.
Musculoskeletal assessment
Using electronic scales and a wall-mounted Harpenden stadiometer, weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, and BMI was calculated (kg/m2). Using dual-energy X-ray absorptiometry (DXA, Lunar Prodigy Pro), areal bone mineral density (BMD) was measured at the femoral neck, and measures of total and regional body fat and lean tissue mass were derived from the whole-body scans. DXA-derived lean mass was used as a surrogate measure for skeletal muscle mass. Appendicular lean mass index (ALMI) was calculated as appendicular lean mass divided by height squared (kg/m2); similarly, fat mass index (FMI) was calculated as whole body fat mass divided by height squared (kg/m2). Quantitative calcaneal ultrasound was performed on the left heel using a Lunar Achilles Insight ultrasonometer (GE Lunar, Madison, WI, USA), with measures expressed as speed of sound (SOS), broadband ultrasound attenuation (BUA) and stiffness index (SI) indicating bone quality [23]. Low trauma fractures (excluding fingers, toes, skull and face) from baseline visit until the follow-up visit for participants were ascertained using radiological reports from the radiological imaging centres servicing the region. This method of fracture ascertainment has been previously validated and described in detail [24, 25].
Comorbidity assessment
Comorbid conditions were classified using a modified Charlson comorbidity index (CCI) [26], that took into account the number and seriousness of comorbid diseases. Using a combination of self-reported and measured data, participants were assigned weights for the following conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, moderate or severe liver disease, mild liver disease, diabetes hemiplegia, moderate or severe renal disease, diabetes with end organ damage, any tumour, leukaemia, lymphoma, metastatic solid tumour, and acquired immunodeficiency syndrome (AIDS) [26].
Lifestyle factors and socioeconomic status
Socioeconomic Indexes For Areas (SEIFA) values using 2011 Australian Bureau of Statistics (ABS) census data were determined using ABS software (these rank areas in Australia according to relative socio-economic advantage and disadvantage), which were ranked in quintiles [16] and, due to small numbers, collapsed into three groups of low (quintile 1 and 2), medium (quintile 3) and high (quintiles 4 and 5). Data regarding current smoking status (yes/no) were derived from a questionnaire. Alcohol consumption exceeding 30 g/day was derived from the Victorian Cancer Council Food Frequency Questionnaire [18].
Statistical analyses
Descriptive characteristics of participants were presented as median (IQR) or mean (±SD) or n (%). Participants’ characteristics for frail, pre-frail, and robust groups were compared using analysis of variance (ANOVA) for parametric data, Kruskal-Wallis test for non-parametric data and chi-square test for categorical factors.
Exploratory analyses that investigated the contribution of muscle and bone parameters to frailty included binary logistic regression models where dichotomised frailty (yes/no; frailty vs pre-frail and robust combined) was considered the outcome. The area under the receiver characteristic curves (AUROCs) was also calculated. In addition, ordinal logistic regression models were used, where frailty was considered in three groups (frail, pre-frail and robust). For these statistical models, BMD, BUA, SOS, SI, ALMI and HGS measures were converted to standard deviation (SD) units using published reference data [19, 27,28,29,30]. The following characteristics were included in multivariable models: age, weight and height or BMI, smoking status, alcohol, prior fracture, FMI, SES and CCI and retained if p < 0.05. Somers’ D nonparametric ordinal association was calculated as a post-hoc measure in ordinal logistic models. These confounders were included as studies have shown association between these factors and frailty [6, 11, 31,32,33]. The estimated Somers’ D associations were adjusted for the characteristics included in the model [34]. Values closer to ±1 for Somers’ D suggest the higher contribution of a musculoskeletal factor in relation to frailty status and values tending towards zero in either direction indicate lower contribution of a musculoskeletal factor. Non-linearity assumption of the logistics models was assessed for key continuous covariates through comparing the linear trend logistic model with alternative nonlinear model assuming square and cubic nonlinear structure [35]. Boxplots were also investigated to explore the association between the frailty items and musculoskeletal parameters. Data for men and women were analysed separately. Minitab (Version 18, State College, PA, USA) and STATA (Version 16, College Station, Texas, USA) statistical software were used for statistical analyses.
