Abstract
Background
FGF23 has been associated with frailty and functional performance in older individuals, but the association to sarcopenia is unknown.
Objectives
To investigate the association between FGF23, frailty, sarcopenia and fractures in older community dwelling women.
Design
Prospective longitudinal cohort study.
Setting
Malmö, Sweden.
Participants
995 75-year-old women, followed prospectively for ten years, with re-investigations after five (n=667) and ten (n=324) years.
Measurements
C-terminal levels of FGF23 were measured and a frailty index of ‘deficits in health’ created. Sarcopenia was defined by low muscle mass and strength and “probable sarcopenia” by low muscle mass only. Incident fractures were continuously registered for 10-years. Based on tertiles of FGF23, odds ratio for frailty, sarcopenia and probable sarcopenia was investigated using logistic regression models adjusted for: eGFR, PTH, calcium, vitamin D and phosphate. Fracture-free survival during 10-year follow-up was depicted using Kaplan Meier curves.
Results
While fracture-free survival did not differ between tertiles, women in the highest tertile of FGF23 had lower muscle strength and gait speed, and higher proportion with impaired mobility at baseline. At age 75, these women had higher odds of also being frail (ORadj 1.6 (95% CI 1.1–2.4)) and suffering from probable sarcopenia (ORadj 1.8 (95% CI 1.1–3.1)), but not sarcopenia. At follow-up the association between FGF23 and probable sarcopenia was not evident. While the association with frailty was attenuated at age 80 after adjustment (ORadj 1.6 (95% CI 1.0–2.5)), women in the highest tertile had higher odds of being frail at age 85 (ORadj 3.4 (95% CI 1.7–6.6)).
Conclusions
FGF23 may be a promising clinical marker for muscle strength, functional performance, and frailty in older women, but not for future fragility fractures. Whether FGF23 is also associated with sarcopenia requires further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world has an ageing population that is growing rapidly. Globally, the proportion of individuals aged 60 or older is anticipated to more than double by 2050, and in Europe, more than one third of the population is estimated to be over 60 years old in 2050 (1). This expected shift in demography prompts the need to promote healthy ageing, thus, the research society has striven to find novel approaches to common disabling conditions of ageing. One such strategy points towards biomarkers to identify and possibly treat such conditions. One particular biomarker in the spotlight is Fibroblast Growth Factor 23 (FGF23).
The hormone FGF23 is a protein produced by osteoclasts and osteocytes which acts in the parathyroid, bone, heart and kidney. In the kidney, FGF23 regulates phosphate and vitamin D homeostasis through two major pathways. Firstly, by decreasing renal phosphate reabsorption and lowering levels of calcitriol, the active form of vitamin D (figure 1), and secondly by increasing renal reabsorption of calcium. FGF23 signalling mostly occurs through FGF receptors in combination with the co-receptor Klotho (2–6). High levels of FGF23 have been linked to cardiovascular outcomes, mortality (7, 8) and possibly fractures, although studies are inconclusive (9, 10).
However, recent findings indicate a broader range of associations. FGF23 has been proposed as a novel muscle biomarker, with increasing levels associated with progressive muscle deterioration in amyotrophic lateral sclerosis (11). In addition to being associated with functional performance in an older population (12, 13), our own proteomic studies indicate that FGF23 is a key protein associated with frailty, encompassing the functional decline in multiple organ systems (14) (unpublished data, manuscript in revision).
Muscle and bone are both secretory endocrine organs that interact, affecting each other’s functions (15) beyond the biomechanical interaction. Given that preservation of muscle function is essential in maintaining independence and quality of life into old age, it is not surprising that FGF23, a bone mineralization hormone, is emerging as a biomarker for musculoskeletal health. It is well established that in frailty, the deterioration of the musculoskeletal system plays a major part, resulting in fragility fractures (16). Likewise, sarcopenia, the state of low muscle mass and muscle function (17), is associated with both falls and fractures (18). Thus, when addressing musculoskeletal health in the elderly, it is apparent that the complex syndrome of frailty is closely linked to sarcopenia, with the two concepts overlapping (19).
