Abstract
Background
Idiopathic Pulmonary Fibrosis (IPF) is a progressive fibrotic lung disease. However, the field of quantitative CT scan analysis in conjunction with pulmonary function test for IPF patients remains relatively understudied. In this study, we evaluated the diagnostic value of features derived high-resolution computed tomography (HRCT) for patients with IPF and correlated them with pulmonary function tests.
Methods
We retrospectively analyzed the chest HRCT images and pulmonary function test results of 52 patients with IPF during the same period (1 week) and selected 52 healthy individuals, matched for sex, age, and body mass index (BMI) and with normal chest HRCT as controls. HRCT scans were performed using a Philips 256-row Brilliance iCT scanner with standardized parameters. Lung function tests were performed using a Jaeger volumetric tracer for forced vital capacity (FVC), total lung capacity (TLC), forced expiratory volume in first second (FEV1), FEV1/FVC, carbon monoxide diffusing capacity (DLCO), and maximum ventilation volume (MVV) metrics. CT quantitative analysis, including tissue segmentation and threshold-based quantification of lung abnormalities, was performed using 3D-Slicer software to calculate the percentage of normal lung areas (NL%), percentage of ground-glass opacity areas (GGO%), percentage of fibrotic area (F%) and abnormal lesion area percentage (AA%). Semi-quantitative analyses were performed by two experienced radiologists to assess disease progression. The aortic-to-sternal distance (ASD) was measured on axial images as a standardized parameter. Spearman or Pearson correlation analysis and multivariate stepwise linear regression were used to analyze the relationship between the data in each group, and the ROC curve was used to determine the optimal quantitative CT metrics for identifying IPF and controls.
Results
ROC curve analysis showed that F% distinguished the IPF patient group from the control group with the largest area under the curve (AUC) of 0.962 (95% confidence interval: 0.85–0.96). Additionally, with F% = 4.05% as the threshold, the Youden’s J statistic was 0.827, with a sensitivity of 92.3% and a specificity of 90.4%. The ASD was significantly lower in the late stage of progression than in the early stage (t = 5.691, P < 0.001), with a mean reduction of 2.45% per month. Quantitative CT indices correlated with all pulmonary function parameters except FEV1/FVC, with the highest correlation coefficients observed for F% and TLC%, FEV1%, FVC%, MVV% (r = − 0.571, − 0.520, − 0.521, − 0.555, respectively, all P-values < 0.001), and GGO% was significantly correlated with DLCO% (r = − 0.600, P < 0.001). Multiple stepwise linear regression analysis showed that F% was the best predictor of TLC%, FEV1%, FVC%, and MVV% (R2 = 0.301, 0.301, 0.300, and 0.302, respectively, all P-values < 0.001), and GGO% was the best predictor of DLCO% (R2 = 0.360, P < 0.001).
Conclusions
Quantitative CT analysis can be used to diagnose IPF and assess lung function impairment. A decrease in the ASD may indicate disease progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Idiopathic pulmonary fibrosis (IPF) is an unexplained, irreversible, chronic, progressive interstitial lung disease characterized by a progressive decline in lung function for which there is a lack of effective treatment and a low survival rate [1, 2]. The prevalence of IPF is increasing in elderly patients, with a median survival of 3–5 years, and high-resolution CT (HRCT) is the most accurate test for noninvasive diagnosis of IPF [3]. IPF manifests as usual interstitial pneumonia (UIP) on HRCT images or surgical lung biopsy [4].
Lung function can reflect the disease progression of patients with IPF, assess the severity of the disease, and predict the disease prognosis. However, pulmonary function tests require the active cooperation of patients [5]. In the process of testing, test results are seriously affected when patients do not cooperate for personal reasons. For patients with serious conditions, it may even be impossible to complete the pulmonary function test. Therefore, qualified pulmonary function tests are not applicable to elderly patients who cannot cooperate and those with acute exacerbation or an advanced stage of IPF.