Results
Men
The participants’ descriptive characteristics for the whole group and according to frailty status are displayed in Table 1. Frail men were older, had a higher mean BMI and were more likely to have higher mean FMI. Frail men were also under-represented in the highest SES tertile. No other differences were observed.
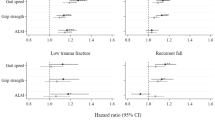
In unadjusted binary logistic models, SI, ALMI and HGS were associated with frailty in men (Table 2). The association was sustained after adjustments for relevant characteristics, where men with higher measures were less likely to be frail; SI (OR 0.73 95%CI 0.53–1.01, p = 0.06), ALMI (OR 0.48 95%CI 0.34–0.68, p < 0.001) and HGS (OR 0.11 95%CI 0.06–0.22, p < 0.001) (Fig. 2). No other associations were observed. Unadjusted ordinal logistic models showed similar results and these associations were sustained in adjusted models (Table 4).
Muscle parameters contributed more to frailty than bone parameters (Tables 2 and 4). HGS made a greater contribution to frailty than ALMI (AUROC 0.85 and 0.77 respectively, p < 0.01) (Table 2). This was also observed in the ordinal regression model, where HGS had a stronger effect (Somers’ D correlation of − 0.49 (− 0.57- (−) 0.41), p < 0.01) (Table 4), meaning that HGS improved our prediction of frailty status by 49%, greater than the 36% observed for ALMI. Of the bone parameters, only SI contributed to the model and the contribution after adjusting for age and BMI was 0.71 (AUROC) (p = 0.06).
For femoral neck BMD, lower mean scores were associated with the following frailty items; low physical activity, fatigue, slowness and low HGS. Weight loss was associated with higher mean femoral neck BMD. Similar results were observed for ALMI and heel ultrasound measures (BUA, SOS, SI), where lower mean scores were associated with low physical activity, fatigue, slowness and low HGS, while the inverse was true for weight loss. (Supplementary Fig. 1). Overall, for low physical activity, SI median scores had the greatest percentage decrease (13.0%) in median score between the yes and no groups compared to the other musculoskeletal parameters. For fatigue, ALMI had the greatest percentage decrease (1.7%) while for slowness, SI had the greatest percentage decrease (10.1%). For low HGS, ALMI had the greatest percentage decrease (5.7%). Lastly, for weight loss, SI had the greatest percentage increase of 7.4% between the no and yes groups. In correlation tests for frailty items, slowness (as measured by TUG) showed a strong correlation (Supplementary Table 1) and thus, TUG was modelled as binary logistic regression models. The TUG models were inferior in the AUROC for the ALMI model (from 0.77 to 0.76 (p < 0.001)) and the HGS model (from 0.85 to 0.79 (p < 0.001)).
Women
Frail women were also older and shorter and had a higher mean BMI than pre-frail and robust women. They were more likely to have a prior fracture and a higher number of comorbid conditions (Table 1).
In unadjusted binary logistic models, BMD and HGS were associated with frailty (Table 3). After adjustments for age, weight and height, only HGS was associated with frailty, where women with greater HGS were less likely to be frail (OR 0.30, (95%CI 0.20–0.45) p < 0.001) (Fig. 2). No other associations were observed (Table 3). In unadjusted ordinal logistic models, HGS was associated with frailty, while an association that did not achieve statistical significance was observed with ALMI. After adjustments for age, height, FMI and SES, HGS and ALMI were associated with frailty (Table 4).
Forest plot of odds ratios (OR 95% confidence interval) of binary logistic multivariable models (which included age, weight and height or body mass index, smoking status, alcohol, prior fracture, fat mass index, socioeconomic status and Charlson comorbidity index) the contribution of musculoskeletal factors (bone and muscle) to frailty. *Footnotes: BMD = bone mineral density at femoral neck, SOS = speed of sound, BUA = broad-band ultrasound attenuation, SI = stiffness index, ALMI = appendicular lean mass index, HGS = handgrip strength. *Indicates significant difference p < 0.05
In binary logistic regression models, only HGS was observed to contribute to the model (AUROC 0.83, p < 0.001) (Table 3). However, when frailty was considered as three groups, ALMI was also observed to contribute to the model (p < 0.001). In these ordinal regression models, HGS had a stronger contribution to frailty than did ALMI (Table 4).