In an attempt to dissect these complex interactions related to ageing, this study investigated the association between FGF23, sarcopenia, frailty and musculoskeletal outcomes in a large cohort of identically aged, community dwelling older women, followed for ten years. To our knowledge, no study has yet investigated the association between FGF23 and sarcopenia, including measures both of low muscle mass and function. Our hypothesis was that part of the association between FGF23 and frailty may partially be explained by association between FGF23 and sarcopenia.
Material and Methods
Subjects
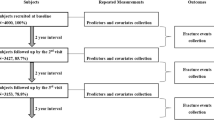
The Osteoporosis Prospective Risk Assessment (OPRA) cohort, previously described (20), is a population-based study, originally designed to study fracture. During 1995–1999 women, being 75 years old at point of invitation, were randomly and without exclusion criteria, selected from the city archives of Malmö and invited by letter to participate. Of the 1604 women approached, 152 stated illness and 376 unwillingness as cause for non-participation, while 32 could not be reached despite several attempts, resulting in 1044 women attending baseline investigation (65% response rate). Participants were followed up twice over a period of ten years. The first follow-up investigation took place after five years (age 80, n=715) and the second after ten years (age 85, n=382). The present study uses data from women with available FGF23 values, corresponding to 995, 667 and 324 at respective visits. Missing values reflect a random loss across the cohort (for example, lack of serum, inability to provide a blood sample, haemolysis or failed analysis) and not a systematic error or selection. The study was approved by the Regional Ethics Committee in Lund (Dnr: 2014804) and in accordance with the Helsinki declaration. All participants provided written informed consent.
General chemistry and FGF23
Non-fasting blood samples were collected between 8 a.m. and 1 p.m, centrifuged and stored at −80°C. Routine blood chemistry was analysed by standard methods at the time of the study (21). Kidney function (estimated glomerular filtration rate, eGFR) was based on cystatin C, using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study equation (22).
Serum levels of FGF23 were measured using the C-terminal assay, which identifies 2 epitopes on the C-terminus part of the hormone, capturing both intact FGF23 and the C-terminal fragments of cleaved and inactivated hormone (23). Samples from all visits (age 75, 80 and 85) were analysed in batch in 2016 using ELISA (rev.no. 150407, Biomedica Medizinprodukte GmbH & Co KG, A-1210 Wien, Divischgasse 4). Intra-assay CVs were: ≤12% at 6 pmol/L and ≤6% at 10 pmol/L. Inter-assay CVs were: ≤10% at 6 pmol/L and ≤5% at 10 pmol/L.
Muscle mass, muscle strength, and function
Muscle mass was measured by dual-energy x-ray absorptiometry (DXA) using the same machine (GE Lunar, Madison, WI) throughout the study. Appendicular skeletal lean mass (ASM) was adjusted for body size by dividing with height squared (ASM/height2, kg/m2). The threshold for low muscle mass was <5.5 kg/m2.
Muscle strength was measured using knee strength, as hand grip strength (HGS) was not collected at all visits and isometric torque methods are suggested as an alternative by the European Working Group on Sarcopenia in Older People (EWGSOP2) (24). At all visits knee extension was tested using a computerized isokinetic dynamometer (Biodex Medical Systems, v4.5.0, Biodex Corporation, New York). The best out of three maximum isometric contractions (knee extension at 90°) lasting 5 seconds each was recorded (Nms). We identified a threshold for low knee strength equating to a HGS threshold of <16kg, previously applied in defining sarcopenic participants (17), using two steps:
Step 1: Using low gait speed (≤0.8 m/s) as the dependent variable, as suggested by Alley et al (25), and maximum repetition knee extension (Nms) as the scale dependent variable, we performed a Classification and Regression Tree (CRT) analysis to establish a clinically relevant threshold for knee strength that would closely mirror a HGS <16kg. The CRT segmented the data and identified the knee strength threshold with the strongest relationship to low gait speed. Based on this a threshold for low knee strength at ≤174.24 Nms was identified. As an additional check, we performed CRT analysis again, this time using low HGS (<16kg) as the dependent variable; the identified threshold was <175.9 Nm which is close to the cutoff derived from using the recommended low gait speed.
Step 2: To assess how well the tree structure can be generalized and to ensure selection of the best performing cutoff, a split-sample validation was also performed, in which test- and training sample groups were randomly assigned with 50% of the cohort data in each group. Based on this, a threshold at ≤174.85 Nms was identified in both segments. Taking the average from steps 1 and 2, knee strength <175 Nms was equated to be equivalent to HGS <16kg.
