Abstract
Background
This systematic review gives an overview of guidelines and original publications as well as the evidence on which the currently proposed indication criteria are based. Until now such a state-of-the-science overview was lacking.
Methods
Websites of orthopaedic and arthritis organizations (English/Dutch language) were independently searched by two authors for THA/TKA guidelines for OA. Furthermore, a systematic search strategy in several databases through August 2014 was performed. Quality of the guidelines was assessed with the AGREE II instrument, which consists of 6 domains (maximum summed score of 6 indicating high quality). Also, the level of evidence of all included studies was assessed.
Results
We found 6 guidelines and 18 papers, out of 3065 references. The quality of the guidelines summed across 6 domains ranged from 0.46 to 4.78. In total, 12 THA, 10 TKA and 2 THA/TKA indication sets were found. Four studies stated that no evidence-based indication criteria are available. Indication criteria concerning THA/TKA consisted of the following domains: pain (in respectively 11 and 10 sets), function (12 and 7 sets), radiological changes (10 and 9 sets), failed conservative therapy (8 and 4 sets) and other indications (6 and 7 sets). Specific cut-off values or ranges were often not stated and the level of evidence was low.
Conclusion
The indication criteria for THA/TKA are based on limited evidence. Empirical research is needed, especially regarding domain specific cut-off values or ranges at which the best postoperative outcomes are achieved for patients, taking into account the limited lifespan of a prosthesis.
Similar content being viewed by others
Background
Total hip and knee arthroplasty (THA/TKA) have been widely performed since the 1970s. In 2009 over a million of THA and TKA were carried out in the United States [1]. Osteoarthritis (OA) is the main clinical indication for which these procedures are performed [2]. Due to the ageing society as well as the obesity epidemic, the prevalence of OA is increasing [3]. As a result the procedure rates of THA and TKA are expected to rise, some estimates even indicate a quadruple demand by 2030 [4, 5].
The rise in THA/TKA surgery has important implications for health care costs as well as capacity. As such, it is of utmost importance that patients are carefully selected, and that these procedures are optimally timed to achieve the best possible patient outcomes and that revision surgery is prevented thereby reducing costs and worse outcomes. However, large heterogeneity exists in the patients disease severity at the time of surgery [6, 7]. This can partly be explained by the patient’s own wishes, with some patients preferring a THA/TKA to continue an active lifestyle whereas others request surgery to be able to perform daily living activities. In addition, the attitude of the surgeon towards arthroplasty plays an important role since surgeons with a positive attitude towards this procedure will have higher surgery rates. This may explain why studies on appropriateness criteria for THA/TKA showed that in approximately 20-45 % of patients appropriateness of arthroplasty was considered uncertain [8–11]. All of these may explain to some extent why a substantial proportion of the patients is unsatisfied after THA and TKA (10-30 %), indicating that outcomes are less than expected and/or that expectations were too high [12]. Therefore, evidence-based indication criteria for THA/TKA to guide decision making are warranted to improve optimal timing and patient selection, which is internationally acknowledged [13–18].
Guidelines concerning THA and TKA indications have been published and several studies regarding the appropriateness of THA and TKA have been conducted [3, 13, 19–21]. However, an overview of the evidence on which the proposed indication criteria are based is lacking, to guide decision making on timing of THA and TKA. In the present study the available guidelines and their indication sets for primary THA and TKA will be reviewed. In addition, we assess the quality of these guidelines and the evidence on which the indication sets are based. In the second part a systematic search is conducted of scientific publications containing proposed indication sets for primary THA and TKA in OA or expert opinion.
Methods
Search strategy
Websites of orthopaedic and arthritis organizations (English or Dutch websites) were independently searched by two authors for guidelines concerning primary THA/TKA for OA. When these websites cross-linked to guidelines from other organizations these were also included. All available guidelines published since January 1, 2000 were included. A librarian-assisted search strategy was performed on August 3 2014 to retrieve additional publications on THA/TKA indications. The following databases were searched: Pubmed, MEDLINE, Embase, Web of Science, the COCHRANE Librabry, CENTRAL and CINAHL. Searches were limited to English, Dutch and German language papers published since January 1, 2000 (see Additional file 1).