With the advent of new computer technologies, quantitative CT techniques are developing into viable tools. Quantitative chest CT technology, which allows chest CT images to be reconstructed and calculated by appropriate software, is gradually gaining wide acceptance due to its objectivity and ability to reflect histological features, and has great potential in the assessment of interstitial lung diseases such as IPF [6]. However, few previous studies have performed quantitative CT analysis of IPF to study its relationship with lung function, and most studies are based on visual analysis [7]. Therefore, in this study, we assessed the relationship between quantitative CT analysis of IPF and lung function to determine the value of quantitative CT analysis in predicting lung function impairment in patients with IPF and its clinical application.
Materials and methods
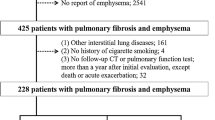
We collected 75 consecutive cases of IPF between February 2021 and February 2024. All patients met the 2018 ATS/ERS/JRS/ALAT guidelines for the diagnosis of IPF. Ultimately, we included 52 patients who underwent pulmonary function tests and high-resolution CT scans within no more than one week between the two tests (Fig. 1). Forty-eight of these patients were diagnosed after multidisciplinary consultation, and four patients had their diagnosis confirmed by surgical lung biopsy. Additionally, 52 healthy individuals matched for sex, age, and body mass index (BMI), and with normalities on chest HRCT were selected as a control group.
The study received approval from the Hospital’s Ethics Committee (Ethics No. YJSKY2023-297). All clinical data were securely extracted from the electronic medical records, ensuring patient confidentiality and compliance with ethical standards.
CT methods
CT scans were performed using a Philips 256-row Brilliance iCT scanner, and all patients underwent respiratory training before scanning, followed by HRCT scanning with breath-holding at the end of maximal inspiration. The patients were placed in the supine position with their hands raised, and the scan was performed from the base of the lungs to the apices. The scanning parameters were as follows: layer thickness, 1 mm; layer spacing, 5 mm; voltage, 120 kV; pitch array, 512 × 512; bone algorithm reconstruction; lung window observation window width, 1,500 Hu, and window position − 600 HU.
Quantitative CT methods
Lung parenchyma segregation was performed using the open source software 3D-Slicer, which automatically segregates the lung parenchyma and manually corrects areas that are inaccurately identified by the software. Images were analyzed by two experienced chest radiologists. Threshold segmentation was used to obtain quantitative CT metrics, setting whole lung pixels between − 1024 and − 200 HU, normal lung tissue pixels between − 950 and − 700 HU, ground-glass opacity pixels between − 700 and − 500 HU, and fibrotic area pixels between − 500 and − 200 HU. The ratios of normal lung pixels, ground-glass opacity pixels, and fibrotic region pixels to whole lung pixels were set as a percentage of normal lung region (NL%), percentage of ground-glass opacity region (GGO%), and percentage of fibrotic region (F%). The sum of the percentage of ground-glass opacity region and the fibrotic region was set as the percentage of abnormal lesion region (AA%) (Fig. 2).
Male patient with IPF, aged 46. (A) Chest HRCT scan demonstrates bilateral ground-glass opacity and reticular patterns, typical for IPF; (B) Automatic segmentation with 3D-Slicer software, where blue represents normal lung tissue, orange indicates ground-glass opacity areas, pink denotes fibrotic regions, and red signifies blood vessels
Semi-quantitative analysis
Thirty-three of the 52 patients underwent regular CT follow-up. Baseline and follow-up CT scans are spaced 1 to 14 months apart. To assess the progression of fibrosis on CT, the follow-up CT images were simultaneously compared by two experienced radiologists who were blinded to the chronological order of the scans. The images were visually analyzed, and disease progression was defined as a decrease in lung volume and an increase in associated signs, such as tractional bronchiectasis, honeycomb, and ground-glass opacity. The final visual analysis screened 22 patients consistent with disease progression.
Data measurement methods
The ASD is defined as the distance from the anterior wall of the aorta to the posterior wall of the sternum on axial films. This parameter was measured by two experienced chest radiologists at baseline and follow-up CT. The HRCT images were standardized using the pulmonary trunk as an anatomical landmark (Fig. 3).
Pulmonary function test
Pulmonary function tests were performed using a volumetric tracer from Jeager, Germany, to obtain the main indices of the study cases in this group: forced vital capacity (FVC), total lung volume (TLC), forced expiratory volume in the first second (FEV1), FEV1/FVC, diffusion volume (DLCO), and maximum ventilation volume (MVV). The pulmonary function tests were performed with the subject in the sitting position, and the best result of three repeated measurements was taken as the final result.