Sarcopenia was defined in accordance with the updated 2018 guidelines from the EWGSOP (17), as low muscle mass (i.e. ASM<5.5 kg/m2) plus low muscle strength (i.e. knee strength <175 Nms). With reference to the same guidelines, a group of women with probable sarcopenia was defined based on muscle strength alone (i.e. knee strength <175 Nms) without reference to muscle mass.
Muscle function. Gait speed (m/s) was tested at all visits through a 10m walk with one turn (i.e. 2×5m); a higher value indicating a poorer function. The participants wore ordinary shoes with the instruction to walk as fast as they could.
Frailty index
Using the principles described by Searle et al (26) and described in full elsewhere (27), a frailty index (Fl) was created using data collected at each visit (age 75, 80 and 85). Concisely, this frailty index contains thirteen variables covering several physiological domains (time spent outdoors, daily physical activity, balance, walking speed, number of steps taken, muscle strength, diabetes, cancer/severe disease, diseases affecting balance, self-reported fall risk, polypharmacy, CRP and creatinine). The index represents ‘deficits in health’, scored from 0.0–1.0; the higher the score, the more frail. The FI correlated very strongly (r = 0.80) to a full 40-variable index (28), created for the two follow-up visits, and both the 13- and 40-variable frailty index show similar capacity to predict mortality (27). The frailty index was used as a continuous variable, but also using a threshold ≥ 0.25 to define frail individuals. This empirical cutoff is supported by other studies (29, 30).
Falls and impaired mobility
At each visit, all women completed an extensive questionnaire, from which information about falls and mobility was collected. Falls were defined as having had a fall within the previous year (yes/no). Impaired mobility was defined as using a walking aid inside or outside or moving with other people or bedbound.
Fractures
Incident fractures (hip or major osteoporotic, the latter defined as any of the following: hip, vertebra, distal radius, shoulder), were followed for ten years through the X-ray files at the Radiology Department, Malmö, Skåne University Hospital. Since the department of orthopedics was the sole unit treating fractures in the catchment area, information loss was exceptionally low (31). Osteoporotic fractures defined as any of the following: hip, vertebra, distal radius, shoulder. Pathological and high energy fractures were excluded.
Statistics
Descriptive fdata is presented as mean with standard deviation or median with interquartile range, as appropriate. In case of non-normally distributed data, non-parametric tests were performed. At all three visits (age 75, 80 and 85), women were stratified into tertiles of FGF23 (T1 = lowest FGF23 level; T3 = highest FGF23 level). Overall comparisons between tertiles of FGF23 with outcomes frailty, sarcopenia, muscle strength, muscle function and muscle mass, were performed using ANOVA, Kruskal-Wallis or Chi-square test as appropriate.
To investigate if women in the highest FGF23 tertile had higher odds of a) sarcopenia, b) probable sarcopenia and c) frailty, logistic regression analyses were performed at age 75, and then repeated at age 80 (based on FGF23 measurements at age 80) and 85 (based on FGF23 measurements at age 85). These analyses are presented unadjusted (model 1) and adjusted for: PTH, vitamin D, estimated kidney function (eGFR), phosphate and calcium (model 2). Additionally, to investigate if FGF23 at baseline (age 75) was associated with outcomes at follow up, longitudinal analyses were performed. Using logistic regression analyses and tertiles of FGF23 at age 75, association with sarcopenia, probable sarcopenia and frailty at age 80 and 85 was investigated. Data is presented unadjusted and adjusted for PTH, vitamin D, estimated kidney function (eGFR), phosphate and calcium at age 75. Kaplan Meier curves describe fracture free survival during the ten-year study period (i.e. from age 75–85) for hip and major osteoporotic fractures. P-value for difference was calculated using the log rank test.
All statistical analyses were performed using SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY). A p-value <0.05 was considered nominally significant.