Selection of publications
First titles and abstracts were independently screened by two authors (MG/SH).The full-text articles were reviewed by MG and were included when the following criteria were met: studies reporting about indication criteria and/or appropriateness of decision tools for primary THA/TKA in OA. Papers involving guidelines on unicompartimental replacements, resurfacing or revision of THA/TKA were excluded if no separate indications for primary THA/TKA were provided. Also papers on prioritizing tools to reduce waiting times were excluded unless these reported on the appropriateness of surgery as all of these patients on the waiting list already have an indication and variables determining priority are not necessarily the same as variables determining an indication for surgery.
The included papers where checked by a second author (SH). If disagreement existed the authors tried to reach consensus, when necessary a third author had the decisive vote (PM). When a guideline was also published as a scientific paper, only the guideline was included.
Data extraction
The following information was extracted from the guidelines by MG: orthopaedic or arthritis organization, publication date, indication criteria and the level of evidence on which indication criteria were based (see below). We extracted the following information from the publications: first author, publication date, country where the indication criteria were developed, the organization(s) that initiated the development of the criteria, study type, indication criteria and the level of evidence on which indication criteria were based. The level of evidence was scored according to the following the criteria [3]:
-
➢ Ia evidence from meta-analysis of randomized controlled trials
-
➢ Ib evidence from at least one randomized controlled trial
-
➢ IIa evidence from at least one controlled study without randomization
-
➢ IIb evidence from at least one well-designed quasi-experimental study
-
➢ III evidence from at least one non-experimental descriptive study, such as comparative studies, correlation studies, and case-control studies
-
➢ IV evidence from expert committee reports or opinions or clinical experience of respected authorities or both
Data extraction and level of evidence score was checked by SH.
Quality of the guidelines
Guideline quality was assessed with the validated AGREE-II instrument (Appraisal of Guidelines for Research and Evaluation, Dutch version) [22]. This instrument evaluates the process of practice guideline development and the quality of reporting. Two authors independently scored the guidelines according to the AGREE-II protocol (MG/SH). When large differences existed the authors tried to reach consensus, when necessary a third author had the decisive vote (PM).
The AGREE-II consists of six quality domains: 1) scope and purpose, 2) stakeholder involvement, 3) rigour of development, 4) clarity of presentation, 5) applicability and 6) editorial independence. Each domain entails several questions which are rated from 1 (lowest score) to 7 (highest score), with 1 rated for items with no clear discussion or no specific information, 7 for exceptional reporting quality, 2–6 for items not fully meeting the AGREE-II criteria. Scaled domain scores were calculated using the following formula:
The scores will always lie between 0 and 1, with scores closer to 1 indicating higher quality. The scaled domain scores from the two authors were averaged to obtain one quality score for each domain. We summed the scaled domain scores across the 6 domains to obtain 1 overall guideline score. The maximum summed score was thus 6, indicating high quality.
Results
Across guidelines and studies, 12 THA, 10 TKA and 2 THA/TKA indication sets were found.
Guidelines
We found six guidelines concerning THA, of which three specific OA guidelines (EULAR [20], NICE [23] and OARSI [3]). In addition, five guidelines concerning TKA were found, of which four OA specific guidelines (BOA [24], EULAR [19], NICE [23] and OARSI [3]) (Table 1).
Indication criteria concerning THA and TKA
Most indication criteria consisted of the following three domains: pain, function and radiological changes, with the prerequisite that pain could not be controlled by conservative therapy (Table 1). Specific cut-off values or ranges for pain and function were not reported. For radiological changes only the BOA TKA guideline reported a cut-off value (Kellgren Lawrence grade ≥ III). The evidence on which the indication criteria were based was rated as low quality evidence (level IV).