Statistical methods
The Shapiro–Wilk method was used to test the normality of the measurement data, with normally distributed data expressed as the mean ± standard deviation and non-normally distributed data expressed as M (P25–P75). Correlations between quantitative CT indices and lung function parameters were assessed using Spearman or Pearson correlation analysis. The diagnostic efficacy of quantitative CT indices for IPF was assessed using subject operating characteristic curves, and the optimal thresholds, the Youden’s J statistic, sensitivity, specificity, and area under the curve (AUC) were calculated. Multiple stepwise linear regression analyses were used to determine the optimal CT quantitative index for predicting lung function. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed using SPSS 26.0.
Results
Study population
Fifty-two patients who underwent pulmonary function tests and HRCT within 1 week and 52 healthy patient controls were included. Data relating to age, sex, BMI, smoking status, ASD, quantitative chest CT, and pulmonary function are shown in Table 1. Differences between the two groups in terms of sex composition ratio, age, and BMI were not statistically significant. There was a significantly larger number of smokers among the patients in the IPF group than in the control group (P < 0.05). The ASD was significantly higher in the healthy control group (P < 0.05). CT quantitative indices showed that the NL% was significantly lower in the IPF group than in the healthy control group, and GGO%, F%, and AA% were significantly higher in the IPF group than in the control group (P < 0.001). Of the 33 patients followed up, 11 showed no evidence of progressive fibrosis. Follow-up CT scans did not show any reduction in fibrosis in the 33 patients.
Comparison and correlation analysis of smoking in IPF group and healthy controls
The IPF group contained significantly more smokers than in the control group (P < 0.05), and there was a significant positive correlation with morbidity (P < 0.05) (Table 2).
Comparison of aortic-to-sternal distance before and after disease progression in patients with IPF
As shown in Table 3, the ASD was significantly lower in the late stage of progression than in the early stage (t = 5.691, P < 0.001), with an average monthly reduction of 2.45%.
ROC curve analysis results
The CT quantitative indexes NL%, GGO%, F%, and AA% were used as diagnostic indexes to classify patients with IPF and healthy controls according to the ROC curve. The results showed that the F% discriminated between the two groups, with the largest AUC of 0.962 (95% confidence interval: 0.925 ~ 0.998), and the Youden’s J statistic with a threshold value of F% = 4.05% was 0.827, with a sensitivity of 92.3% and specificity of 90.4% (Fig. 4; Table 4).
Correlation between quantitative chest CT indices and lung function in patients with IPF
As shown in Table 5, the correlations between quantitative CT indices and pulmonary function parameters beyond the initial FEV1/FVC were statistically significant, with the highest correlation coefficients between F% and TLC%, FEV1%, FVC%, MVV% (r = − 0.571, − 0.520, − 0.521, − 0.555, P-values < 0.001). GGO% was significantly correlated with DLCO% (r = − 0.600, P < 0.001).
Multiple stepwise first regression analyses were performed to determine whether lung function indices could be predicted from quantitative CT analysis data. F% was the best predictor of TLC%, FEV1%, FVC%, and MVV% (R2 = 0.301, 0.301, 0.300, and 0.302, all P-values < 0.001), while GGO% was the best predictor of DLCO% (R2 = 0.360, P < 0.001) (Table 6).
Discussion
Currently, there is no consensus on the threshold definition; however, most scholars now recognize the idea that − 950 to − 700 HU is a normal lung pixel [8]. Stefano et al. [9]. found that the optimal upper lung threshold CT value for the diagnosis of IPF was − 200 HU by comparing the diagnostic values of − 200 HU, 0, and 200 HU as the upper threshold for IPF. Ash et al. [10]. considered that the lung volume should be from − 1024 to − 200 HU. Matsuoka et al. [11]. have defined the extent of interstitial lung disease as areas of high attenuation by selecting a rage from − 700 to 0 HU. In their study, they found that the range of − 500 HU to 0 HU has a higher correlation with fibrosis, and the lung attenuation in the range of − 700 to − 500 HU is strongly correlated with the extent of ground-glass opacity. Therefore, we set the whole lung pixels between − 1024 and − 200 HU, normal lung tissue pixels between − 950 and − 700 HU, ground-glass opacity pixels between − 700 and − 500 HU, and fibrotic region pixels between − 500 and − 200 HU.