Results
In the OPRA cohort, levels of FGF23 were non-normally distributed and increased slightly over the ten-years of follow-up, from age 75 to 85 (median FGF23 level 0.8 (0.7) pmol/L at 75y and 0.9 (1.1) pmol/L at 85y) (table 1). Women who attended the first follow-up at 80y had lower median FGF23 values at 75y compared to those who did not attend (0.7 (0.6) vs 0.9 (1.1) pmol/L, p<0.001). However, at the second follow-up at 85y, FGF23 levels at age 80 were similar in those who did and those who did not attend (1.1 (0.9) vs 1.1 (1.0) pmol/L, p=0.761). For the analyses in this study, women were divided into tertiles of FGF23 (T3 highest tertile; T1 lowest tertile). While a majority of women (58%) in T3 of FGF23 at baseline remained in that category at the first follow up (age 80), 28% were now classed in T2 and 13% in T1. Correspondingly, 57% of the women in T3 at age 80 were also in T3 at age 85, with 36% being categorized in T2 and 7% in T1.
Using the stringent definition of the latest EWGSOP guidelines, prevalence of sarcopenia at baseline (age 75) was low, only 3%. At the first follow up (age 80), prevalence increased to 5% and at the second follow up (age 85), prevalence had reached 9%. Similarly, prevalence of probable sarcopenia increased during the ten year follow up from 13% at age 75 to 22% at age 85, as did proportion of frail individuals (23% age 75; 57% age 85). Correspondingly, percentage of women with a recent history of falls increased during the follow up, while proportion of women with impaired mobility more than tripled, from 1/10 at age 75 to almost 4/10 at age 85 (table 1).
FGF23, muscle parameters and frailty
Table 2 show frailty, sarcopenia, muscle strength, muscle function and muscle mass stratified by tertiles of FGF23 at age 75, 80 and 85. Looking at cross sectional data from age 75, prevalence of probable sarcopenia differed between tertiles; 19% in T3 compared to 9% in T1 (p<0.001 for overall difference between tertiles). Women in the highest FGF23 tertile had a higher frailty index (median 0.19 in T3 vs 0.14 in T1, p<0.001 for overall difference between tertiles) and correspondingly, a higher proportion of frail individuals (32% in T3 vs 17% in T1, p<0.001 for overall difference). Muscle strength was also lower (mean 258 Nms in T3 vs 277 in T1, p<0.01 for overall difference), as was gait speed (mean 1.1 vs 1.3 m/s, p<0.001 for overall difference). Compared to tertile 1, tertile 3 had three times higher proportion of women with impaired mobility (17% vs 5%, p<0.001 for overall difference). In contrast, no difference between tertiles was seen in prevalence of sarcopenia, muscle mass or recent history of falls.
At follow-up investigations (age 80 and 85), the observed difference in frailty and functional performance was also apparent; using cross sectional data at age 80 women in the highest tertile of FGF23 had higher frailty index compared to women in the lowest tertile (median 0.25 vs 0.20, p<0.001) and correspondingly higher proportion of frail individuals (49% vs 32%, p<0.001), in addition to lower gait speed (mean 1.0 vs 1.1, p=0.041) and higher proportion of individuals with impaired mobility (26% vs 17%, p=0.015)(table 2). However, the association between FGF23 and muscle strength and probable sarcopenia was attenuated. Comparable results were observed using cross sectional data at age 85.
Supplementary table 1 show markers of mineral metabolism and kidney function by FGF23 tertile. As expected, and consistent at ages 75, 80 and 85, in the tertile with highest FGF23, eGFR levels were lowest, while conversely PTH levels were higher.
FGF23 and musculoskeletal outcomes
FGF23 has been suggested as a novel biomarker for frailty and musculoskeletal outcomes. In the OPRA cohort, women in the highest FGF23 tertile at baseline were not at increased odds of having sarcopenia (p=0.070), but they had higher odds of suffering from probable sarcopenia (ORadj 1.8 (95% CI 1.1–3.1)) and of being frail (ORadj 1.6 (96% CI 1.1–2.4)) compared to women in T1. At follow-up, using cross sectional data from age 80 and 85, the association between FGF23 and probable sarcopenia was not evident. While the association with frailty was attenuated at age 80 after adjustment, women in T3 had higher odds of being frail at age 85 (ORadj 3.4 (96% CI 1.7–6.6)) (table 3).
Longitudinally, FGF23 at age 75 was associated with neither sarcopenia, nor probable sarcopenia at age 80 or 85. However, women in the highest tertile of FGF23 at age 75 were at increased odds of also being frail at age 80 (OR 1.6 (96% CI 1.1–2.4) and 85 (OR 1.7 (96% CI 1.0–2.8), although after adjustments these associations were attenuated and nonsignificant (data not shown).