Quality of the guidelines
The quality of the guidelines differed considerably between the AGREE-II domains and the guidelines (Fig. 1). The ranges of the scaled domain scores were: scope and purpose 0.06-0.81, stakeholder involvement 0.19-0.75, rigour of development 0.03-0.88, clarity of presentation 0.33-0.89, applicability 0-0.50, editorial independence 0-0.96. Low scores were frequently attained in the editorial independence domain due to no clear statement on the influence of the funding body and competing interests. In addition, low scores were often attained in the applicability domain, due to no clear statements on monitoring/auditing criteria of the guideline or facilitators and barriers to the application of the guideline. The OARSI and NOV guidelines attained the highest overall scores, 4.78 and 4.46 respectively. This is explained because both guidelines were developed according to the AGREE-II. The lowest scores were attained by the NZ guidelines, THA (0.84) and TKA (0.46). These guidelines primarily consisted of a, from the BOA guidelines derived, summary of statements concerning THA/TKA but limited information on the required 6 domains.
AGREE II guideline quality scores. Panel (a) AGREE II quality scores of the guidelines concerning hip replacement. Panel (b) AGREE II quality scores of the guidelines concerning knee replacement. Panel (c) AGREE II quality scores of the guidelines concerning joint replacement in osteoarthritis. Domain 1: scope and purpose, domain 2: stakeholder involvement, domain 3: rigour of development, domain 4: clarity of presentation, domain 5: applicability, domain 6: editorial independence
Although the process of guideline development and quality of reporting differed considerably between the guidelines, the given indication criteria for primary THA and TKA are similar across guidelines (pain, function, radiological changes). Hence, it seems that guideline quality did not influence the main domains included in the indication sets.
Publications
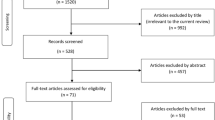
Our literature search yielded 3065 references (Fig. 2), the full-text of 88 papers was assessed on eligibility. Of these 70 were excluded mainly because no indication criteria for THA/TKA in OA patients were reported. Finally, 18 papers were included (12 reviews/6 original studies).
Reviews
Of the included 12 reviews, only 2 were systematic reviews (Table 2) [25, 26]. Furthermore, only 2 reviews focussed on indications for THA/TKA as their main topic [27, 28]. In addition, 1 review investigated the indications for THA/TKA referral [29]. Other topics on which the reviews focussed were management of THA/TKA [30–32], effectiveness of THA/TKA [26] and state of the art overviews of THA/TKA [14, 33, 34].
Pain not responsive to conservative treatment, in patients who have functional limitations and radiographic evidence of joint degeneration was most often reported as THA/TKA indication (Table 2). No specific cut-off values were mentioned. It was often not stated if deviations in all these domains should be apparent, or which combinations should be apparent to indicate THA or TKA. Furthermore, the evidence behind all these indication criteria was very low (level IV). In 3 of the reviews the experts explicitly stated that no appropriate indication sets are available for performing THA/TKA.
Original publications
Three original publications reported on TKA [8, 9, 35] and 3 on THA [10].
Yamabe et al. [35] considered severe cartilage defects as an optimal indication for TKA. In their discussion section they also included pain but no referral was made to any evidence or the way these indications were established.
The other 5 included original studies investigated decision tools to assess the appropriateness of TKA (n = 2) [8, 9] or THA (n = 3) [10, 11, 36] in OA patients.
TKA appropriateness
Two studies evaluated algorithms to assess TKA appropriateness [8, 9]. The Escobar algorithm was established using the RAND/UCLA appropriateness method, in which expert opinion is combined with available scientific evidence [37]. The following variables where taken into account in different combinations: symptomatology, radiology, age, mobility and stability, previous surgical management and localization. Symptomatology and radiology were the largest contributors in explaining the variability of appropriateness in their model. Table 3 depicts various scenarios in which TKA was considered inappropriate, uncertain or appropriate [9]. However, appropriateness was rated uncertain in a high percentage of scenarios (24.5 %). Another study showed that patients with their TKA rated as appropriate were more likely to achieve better health-related quality of life than patients for whom the TKA was rated as inappropriate [38].