Early diagnosis of IPF is critical to patient prognosis. Currently, the diagnosis of IPF relies on HRCT, pathology, and pulmonary function tests. Oldham et al. [12] noted that patients with IPF usually received a correct diagnosis after seeing multiple doctors and waiting more than a year. In another study [13], they found that the time between the onset of symptoms and obtaining a confirmed diagnosis in patients with IPF was usually between 4 and 5 years. Overall, our findings and the cited studies suggest that it is crucial to make a correct diagnosis of IPF as early as possible. HRCT is the most meaningful test for the diagnosis of IPF. HRCT can be used to visualize abnormal lung changes. This study shows that the CT quantitative index F% value of 4.05% is the best threshold value to identify IPF in healthy controls. In healthy controls, the CT quantitative index was mainly related to the fallout effect. Ostridge et al [14] performed quantitative CT analysis on subjects with normal lung function and found that emphysema may also occur in people with normal lung function. Therefore, when the F% of patients is higher than 4.05%, it is clinically advisable to be vigilant for the possibility of interstitial lung disease, with a higher likelihood of IPF.
PFT is important for assessing disease severity and predicting the risk of disease exacerbation or death in patients with interstitial lung disease [15]. A decrease in lung function, especially FVC, has been shown to be significantly associated with an increased risk of death in IPF [16], and the guidelines [17] also suggest that lung function is an important method for assessing the disease progression and predicting the disease prognosis in IPF. The results of the present study showed that CT quantitative indices correlated with lung function parameters except FEV1/FVC. FEV1/FVC is used to assess airflow obstruction in COPD and correlates with emphysema parameters analyzed by CT quantitative analysis [18], whereas IPF usually manifests itself as a restrictive ventilatory disorder, which may be the reason why it did not correlate with CT quantitative indices in the present study. Koh et al. [19] found that changes in CT quantification and FVC were related to visual assessment of interstitial lung disease progression by deep learning. A related study [20] found that FVC reliably predicted mortality in patients with IPF. In this study, the relationship between CT quantitative indexes and each lung function parameter was analyzed by modeling separately. The results showed that F% was the best predictor of FVC%, and GGO% was the best predictor of DLCO%, which indicated that CT quantitative analysis could assess lung function impairment to a certain extent and could be used as a supplement to pulmonary function tests. The accuracy of PFT depends on the patient’s own cooperation, whereas HRCT requires much less patient cooperation than PFT. Therefore, quantitative CT analysis may be more useful when the patient is in poor physical condition or has poor cooperation. Quantitative CT analysis allows not only for specific quantification of the percentage of each lesion in the lungs but also for assessment of lung function impairment.
The most commonly used lung function parameters for prognostic and outcome assessment of interstitial lung disease are FVC% and DLCO%. Andersson et al. [21]. found a significant correlation between the degree of interstitial lung disease assessed by HRCT via FVC% and DLCO% in patients with anti-synthetase syndrome, but not with disease staging. This suggests that FVC% and DLCO% are appropriate outcome measures for assessing ILD associated with antisynthetase syndrome. Du et al. [22]. found that fibrotic area percentage and DLCO% were significantly associated with survival. Our results showed that CT quantitative indices correlated with both FVC% and DLCO%, with the highest correlation coefficients observed between F% and FVC% and between GGO% and DLCO%; therefore, we hypothesize that CT quantification is also suitable for prognostic and efficacy assessment in IPF.
Harmful substances in cigarettes such as nicotine, which promotes alveolar epithelial and vascular endothelial damage, play a key role in the evolution of fibrosis [23]. In this study, smoking was found to be positively correlated with the prevalence of the disease (P < 0.05); however, the correlation was weak (r = 0.266), suggesting that smoking is a possible pathogenetic factor in patients with IPF.