Looking at fractures, 126 women suffered a first hip fracture, while 342 women suffered a first osteoporotic fracture during the ten-year follow-up period. There was no difference in number of first hip or osteoporotic fractures based on tertile of FGF23 at age 75 (p for difference =0.420 for hip fracture; p=0.787 for osteoporotic fractures, data not shown). Correspondingly, fracture free survival during the 10yr follow-up did not differ between FGF23 tertiles at age 75 (figure 2).
Discussion
This study investigates the association between FGF23, sarcopenia, frailty and musculoskeletal outcomes over ten years in older community dwelling women. The main finding was that, not only did women with the highest levels of FGF23 have increased odds of being frail, FGF23 was also associated with probable sarcopenia and lower muscle strength at baseline investigation, and reduced functional performance throughout the investigation period.
Our observation that FGF23 was associated with lower gait speed and impaired mobility is in line with a recent cross-sectional study showing that FGF23 level is associated with lower muscle strength in individuals over the age of 80 (13). The present study shows that the association between FGF23 and muscle may be related to quality and not quantity since baseline data from OPRA shows correlation between FGF23 and muscle strength but not muscle mass. Muscle quality is an important aspect of ageing, and it has been reported that muscle strength, but not muscle mass, is associated with mortality (32). The lack of association between FGF23 and muscle strength at ages 80 and 85 may be attributed to the reduced number of women attending follow-up.
To the authors’ knowledge, this is the first study investigating association between FGF23 and sarcopenia. Contrary to our initial hypothesis, no association between FGF23 and sarcopenia (defined by low muscle mass and strength) was apparent. This may be attributed to the low number with sarcopenia in the cohort (3 percent at baseline) since, when using the less stringent definition, women in the highest tertile of FGF23 had increased odds of ‘probable sarcopenia’ (defined by muscle strength alone). This supports the notion of a relationship between FGF23 and muscle quality but warrants further investigation in a larger cohort.
There are several hypotheses regarding the mechanism behind FGF23 and muscle function, one being that FGF23, through inhibiting the action of vitamin D, thereby affects proximal muscles (12). Another relates to the effect of high phosphate levels (which in turn increases FGF23) that have been inversely associated with muscle strength (33). In the OPRA cohort, while there was no difference in phosphate level, women in the highest tertile of FGF23 did have lower vitamin D levels, albeit only at baseline. However, the association between FGF23 and probable sarcopenia, although attenuated, remained after adjustment for vitamin D and phosphate in addition to eGFR, PTH and calcium.
The question of whether FGF23 has a direct effect on skeletal muscle, although highly interesting, cannot be answered in this population-based study. An investigation in rats showed that even though skeletal muscle expressed receptors involved in FGF23 signalling, no direct effect of FGF23 on skeletal muscle was apparent (34). Tentatively, our findings indicate that the association between FGF23 and muscle strength cannot solely be explained through the role of FGF23 in phosphate and vitamin D regulation.
In contrast to a study in individuals aged 80 or over (13), FGF23 was not associated with having recently fallen, somewhat surprising, given the association between FGF23 and muscle strength. In both studies falls are self-reported, therefore the possibility of recollection bias cannot be excluded. As with falls, we found no difference in fracture-free survival based on FGF23 tertile. Although some previous studies report increased fracture risk (35), results are conflicting, possibly dependent on kidney function (9, 10, 36, 37). At present there is insufficient evidence to suggest that FGF23 plays a significant role in age-related osteoporosis (38); our results support this conclusion as we found no difference in osteoporotic fractures between FGF23 tertiles.
Throughout the ten-year follow-up, association between quantitatively measured frailty and FGF23 was observed. This study is not the first investigating FGF23 as a marker for frailty. In community dwelling individuals (n=2977; mean age 78) serum FGF23 was associated both with frailty and pre-frailty defined through Fried’s frailty phenotype (12). In individuals with chronic kidney disease (n=2376) higher serum FGF23 was associated with both quantitatively measured frailty and falls (39), while an unpublished proteomic study in this cohort suggests FGF23 as a core marker of frailty (unpublished data, manuscript in revision). The link between FGF23 and frailty may in part be explained by the association between FGF23 and muscle strength and functional outcomes, since deterioration of the musculoskeletal system is a key component of frailty. However, FGF23 may also be a broader marker of general health, which is captured by the frailty index.