Riddle et al. modified the Escobar algorithm to attain a decision tool for US patients [8]. They used the Kellgren Lawrence score rather than the Ahlbäck classification and quantified symptomatology using the Western Ontario and McMaster osteoarthritis index (WOMAC). In 21.7 % of patients appropriateness of TKA was rated as uncertain.
THA appropriateness
Quintana et al. developed three THA appropriateness algorithms in OA patients [10, 11, 36]. Two were established using the RAND/UCLA appropriateness method. These algorithms took the following variables into account: age, surgical risk, previous nonsurgical treatments, pain and functional limitation. Table 4 depicts various scenarios in which THA was considered inappropriate, uncertain or appropriate [10]. In both algorithms, appropriateness was rated uncertain in a large part of patients, 46.2 % and 32.4 %. Both algorithms were validated in a population of OA patients scheduled for THA [10, 11]. Patients rated as appropriate THA candidates had better outcomes at 3 months on the WOMAC stiffness and functional limitation domains compared to inappropriate candidates.
The other algorithm was based on the WOMAC as they wanted to develop a tool based on a disease specific instrument rather than on expert opinion [36]. Surgical risk, pre-intervention pain and functional limitations were found to significantly predict changes in the WOMAC pain domain 6 months after THA and pre-intervention functional limitations predicted changes in the functional limitation domain [36]. In addition, by means of a classification and regression tree analysis a summary tree was constructed. THA was rated as appropriate when pain was qualified as severe (according to the pain and limitation short scales), when WOMAC pain pre-intervention score was >60 or when WOMAC functional limitation pre-intervention was >60 with pain pre-intervention >40. Surgical risk was not included in the decision tree. However, the authors stated that one should be aware that higher surgical risk often results in a worse outcome and that conservative treatment should always be performed before considering THA. Again this decision tool was validated in a THA cohort. They assessed sensitivity and specificity of being classified as appropriate compared with the appropriateness based on the minimal clinical important difference values (gain in WOMAC 6 months after THA, pain domain ≥30, function domain ≥25). A sensitivity of 95.0 % and a specificity of 41.0 % were found, suggesting that it seems difficult to identify the non-appropriate cases.
Discussion
In this systematic review we examined the quality and evidence base of existing indication criteria and guidelines for primary THA and TKA in OA patients. Across guidelines and publications we found, 12 THA, 10 TKA and 2 THA/TKA indication sets. Only 6 guidelines included indication criteria for THA/TKA with differing quality. Overall quality of the guidelines summed across the 6 domains ranged from 0.46 to 4.78. Low scores were frequently attained in the editorial independence domain and the applicability domain. High scores were often attained in the clarity of presentation domain. In the additional 12 reviews and 6 original publications most indication criteria included the following three domains: pain, function and radiological changes. Frequently a prerequisite was that conservative treatment had been insufficient in controlling pain. However, domain specific cut-off values or ranges were mostly not reported. Also, it was often not stated if pain, functional disability and radiological changes should all exist, or which combinations of domain-specific deviations should be apparent to indicate THA or TKA. The level of evidence was low (level IV).
We were not able to discriminate between high and poor quality guidelines as the AGREE-II has not given a set of rules to define a high quality guideline. Given the low scores in the applicability and the editorial independence domains, we advise guideline developers to pay more attention in reporting these issues. A limitation of the current study may be that the scoring of guidelines according to the AGREE-II is not completely objective, even though the manual clearly articulates how each item should be scored including the criteria and considerations for each item. However, the weighting of criteria and considerations in the overall scoring of the item is not mentioned, which could introduce inter-observer variability. To cope with this, the AGREE-II proposes to use more than one observer, which is why the guidelines were scored independently by two investigators and compared to reach consensus (with or without a decisive vote of a third investigator). As such, we tried to minimalize subjectivity.
Another limitation of this study is that we restricted our search strategy to English, Dutch and German language papers. In addition, our website search of orthopaedic and arthritis organizations was restricted to English and Dutch websites. Therefore we may have missed part of the existing literature on indication criteria for THA/TKA.