Overall boxplots that explored the association between the frailty items and musculoskeletal parameters did not reveal any apparent associations or trends across the musculoskeletal parameters. (Supplementary Fig. 2).
In correlation tests for frailty items, slowness (as measured by TUG) showed a strong correlation (Supplementary Table 2) and thus, TUG was modelled as binary logistic regression models. The TUG models were inferior compared with the HGS models (AUROC decrease from 0.83 to 0.74 (p = 0.001)).
For all models with significant exposure (i.e., for men: SI, ALMI and HGS and for women: ALMI and HGS), an inclusive model that included important potential confounders irrespective the p-values were employed. A link-test was utilised to test for any potential regression model specification error [36]. The link-test tests the idea that if a regression equation is properly specified no additional independent variables that are significant can be found except by chance. In addition, the square and cubic terms were added to the logistics models to test for potential nonlinearity. In conclusion none of the link-tests or testing the joint square and cubic terms were significant, and as such we did not detect any strong evidence of lack of regression goodness of fit. Furthermore, multivariate regression models that included necessary covariates were constructed, however, the conclusion did not substantially change by these adding additional factors (Supplementary Table 2-14).
Discussion
Our study suggests that muscle parameters make a greater contribution to frailty compared to bone parameters for men and women. For men, when frailty was considered as a binary outcome, muscle measures (lean mass and HGS) contributed to models of frailty more than bone measures (BMD, BUA, SOS and SI). Similar results were observed when frailty was considered in the three groups (robust, pre-frail and frail). For women, considering frailty as a binary outcome, only HGS contributed to the models. However, when frailty was considered in three groups, lean mass and HGS contributed to the models. No bone measures were observed to contribute to frailty in women.
The clinical signs of frailty include weight loss, reduced physical activity, balance and gait speed, reduced cognitive function and altered state of nutrition, and our data suggests contributions by parameters of sarcopenia and a lesser contribution from osteoporosis [8]. In a study of 3231 European men aged 40-79 yr, pre-frail and frail men had lower SOS and BUA and frail men had a lower femoral BMD compared to robust men [37]. Our study indicates that SI, a mathematical combination of SOS and BUA, was associated with frailty in men [23]. No association was observed with femoral neck BMD in our study which concurs with another study including 392 community dwelling men age 58–95 yr which reported that although femoral neck BMD was associated with frailty, this association was attenuated after adjustment for age [38]. Another study of 235 community-dwelling older women observed frailty, defined by the Fried phenotype and a self-reported Vulnerable Elders Survey (VES-13), was not correlated with hip or spine BMD, although frail women (defined by VES-13) had lower hip and spine BMD after one year [14]. While in a longitudinal study of 75 yr old community-dwelling women, frailty was associated with low BMD and higher mortality risk [39]. Similar observations were made in another study including 257 community-dwelling participants of the Women Health and Aging Study II, with higher rates of frailty among those with severe osteoporosis/osteopenia as defined in terms of low BMD [13].
Sarcopenia, defined as age-related loss of muscle mass and a decline in muscle function [40] has overlapping constructs with frailty [41,42,43]. Overall, our data were observed to suggest that muscle parameters in both men and women as major contributors to frailty as compared to bone parameters. Indeed HGS contributed the most to the models compared to other musculoskeletal parameters, which is likely to reflect the consideration of HGS as a marker of weakness in the Fried definition of frailty [6]. The use of a different tool such as the Rockwood frailty index of deficit accumulation [7] may present different results as the focus of this tool is both physical aspects of frailty as well as psychosocial domains. At present, sufficient data were not available in our study to calculate the frailty index of deficit accumulation to test this theory.
During ageing there is concomitant deterioration of muscle and bone that may manifest in the form of frailty [12, 44]. Although both sarcopenia and osteoporosis contribute to frailty secondary to age-related changes in body composition, hormonal imbalance and chronic inflammation [8, 12, 45], parameters of osteoporosis appeared to contribute less than measures of sarcopenia. Our models, at least in men, suggest that individuals with marked deficits in both bone and muscle (osteosarcopenia) are more likely to be frail. However, with advancing age, there is an integrated and progressive decline of both muscle and bone [9], making it difficult to make a clear distinction of their individual contributions to frailty.