To our knowledge, this is the first study investigating the association between fibroblast growth factor 23 (FGF23) and sarcopenia, and limitations are acknowledged.
Firstly, muscle strength was estimated from knee strength, in the absence of hand grip strength at all visits. However, although hand grip strength is the preferred measuring tool, knee strength is also a validated method for measuring muscle strength and may even be a more appropriate method in older women since it decreases more than arm strength with age (40, 41).
Also, FGF23 was measured in samples archived for twenty-one to twenty-seven-years. A previous study has found that long-term storage at -80°C induced slight changes (generally a decline) in FGF23 levels (42). Even if this is a possibility, we have been rigorous across the study period with regard to optimal handling, storage and processing, therefore any declines should be homogenous across all samples.
Furthermore, and similar to many previous studies, we utilized C-terminal FGF23 which detects both the intact biologically actively FGF23 and the C-terminal fragments. C-terminal FGF23 levels may be affected by iron deficiency and inflammation, through increased cleavage (43), which does not affect levels of intact FGF23. However, the longitudinal design of this study with multiple measurements of FGF23 over time, is a proxy for replication, which makes this population-based study unique. In addition, analyses are adjusted for estimated kidney function based on cystatin C, an endogenous marker not affected by low muscle mass or dietary intake.
The study lacks measurement of co-receptor klotho, which in this instance would have been an advantage since FGF23 signalling mostly occurs in combination with klotho. Moreover, the advanced age of participants, in combination with long follow-up time, resulted in lower participation numbers at the re-visits, but this is unavoidable in this age group due to natural reasons. Also, while study participants were randomly selected without exclusion criteria, they might be healthier than those who declined (44), which has been described in older populations (45), possibly reflected in the low number of women with sarcopenia. Nevertheless, participation rate was high (65%), thus increasing the probability that we have a representative sample of typical 75-year-old women. Importantly, all participants were at the same age at inclusion, which reduces confounding of chronological age with respect to the accumulated health deficits captured by the frailty index. Lastly, since all participants in the OPRA cohort were older females, the results may not be applicable to other ages and men, and will be an interesting field for future studies.
In conclusion, our results indicate that FGF23 may be a promising marker for muscle strength, functional performance, and frailty in older women, but not for future fragility fractures. Whether FGF23 is also associated with sarcopenia requires further investigation.
References
UnitedNations. The World Population Prospects: 2015 Revision 2015 [Available from: https://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html.
Erben RG. Physiological Actions of Fibroblast Growth Factor-23. Front Endocrinol (Lausanne). 2018;9:267. https://doi.org/10.3389/fendo.2018.00267.
Haffner D, Leifheit-Nestler M. Extrarenal effects of FGF23. Pediatr Nephrol. 2017;32(5):753–65. https://doi.org/10.1007/s00467-016-3505-3.
Ho BB, Bergwitz C. FGF23 signalling and physiology. J Mol Endocrinol. 2021;66(2):R23–r32. https://doi.org/10.1530/jme-20-0178.
McGuigan FE, Malmgren L. Bone health as a co-morbidity of chronic kidney disease. Best Pract Res Clin Rheumatol. 2022:101760. https://doi.org/10.1016/j.berh.2022.101760.
Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35. https://doi.org/10.1359/jbmr.0301264.
Lutsey PL, Alonso A, Selvin E, et al. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3(3):e000936. https://doi.org/10.1161/jaha.114.000936.
Sharma S, Katz R, Dubin RF, et al. FGF23 and Cause-Specific Mortality in Community-Living Individuals-The Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2021;69(3):711–7. https://doi.org/10.1111/jgs.16910.
Isakova T, Cai X, Lee J, et al. Associations of FGF23 With Change in Bone Mineral Density and Fracture Risk in Older Individuals. J Bone Miner Res. 2016;31(4):742–8. https://doi.org/10.1002/jbmr.2750.
Kanda E, Yoshida M, Sasaki S. Applicability of fibroblast growth factor 23 for evaluation of risk of vertebral fracture and chronic kidney disease-mineral bone disease in elderly chronic kidney disease patients. BMC Nephrol. 2012;13:122. https://doi.org/10.1186/1471-2369-13-122.