Irrespective of the quality of individual guidelines, the same domains concerning THA/TKA indications were reported across most guidelines. Based on the design of included studies, the highest level of evidence was reported by the OARSI and EULAR guidelines (only non-experimental studies, level III evidence). The evidence on which indication sets were based came from studies investigating the effectiveness and safety of THA/TKA, but these studies did not specifically address THA/TKA indication sets. Therefore the evidence from these guidelines was rated as level IV evidence, so that the evidence on which indication criteria are based, is low quality evidence.
Looking at other literature, most of the reviews also did not specifically focus on THA/TKA indications and none of the systematic reviews did. Moreover, in 2 of 3 reviews with THA/TKA indication as the main topic it was concluded that no conclusive evidence on THA/TKA indications are currently available. Furthermore, few original papers investigating THA/TKA indications were found, which may be partly due to the employed language restrictions, possibly resulting in language bias. Four of five original studies came from the same group and four were based on the RAND/UCLA method [8–11]. Although this is a respected approach, the limitation is that the indication set is mainly based on expert opinion if little research is available. Thus, even with an optimal composition of experts in the panel, the level of evidence will still be low. This is currently the case for THA/TKA indication sets. In addition, within the proposed THA/TKA decision tools, the appropriateness of surgery was rated as uncertain in many patients. This makes these decision tools difficult to use in daily practice, as patients with appropriateness rated as uncertain may have similar improvements in health outcomes as patients rated as appropriate. Therefore, no evidence-based indications concerning THA/TKA are currently available which can be uniformly used in daily practice.
Nonetheless, when indications were reported, the same domains were included. Hence, although evidence based studies are lacking, expert opinion seems reasonably consistent. This is promising as these domains may give clues to the targets on which future research for THA/TKA indications should focus. It seems evident that pain, function, radiological changes and failed conservative therapy should be part of future studies on THA/TKA indications. The research of indication criteria is, however, difficult. One of the difficulties is that pain and function are relatively subjective measures both when reported by the patient and when judged by the physician. This is illustrated by the fact that although consensus on the indication domains seems to exist, disease severity greatly varies at the time of surgery across different centres in Europe and Australia [6, 7]. This suggests no agreement on the cut-off values or ranges within these domains or between combinations of domains as an indication for surgery. Another difficulty is that it is not possible to conduct controlled trials with the timing of surgery randomized, so that other designs are needed. As a consequence the highest level of evidence is not likely to be obtained, but likely to be relatively low given mainly observational studies (level II and III). However, outcomes of observational studies can be valid and may provide similar results as RCTs. For instance, meta-analyses comparing RCTs and observational studies of treatment effects found no large systematic differences [39]. Furthermore, randomization will avoid confounding by indication but this can also be achieved with advanced statistical analyses and pseudo-randomization in observational studies. To obtain the best possible evidence, we should try to identify predictors for a (less than) good outcome after THA/TKA. With the identified predictors we might be able to simulate with mathematical modelling at which cut-off points surgery has the best postoperative outcomes, taking into account the limited lifespan of a prosthesis and the fact that revision surgery mostly has worse outcomes than primary surgery.
Conclusions
In conclusion, our current study gives an overview of the available evidence base of THA/TKA indication criteria in both guidelines and original studies. We showed that the currently available THA/TKA indication criteria are based on limited and low quality evidence. Hence, empirical research on this topic is needed, especially regarding domain specific cut-off values or ranges at which the best postoperative outcomes are achieved.
Abbreviations
- AGREE-II:
-
Appraisal of Guidelines for Research and Evaluation instrument, Dutch version
- OA:
-
Osteoarthritis
- THA:
-
Total hip arthroplasty
- TKA:
-
Total knee arthroplasty
- WOMAC:
-
Western Ontario and McMaster osteoarthritis index
References
Lawson EH, Gibbons MM, Ingraham AM, Shekelle PG, Ko CY. Appropriateness criteria to assess variations in surgical procedure use in the United States. ArchSurg. 2011;146:1433–40.