The strengths of this study include the use of objective measures for bone and muscle parameters and that participants were from an unselected population. Our study also had some limitations including the use of a modified Fried phenotype, some self-reported data and the use of DXA-derived lean mass as a surrogate for muscle mass. Although different dynamometers were used in assessing HGS in men and women, the data were harmonised by converting Vernier values into Jamar equivalents. Participants were pre-dominantly Caucasian, thus, our results may not be applicable to other populations. As data were cross-sectional, no inferences can be drawn about causality.
Conclusion
From the parameters measured in this study, our data suggest that muscle deficits may contribute more to frailty than bone deficits (however due to the cross-sectional nature of the study, causality cannot be conferred), and this was observed for both men and women. From these observations, interventions for addressing the trajectory of frailty might be targeted to improve muscle health, although this conjecture would need to be investigated using prospective data from a longitudinal study as well as data from intervention studies targeting muscle and/or bone. We suggest further studies should be performed using other definitions frailty and in larger diverse populations to obtain a more comprehensive overview of the contributions of musculoskeletal parameters to frailty.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to data privacy rules but are available from the corresponding author on reasonable request.
Abbreviations
- AIDS:
-
Immunodeficiency syndrome
- ABS:
-
Australian Bureau of Statistics
- ALMI:
-
Appendicular lean mass index
- ANOVA:
-
Analysis of variance
- AUROCs:
-
Area under the receiver characteristic curves
- BMD:
-
Lower bone mineral density
- BMI:
-
Body mass index
- BUA:
-
Broadband ultrasound attenuation
- CCI:
-
Charlson Comorbidity Index
- DXA:
-
Dual-energy X-ray absorptiometry
- FMI:
-
Fat mass index
- GOS:
-
Geelong Osteoporosis Study
- HGS:
-
Handgrip Strength
- IQR:
-
Interquartile range
- OR:
-
Odds Ratio
- SD:
-
Standard deviation
- SEIFA:
-
Socioeconomic Indexes For Areas
- SES:
-
Social economic status
- SI:
-
Stiffness index
- SOS:
-
Speed of Sound
- TUG:
-
Timed Up and Go
- VES-13:
-
Vulnerable Elders Survey
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62.
Iqbal J, Denvir M, Gunn J, Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62 Available from: http://www.thelancet.com/journals/a/article/PIIS0140-6736(13)61203-9/fulltext.
Tembo MC, Holloway-Kew KL, Sui SX, Dunning T, Low ACH, Yong SJ, et al. Prevalence of frailty in older men and women: cross-sectional data from the Geelong osteoporosis study. Calcif tissue Int. 2020;107:220–9. Available from:. https://doi.org/10.1007/s00223-020-00713-3.
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54(6):991–1001.
Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14(6):392–7 [cited 2017 Jul 17]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1525861013001825.
Fried L, Tangen C, Walston J, Newman A, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56(3):M146–56.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7.
Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne). 2019;10(APR):1–10.
Pasco JA. Age-related changes in muscle and bone. In: Dusque G, editor. Osteosarcopenia: Bone, Muscle and Fat Interactions. Springer International Publishers; 2019. p. 45–71. https://doi.org/10.1007/978-3-030-25890-0.
Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46(11):849–53.
Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: Two sides of the same coin. Front Aging Neurosci. 2014;6(JUL):1–4.
Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol. 2014;28(3):395–410. Available from:. https://doi.org/10.1016/j.berh.2014.07.005.
Frisoli A, Chaves PH, Ingham SJMN, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s health and aging study (WHAS) II. Bone. 2011;48(4):952–7. Available from:. https://doi.org/10.1016/j.bone.2010.12.025.
Sternberg SA, Levin R, Dkaidek S, Edelman S, Resnick T, Menczel J. Frailty and osteoporosis in older women - a prospective study. Osteoporos Int. 2014;25(2):763–8.
Ma SL, Oyler J, Glavin S, Alavi A, Vokes T. Self-reported frailty is associated with low calcaneal bone mineral density in a multiracial population of community-dwelling elderly. Osteoporos Int. 2009;20(11):1837–46.