The research area of exploring quantifiable indicators of lung volume loss that can be used as routine imaging tools to monitor disease progression is both attractive and promising. The development of such a biomarker would not only provide clinicians with an easier and more direct means to assess the extent of lung disease but also help to identify the risk of disease progression at an earlier stage, thus guiding more timely interventions. In our study, we found that the ASD progressively shortens as the disease progresses, with an average reduction of 2.45% per month, which provides new evidence of disease progression. This may be due to increased fibrosis and volume loss in progressive IPF, leading to a reduction in the anterior-posterior anatomical distance of the thorax. This may also be related to variations in the patient’s inspiratory volume. Robbie et al. [24]. found that the aortic sternal distance specifically decreased by 25% per year, providing evidence of disease progression. This aligns with our findings.
Currently, quantitative chest CT techniques are more widely used to assess the severity of emphysema in patients with chronic obstructive pulmonary disease [25, 26]. However, there are fewer studies related to quantitative chest CT techniques in the field of interstitial lung disease. Many visual scoring systems have been used to assess the severity of fibrotic lung disease [27]. However, these methods are time-consuming, labor-intensive, and subjective, in addition to having poor interobserver variability. The quantitative CT analysis of images of patients with IPF not only takes little time and is reproducible but has the ability to quantify the data generated, and has many applications in future studies of this disease [28].
Despite its strengths, this study also has some limitations. First, this was a retrospective study with a small sample size study that included clinically indicated CT examinations, which may have led to the inclusion of patients with more advanced disease, who are more susceptible to bias influenced by their subjective wishes. Second, the differentiation between IPF and other interstitial lung diseases often relies on the honeycombing signs. However, the threshold segmentation method used in this study is incapable of quantifying honeycombing appearances. Third, the assessment of disease progression in this study primarily relied on semi-quantitative/visual analysis, lacking clinical standards and data. In our next study, we will further follow-up the cases, incorporate more clinical data, and conduct a multicenter, large-sample study to delve into the role of quantitative CT analysis in the prognosis of patients with IPF and the value of its application in other interstitial lung diseases.
Conclusions
In conclusion, we have demonstrated that quantitative CT analysis can be used for the diagnosis of IPF and can assess the impairment of lung function, and the reduction of ASD can provide evidence of disease progression. Quantitative chest CT is of great value in the diagnosis, assessment, and prognosis of IPF because of its advantages of noninvasiveness, quantification, objectivity, simplicity, and reliability, and it is worthwhile to promote its use in clinical practice.
Data availability
The datasets used in the present study are available from the first author and corresponding authors on reasonable request.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- PFT:
-
Pulmonary function test
- BMI:
-
Body mass index
- FEV1:
-
Forced expiratory volume in first second
- FVC:
-
Forced vital capacity
- TLC:
-
Total lung capacity
- DLCO:
-
Carbon monoxide diffusing capacity
- MVV:
-
Maximum ventilation volume
- ASD:
-
Aortic-to-sternal distance
References
Althobiani MA, Russell A-M, Jacob J, Ranjan Y, Folarin AA, Hurst JR, et al. Interstitial lung disease: a review of classification, etiology, epidemiology, clinical diagnosis, pharmacological and non-pharmacological treatment. Front Med. 2024;11:1296890.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primer. 2017;3:17074.
Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22:197.
Sverzellati N. Highlights of HRCT imaging in IPF. Respir Res. 2013;14(Suppl 1):S3.
Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of Spirometry 2019 Update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–88.
Chen A, Karwoski RA, Gierada DS, Bartholmai BJ, Koo CW. Quantitative CT analysis of diffuse lung disease. Radiographics. 2020;40:28–43.
Salisbury ML, Lynch DA, Van Beek EJR, Kazerooni EA, Guo J, Xia M, et al. Idiopathic pulmonary fibrosis: the Association between the adaptive multiple features method and fibrosis outcomes. Am J Respir Crit Care Med. 2017;195:921–9.
Ohkubo H, Kanemitsu Y, Uemura T, Takakuwa O, Takemura M, Maeno K, et al. Normal lung quantification in Usual interstitial pneumonia pattern: the impact of threshold-based Volumetric CT Analysis for the staging of idiopathic pulmonary fibrosis. PLoS ONE. 2016;11:e0152505.
Stefano A, Gioè M, Russo G, Palmucci S, Torrisi SE, Bignardi S, et al. Performance of Radiomics Features in the quantification of idiopathic pulmonary fibrosis from HRCT. Diagnostics. 2020;10:306.