Robertsson O, Bizjajeva S, Fenstad AM, Furnes O, Lidgren L, Mehnert F, Odgaard A, Pedersen AB, Havelin LI. Knee arthroplasty in Denmark, Norway and Sweden. A pilot study from the Nordic Arthroplasty Register Association. Acta Orthop. 2010;81:82–9.
Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartilage. 2008;16:137–62.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5.
Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–12.
Ackerman IN, Dieppe PA, March LM, Roos EM, Nilsdotter AK, Brown GC, Sloan KE, Osborne RH. Variation in age and physical status prior to total knee and hip replacement surgery: a comparison of centers in Australia and Europe. Arthritis Rheum. 2009;61:166–73.
Dieppe P, Judge A, Williams S, Ikwueke I, Guenther KP, Floeren M, Huber J, Ingvarsson T, Learmonth I, Lohmander LS, et al. Variations in the pre-operative status of patients coming to primary hip replacement for osteoarthritis in European orthopaedic centres. BMC Musculoskelet Disord. 2009;10:19.
Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66:2134–43.
Escobar A, Quintana JM, Arostegui I, Azkarate J, Guenaga JI, Arenaza JC, Garai I. Development of explicit criteria for total knee replacement. IntJ Technol Assess Health Care. 2003;19:57–70.
Quintana JM, Arostegui I, Azkarate J, Goenaga JI, Elexpe X, Letona J, Arcelay A. Evaluation of explicit criteria for total hip joint replacement. J Clin Epidemiol. 2000;53:1200–8.
Quintana JM, Azkarate J, Goenaga JI, Arostegui I, Beldarrain I, Villar JM. Evaluation of the appropriateness of hip joint replacement techniques. Int J Technol Assess Health Care. 2000;16:165–77.
Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435.
Ghomrawi HM, Schackman BR, Mushlin AI. Appropriateness criteria and elective procedures--total joint arthroplasty. N Engl J Med. 2012;367:2467–9.
Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet. 2012;380:1768–77.
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, Beard DJ. Knee replacement. Lancet. 2012;379:1331–40.
Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701–12.
Gossec L, Paternotte S, Bingham III CO, Clegg DO, Coste P, Conaghan PG, Davis AM, Giacovelli G, Gunther KP, Hawker G, et al. OARSI/OMERACT initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. An OMERACT 10 Special Interest Group. J Rheumatol. 2011;38:1765–9.
Hawker G, Bohm E, Conner-Spady B, De CC, Dunbar M, Hennigar A, Loucks L, Marshall DA, Pomey MP, Sanmartin C, Noseworthy T. Perspectives of canadian stakeholders on criteria for appropriateness for total joint arthroplasty in patients with hip and knee osteoarthritis. Arthritis Rheumatol. 2015;67:1806–15.
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145–55.
Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, Hauselmann HJ, Herrero-Beaumont G, Jordan K, Kaklamanis P, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64:669–81.
Swierstra BA, Vervest AM, Walenkamp GH, Schreurs BW, Spierings PT, Heyligers IC, van Susante JL, Ettema HB, Jansen MJ, Hennis PJ, et al. Dutch guideline on total hip prosthesis. Acta Orthop. 2011;82:567–76.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–42.
Osteoarthritis: Care and management in adults [http://www.nice.org.uk/guidance/cg177]
Commissioning guide: Painful osteoarthritis of the knee [http://www.boa.ac.uk/pro-practice/painful-osteoarthritis-of-the-knee-commissioning-guide-2/]
Altman RD, Abadie E, Avouac B, Bouvenot G, Branco J, Bruyere O, Calvo G, Devogelaer JP, Dreiser RL, Herrero-Beaumont G, et al. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthr Cartilage. 2005;13:13–9.
Total knee replacement: an evidence-based analysis. OntHealth Technol Assess Ser 2005, 5:1-51.