Pasco JA, Nicholson GC, Kotowicz MA. Cohort profile: Geelong osteoporosis study. Int J Epidemiol. 2012;41(6):1565–75.
LEE V, Ravelli AC, Dongelmans PC, Deurenberg PA, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exer. 1991;23:974–9 Available from: http://journals.lww.com/acsm-msse/abstract/1991/08000/a_physical_activity_questionnaire_for_the_elderly.15.aspx%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/1956274.
Giles G. Dietary questionnaire for epidemiological studies (version 2). Melbourne: The Cancer Council Victoria; 1996.
Sui SX, Holloway-Kew KL, Hyde NK, Williams LJ, Tembo MC, Mohebbi M, et al. Handgrip strength and muscle quality in Australian women: cross-sectional data from the Geelong osteoporosis study. J Cachexia Sarcopenia Muscle. 2020;11(3):690–7.
Sui SX, Holloway-Kew KL, Hyde NK, Williams LJ, Tembo MC, Leach S, et al. Definition-specific prevalence estimates for sarcopenia in an Australian population: the Geelong Osteoporosis Study. JCSM Clin Reports. 2020; Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1002/crt2.22.
Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8 Available from: http://doi.wiley.com/10.1111/j.1532-5415.1991.tb01616.x.
Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol Ser A Biol Sci Med Sci. 2013;68(4):441–6.
Taal MW, Cassidy MJD, Pearson D, Green D, Masud T. Usefulness of quantitative heel ultrasound compared with dual-energy X-ray absorptiometry in determining bone mineral density in chronic haemodialysis patients. Nephrol Dial Transplant. 1999;14(8):1917–21.
Pasco JA, Henry MJ, Gaudry TM, Nicholson GC, Kotowicz MA. Identification of incident fractures: the Geelong osteoporosis study. Aust N Z J Med. 1999;29(2):203–6 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10342018.
Pasco JA, Lane SE, Brennan-Olsen SL, Holloway KL, Timney EN, Bucki-Smith G, et al. The epidemiology of incident fracture from cradle to senescence. Calcif Tissue Int. 2015;97(6):568–76.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Gould H, Brennan SL, Nicholson GC, Kotowicz MA, Henry MJ, Pasco JA. Calcaneal ultrasound reference ranges for Australian men and women: the Geelong osteoporosis study. Osteoporos Int. 2013;24(4):1369–77.
Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong osteoporosis study. Calcif Tissue Int. 2014;94(4):363–72.
Henry MJ, Pasco JA, Korn S, Gibson JE, Kotowicz MA, Nicholson GC. Bone mineral density reference ranges for Australian men: Geelong osteoporosis study. Osteoporos Int. 2010;21(6):909–17.
Henry MJ, Pasco JA, Pocock NA, Nicholson GC, Kotowicz MA. Reference ranges for bone densitometers adopted Australia-wide: Geelong osteoporosis study. Australas Radiol. 2004;48(4):473–5.
Jarosz PA, Bellar A. Sarcopenic obesity: an emerging cause of frailty in older adults. Geriatr Nurs (Minneap). 2009;30(1):64–70 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19226689.
Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46. https://doi.org/10.1186/s12891-017-1403-x.
Poli S, Cella A, Puntoni M, Musacchio C, Pomata M, Torriglia D, et al. Frailty is associated with socioeconomic and lifestyle factors in community-dwelling older subjects. Aging Clin Exp Res. 2016;29(4):1–8.
Newson R. Confidence intervals for rank statistics: Somers’ D and extensions. Stata J. 2006;6(3):309–34.
Papke LE. Econometric methods for fractional response variables with an application to 401 (k) plan participation rates. J Appl Econ. 1996;11(6):619–32.
Pregibon D. Goodness of lik tests for generalized linear models. Appl Stat. 1980;29:15–24.
Cook MJ, Oldroyd A, Pye SR, Ward KA, Gielen E, Ravindrarajah R, et al. Frailty and bone health in European men. Age Ageing. 2017;46:635–41.
Kenny AM, Waynik IY, Smith JA, Fortinsky R, Kleppinger A, McGee D. Association between level of frailty and bone mineral density in community-dwelling men. J Clin Densitom. 2006;9(3):309–14.