Si Y, Kazamel M, Benatar M, et al. FGF23, a novel muscle biomarker detected in the early stages of ALS. Sci Rep. 2021;11(1):12062. https://doi.org/10.1038/s41598-021-91496-6.
Beben T, Ix JH, Shlipak MG, et al. Fibroblast Growth Factor-23 and Frailty in Elderly Community-Dwelling Individuals: The Cardiovascular Health Study. J Am Geriatr Soc. 2016;64(2):270–6. https://doi.org/10.1111/jgs.13951.
Foroni MZ, Cendoroglo MS, Costa AG, et al. FGF23 levels as a marker of physical performance and falls in community-dwelling very old individuals. Arch Endocrinol Metab. 2022. https://doi.org/10.20945/2359-3997000000488.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36. https://doi.org/10.1100/tsw.2001.58.
Lu W, Xiao W, Xie W, et al. The Role of Osteokines in Sarcopenia: Therapeutic Directions and Application Prospects. Front Cell Dev Biol. 2021;9:735374. https://doi.org/10.3389/fcell.2021.735374.
Bartosch P, Malmgren L, Kristensson J, McGuigan FE, Akesson KE. In community-dwelling women frailty is associated with imminent risk of osteoporotic fractures. Osteoporos Int. 2021;32(9):1735–44. https://doi.org/10.1007/s00198-021-05886-7
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169.
Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. https://doi.org/10.1002/jcsm.12411.
Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43–8. https://doi.org/10.1007/s40520-016-0709-0.
Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19(3):386–93. https://doi.org/10.1359/JBMR.0301244.
Malmgren L, McGuigan F, Christensson A, Akesson KE. Reduced kidney function is associated with BMD, bone loss and markers of mineral homeostasis in older women: a 10-year longitudinal study. Osteoporos Int. 2017;28(12):3463–73. https://doi.org/10.1007/s00198-017-4221-y.
Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. https://doi.org/10.1056/nejmoa1114248.
Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–63. https://doi.org/10.1056/nejmoa020881.
Francis P, Toomey C, McCormack W, Lyons M, Jakeman P. Measurement of maximal isometric torque and muscle quality of the knee extensors and flexors in healthy 50- to 70-year-old women. Clin Physiol Funct Imaging. 2017;37(4):448–55. https://doi.org/10.1111/cpf.12332.
Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–66. https://doi.org/10.1093/gerona/glu011.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. https://doi.org/10.1186/1471-2318-8-24.
Bartosch P, McGuigan FE, Akesson KE. Progression of frailty and prevalence of osteoporosis in a community cohort of older women-a 10-year longitudinal study. Osteoporos Int. 2018;29(10):2191–9. https://doi.org/10.1007/s00198-018-4593-7.
Bartosch PS, Kristensson J, McGuigan FE, Akesson KE. Frailty and prediction of recurrent falls over 10 years in a community cohort of 75-year-old women. Aging Clin Exp Res. 2020;32(11):2241–50. https://doi.org/10.1007/s40520-019-01467-1.
Kojima G, Kendrick D, Skelton DA, et al. Frailty predicts short-term incidence of future falls among British community-dwelling older people: a prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:155. https://doi.org/10.1186/s12877-015-0152-7.
Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. https://doi.org/10.1093/gerona/62.7.738.
Jónsson B, Gärdsell P, Johnell O, Redlund-Johnell I, Sernbo I. Remembering fractures: fracture registration and proband recall in southern Sweden. J Epidemiol Community Health. 1994;48(5):489–90. https://doi.org/10.1136/jech.48.5.489.
Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. https://doi.org/10.1093/gerona/61.1.72.
Chen YY, Kao TW, Chou CW, et al. Exploring the Link between Serum Phosphate Levels and Low Muscle Strength, Dynapenia, and Sarcopenia. Sci Rep. 2018;8(1):3573. https://doi.org/10.1038/s41598-018-21784-1.
Avin KG, Vallejo JA, Chen NX, et al. Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab. 2018;315(4):E594–e604. https://doi.org/10.1152/ajpendo.00343.2017.
Mirza MA, Karlsson MK, Mellström D, et al. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res. 2011;26(4):857–64. https://doi.org/10.1002/jbmr.263.