Ash SY, Harmouche R, Vallejo DLL, Villalba JA, Ostridge K, Gunville R, et al. Densitometric and local histogram based analysis of computed tomography images in patients with idiopathic pulmonary fibrosis. Respir Res. 2017;18:45.
Shin Matsuoka. Objective quantitative CT evaluation using different attenuation ranges in patients with pulmonary fibrosis: correlations with visual scores. Int J Respiratory Pulmonary Med. 2016;3(2):0–0. https://doi.org/10.23937/2378-3516/1410049.
Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med. 2014;108:819–29.
Hewson T, McKeever TM, Gibson JE, Navaratnam V, Hubbard RB, Hutchinson JP. Timing of onset of symptoms in people with idiopathic pulmonary fibrosis. Thorax. 2018;73:683–5.
Ostridge K, Wilkinson TMA. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J. 2016;48:216–28.
Oldham JM, Lee CT, Wu Z, Bowman WS, Pugashetti JV, Dao N, et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur Respir J. 2022;59:2101396.
Reichmann WM, Yu YF, Macaulay D, Wu EQ, Nathan SD. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. BMC Pulm Med. 2015;15:167.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198:e44–68.
Koo HJ, Lee SM, Seo JB, Lee SM, Kim N, Oh SY, et al. Prediction of pulmonary function in patients with chronic obstructive Pulmonary Disease: correlation with quantitative CT parameters. Korean J Radiol. 2019;20:683.
Koh SY, Lee JH, Park H, Goo JM. Value of CT quantification in progressive fibrosing interstitial lung disease: a deep learning approach. Eur Radiol. 2023. https://doi.org/10.1007/s00330-023-10483-9.
Richeldi L, Ryerson CJ, Lee JS, Wolters PJ, Koth LL, Ley B, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67:407–11.
Andersson H, Aaløkken TM, Günther A, Mynarek GK, Garen T, Lund MB, et al. Pulmonary involvement in the antisynthetase syndrome: a comparative cross-sectional study. J Rheumatol. 2016;43:1107–13.
Du K, Zhu Y, Mao R, Qu Y, Cui B, Ma Y, et al. Medium-long term prognosis prediction for idiopathic pulmonary fibrosis patients based on quantitative analysis of fibrotic lung volume. Respir Res. 2022;23:372.
Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J. 2012;26:4778–87.
Robbie H, Wells AU, Fang C, Jacob J, Walsh SLF, Nair A, et al. Serial decline in lung volume parameters on computed tomography (CT) predicts outcome in idiopathic pulmonary fibrosis (IPF). Eur Radiol. 2022;32:2650–60.
Konietzke P, Wielpütz MO, Wagner WL, Wuennemann F, Kauczor H-U, Heussel CP, et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol. 2020;30:2502–12.
Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. Am J Roentgenol. 2013;201:W460–70.
Kishaba T, Maeda A, Yamazato S, Nabeya D, Yamashiro S, Nagano H. Radiological and physiological predictors of IPF Mortality. Med (Mex). 2021;57:1121.
Chung JH, Adegunsoye A, Cannon B, Vij R, Oldham JM, King C, et al. Differentiation of idiopathic pulmonary fibrosis from connective tissue disease-related interstitial lung Disease using quantitative imaging. J Clin Med. 2021;10:2663.
Funding
Beijing Kanghua Traditional Chinese and Western Medicine Development Foundation (No. KH–2022–DXJJ–022).
Author information
Authors and Affiliations
Contributions
ZH analyzed and interpreted the patient data and was a major contributor in writing the manuscript. LX, ZX and YY analyzed some of the patient data. ZC provided technical support. ZJ proofread the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received approval from the Hospital’s Ethics Committee (Ethics No. YJSKY2023-297). Verbal and written details were relayed to all participants, and written consent was secured from all.
Clinical trial number
As this study involved standard clinical procedures and assessments without an experimental treatment protocol, it did not require registration with a public clinical trials registry.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Li, X., Zhang, X. et al. Quantitative CT analysis of idiopathic pulmonary fibrosis and correlation with lung function study. BMC Pulm Med 24, 437 (2024). https://doi.org/10.1186/s12890-024-03254-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03254-9