Bartosch P, McGuigan FE, Akesson KE. Progression of frailty and prevalence of osteoporosis in a community cohort of older women—a 10-year longitudinal study. Osteoporos Int. 2018;29(10):2191–9.
Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95(1):139–59.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–23.
Cederholm T. Overlaps between frailty and sarcopenia definitions. Nestle Nutr Inst Work Ser. 2015;83:65–9.
Calvani R, Marini F, Cesari M, Tosato M, Anker SD, Von Haehling S, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle. 2015;6(4):278–86.
Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45(3):M82–8 Available from: https://www.researchgate.net/publication/276280873_Using_web-based_training_to_enhance_perceptual-cognitive_skills_in_complex_dynamic_offside_events.
Tembo MC, Holloway-kew KL, Mohebbi M, Sui SX, Hosking SM, Brennan-olsen SL, et al. The association between a fracture risk tool and frailty: Geelong Osteoporosis Study. BMC Geriatr. 2020;20:196.
Acknowledgements
The authors acknowledge the men and women who participated in the study. The authors thank Professor Graham Giles of the Cancer Epidemiology Centre of The Cancer Council Victoria, for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria, 1996.
Funding
The Geelong Osteoporosis Study (GOS) was funded by the National Health and Medical Research Council (NHMRC) Australia (projects 299831, 251638, 628582). The funding organisation played no role in the design or conduct of the study, in the collection, management, analysis and interpretation of the data, nor in the preparation, review and approval of the manuscript.
MCT and SXS were supported by Deakin Postgraduate Scholarships, KLH-K was supported by Alfred Deakin Postdoctoral Research Fellowship, SLB-O was supported by NHMRC Career Development Fellowships (1107510) and LJW is supported by NHMRC Career Development Fellowship (1064272) and a NHMRC Investigator grant (1174060).
Author information
Authors and Affiliations
Contributions
MCT: Conceptualization, design of methodology, acquisition, data analysis, writing-Original Draft, Writing-Review and Editing. MM: Design of methodology, Formal data analysis, Writing-Review and Editing. KLH-K: Data analysis, Writing-Review and Editing. JG: Data acquisition, Writing-Review and Editing. SXS: Writing-Review and Editing. SB-O: Writing-Review and Editing. LJW: Writing-Review and Editing. MAK: Writing-Review and Editing. JAP: Conceptualization, Methodology, Formal Analysis, Writing-Review and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Human Research Ethics Committee at Barwon Health. Written informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
Monica C. Tembo, Mohammadreza Mohebbi, Kara L. Holloway-Kew, James Gaston, Sophia X Sui, Sharon L. Brennan-Olsen, Lana J. Williams, Mark A. Kotowicz and Julie A. Pasco declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1
. Association between frailty items in the Fried frailty phenotype and musculoskeletal parameters in men. Frailty items; 1 = Low physical activity, 2 = fatigue, 3 = weight loss, 4 = slowness, 5 = low handgrip strength, Heel Ultrasound measures; BUA = broadband ultrasound attenuation, SOS = speed of sound, SI = stiffness index. Supplementary Fig. 2. Association between frailty items in the Fried frailty phenotype and musculoskeletal parameters in women. Frailty items; 1 = Low physical activity, 2 = fatigue, 3 = weight loss, 4 = slowness, 5 = low handgrip strength, Heel Ultrasound measures; BUA = broadband ultrasound attenuation, SOS = speed of sound, SI = stiffness index. Supplementary Table 1. Spearman correlation of frailty items with frailty in men. Supplementary Table 2. Spearman correlation of frailty items with frailty in women. Table 3. Model 1 Femoral BMD. Table 4. Model 2 BUA. Table 5 Model 3 SI. Table 6. Model 4 SOS. Table 7. Model 5 ALMI. Table 8. Model 6 HGS. Table 9. Model 1 Femoral BMD. Table 10 Model 2 BUA. Table 11 Model 3 SI. Table 12 Model 4 SOS. Table 13 Model 5 ALMI. Table 14. Model 6 HGS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tembo, M.C., Mohebbi, M., Holloway-Kew, K.L. et al. The contribution of musculoskeletal factors to physical frailty: a cross-sectional study. BMC Musculoskelet Disord 22, 921 (2021). https://doi.org/10.1186/s12891-021-04795-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04795-4