Jovanovich A, Bùzková P, Chonchol M, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98(8):3323–31. https://doi.org/10.1210/jc.2013-1152.
Lane NE, Parimi N, Corr M, et al. Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2013;28(11):2325–32. https://doi.org/10.1002/jbmr.1985.
Sirikul W, Siri-Angkul N, Chattipakorn N, Chattipakorn SC. Fibroblast Growth Factor 23 and Osteoporosis: Evidence from Bench to Bedside. Int J Mol Sci. 2022;23(5). https://doi.org/10.3390/ijms23052500.
Jovanovich A, Ginsberg C, You Z, et al. FGF23, Frailty, and Falls in SPRINT. J Am Geriatr Soc. 2021;69(2):467–73. https://doi.org/10.1111/jgs.16895.
Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985). 1999;86(1):188–94. https://doi.org/10.1152/jappl.1999.86.L188.
Porto JM, Nakaishi APM, Cangussu-Oliveira LM, et al. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr. 2019;82:273–8. https://doi.org/10.1016/j.archger.2019.03.005.
El-Maouche D, Dumitrescu CE, Andreopoulou P, et al. Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int. 2016;27(7):2345–53. https://doi.org/10.1007/s00198-016-3543-5.
David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–46. https://doi.org/10.1038/ki.2015.290.
Wihlborg A, Åkesson K, Gerdhem P. External validity of a population-based study on osteoporosis and fracture. Acta Orthop. 2014;85(4):433–7. https://doi.org/10.3109/17453674.2014.920987.
Golomb BA, Chan VT, Evans MA, et al. The older the better: are elderly study participants more non-representative? A cross-sectional analysis of clinical trial and observational study samples. BMJ Open. 2012;2(6). https://doi.org/10.1136/bmjopen-2012-000833.
Acknowledgements
Thanks are extended to funders, the research nurses and data management at the Clinical and Molecular Osteoporosis Research Unit, Malmö; and to all the women who kindly participated in the study. Thanks are also extended to Paul Gerdhem and Karl Obrant, for initiating the cohort, to Fiona McGuigan for language editing, to Kristina Åkesson for scientific advice.
Funding
Funding Sources: This work was supported by grants from the Swedish Research Council (2018–02981), Greta and Johan Kock Foundation, A. Påhlsson Foundation, A. Österlunds Foundation, H Järnhardt foundation, King Gustav V 80 year fund, Swedish Rheumatism foundation, Royal Physiographic Society Lund, Skåne University Hospital Research Fund, the Research and Development Council of Region Skåne, Sweden, Maggie Stephens foundation for medical sciences, Anna-Lisa and Sven Eric Lundgrens foundation for medical research and the Swedish Kidney Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Open Access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: Lisa Egund (LE), Tine Kolenda Paulin (TKP), Hampus Ekstubbe (HE), Patrik Bartosch (PB), Linnea Malmgren (LM); 1) Conception or design, or interpretation of the data, or both (LE, TKP, HE, PB, LM); 2) Data analysis (TKP, HE, PB, LM). LM takes responsibility for the integrity of the data analysis; 3) Drafting of the manuscript (LE, TKP, LM) and/or revising it (LE, TKP, HE, PB, LM); 4) Providing intellectual content of critical importance to the work (LE, TKP, HE, PB, LM); 5) Final approval of the version to be published (LE, TKP, HE, PB, LM); 6) Agree to be accountable for accuracy and integrity of the work (LE, TKP, HE, PB, LM).
Corresponding author
Ethics declarations
Disclosure statement: LM has received lecture fees from Amgen. LE, TKP, HE and PB have nothing do disclose.
Ethical standards: The study was approved by the Regional Ethics Committee in Lund (Dnr: 2014804) and in accordance with the Helsinki declaration. All participants provided written informed consent.
Additional information
Sponsors role: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest: LE, TKP, HE, PB and LM declare no conflict of interests.
Electronic supplementary material
Supplementary table 1
. Markers of mineral metabolism and kidney function based on tertiles
Rights and permissions
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Egund, L., Paulin, T.K., Ekstubbe, H. et al. Longitudinal Measurements of FGF23, Sarcopenia, Frailty and Fracture in Older Community Dwelling Women. J Frailty Aging 12, 166–174 (2023). https://doi.org/10.14283/jfa.2023.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2023.22