Dowsey MM, Gunn J, Choong PF. Selecting those to refer for joint replacement: who will likely benefit and who will not? Best Pract Res Clin Rheumatol. 2014;28:157–71.
Schneppenheim M, Jerosch J. Prosthetic replacement of the knee joint for osteoarthritis of the knee and retropatellar osteoarthritis. When is which endoprosthesis indicated? Chirurgische Praxis. 2001;59:275–92.
Mandl LA. Determining who should be referred for total hip and knee replacements. Nat Rev Rheumatol. 2013;9:351–7.
Van Manen MD, Nace J, Mont MA. Management of primary knee osteoarthritis and indications for total knee arthroplasty for general practitioners. J Am Osteopath Assoc. 2012;112:709–15.
Levine ME, Nace J, Kapadia BH, Issa K, Banerjee S, Cherian JJ, Mont MA. Treatment of primary hip osteoarthritis for the primary care physician and the indications for total hip arthroplasty. J Long Term Eff Med Implants. 2013;23:323–30.
Passias PG, Bono JV. Total hip arthroplasty in the older population. Geriatrics Aging. 2006;9:535–43.
Lane NE. Clinical practice. Osteoarthritis of the hip. N Engl J Med. 2007;357:1413–21.
Hanssen AD, Stuart MJ, Scott RD, Scuderi GR. Surgical options for the middle-aged patient with osteoarthritis of the knee joint. J Bone Joint Surg Am Vol. 2000;82A:1768–81.
Yamabe E, Ueno T, Miyagi R, Watanabe A, Guenzi C, Yoshioka H. Study of surgical indication for knee arthroplasty by cartilage analysis in three compartments using data from Osteoarthritis Initiative (OAI). BMC Musculoskelet Disord. 2013;14:194.
Quintana JM, Bilbao A, Escobar A, Azkarate J, Goenaga JI. Decision trees for indication of total hip replacement on patients with osteoarthritis. Rheumatol (Oxford). 2009;48:1402–9.
Park RE, Fink A, Brook RH, Chassin MR, Kahn KL, Merrick NJ, Kosecoff J, Solomon DH. Physician ratings of appropriate indications for six medical and surgical procedures. Am J Public Health. 1986;76:766–72.
Quintana JM, Escobar A, Arostegui I, Bilbao A, Azkarate J, Goenaga JI, Arenaza JC. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med. 2006;166:220–6.
Shrier I, Boivin JF, Steele RJ, Platt RW, Furlan A, Kakuma R, Brophy J, Rossignol M. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–9.
Total knee replacement: A guide to good practice [http://nzoa.org.nz/system/files/total_knee_replacement_practice_guidelines.pdf]
Commissioning guide: Pain arising from the hip in adults [http://www.boa.ac.uk/pro-practice/pain-arising-from-the-hip-in-adults-commissioning-guide/]
Totale Heupprothese [http://www.kwaliteitskoepel.nl/assets/structured-files/2011/Richtlijn+Totale+Heupprothese+2010+zonder+watermerk.pdf]
Total hip replacement: Good practice guidelines [http://nzoa.org.nz/system/files/Total_Hip_Joint_Arthroplasty_Guidelines_0.pdf]
Kirschner S. Indication criteria and epidemiology of joint replacement. Z Evid Fortbild Qual Gesundhwes. 2011;105:143–5.
Acknowledgements
We would like to thank J. Schoones, Librarian, who assisted us with the search strategy.
Funding
Source of funding: The Dutch Arthritis Foundation grant number BP 12-3-401.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
MG and SH performed the selection of the guidelines and articles. MG extracted the data and this data was checked by SH. The guidelines scored by MG and SH in case of large differences and not reaching consensus PM had the decisive vote. MG wrote the article. SH, PM, TV, and RN have critically read and modified the manuscript. All authors read and approved the final version of our article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Search strategy. (DOCX 31 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gademan, M.G.J., Hofstede, S.N., Vliet Vlieland, T.P.M. et al. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord 17, 463 (2016). https://doi.org/10.1186/s12891-016-1325-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-016-1325-z