Abstract
Introduction
Lung cancer is a common malignant tumor, and different types of immune cells may have different effects on the occurrence and development of lung cancer subtypes, including lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD). However, the causal relationship between immune phenotype and lung cancer is still unclear.
Methods
This study utilized a comprehensive dataset containing 731 immune phenotypes from the European Bioinformatics Institute (EBI) to evaluate the potential causal relationship between immune phenotypes and LUSC and LUAD using the inverse variance weighted (IVW) method in Mendelian randomization (MR). Sensitivity analyses, including MR-Egger intercept, Cochran Q test, and others, were conducted for the robustness of the results. The study results were further validated through meta-analysis using data from the Transdisciplinary Research Into Cancer of the Lung (TRICL) data. Additionally, confounding factors were excluded to ensure the robustness of the findings.
Results
Among the final selection of 729 immune cell phenotypes, three immune phenotypes exhibited statistically significant effects with LUSC. CD28 expression on resting CD4 regulatory T cells (OR 1.0980, 95% CI: 1.0627–1.1344, p < 0.0001) and CD45RA + CD28- CD8 + T cell %T cell (OR 1.0011, 95% CI: 1.0007; 1.0015, p < 0.0001) were associated with increased susceptibility to LUSC. Conversely, CCR2 expression on monocytes (OR 0.9399, 95% CI: 0.9177–0.9625, p < 0.0001) was correlated with a decreased risk of LUSC. However, no significant causal relationships were established between any immune cell phenotypes and LUAD.
Conclusion
This study demonstrates that specific immune cell types are associated with the risk of LUSC but not with LUAD. While these findings are derived solely from European populations, they still provide clues for a deeper understanding of the immunological mechanisms underlying lung cancer and may offer new directions for future therapeutic strategies and preventive measures.
Similar content being viewed by others
Introduction
Lung cancer is the second most prevalent cancer worldwide, where non-small cell lung cancer (NSCLC) is the principal pathological phenotype [1]. NSCLC encompass two major subtypes, lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD), based on their cell origin, morphology, and biological characteristics [2]. Research recognizes a number of risk factors that contribute to lung cancer, such as smoking, past pulmonary diseases, air pollutants, and occupational carcinogens [3, 4]. The identification and screening of potentially alterable risk factors are essential to lower the incidence of lung cancer, thereby aiding in its early diagnosis and treatment.
Research has reported that the inflammatory immune mechanism is an important feature of the tumor immune microenvironment (TIME) and is associated with poor prognosis in cancer [5, 6]. The location, type, density, and functional status of immune cells constitute the immune structure of the TIME, which varies among patients with NSCLC [7]. Immune cells may exhibit dual roles in both anti-tumor and pro-tumor effects. For example, CD8+ T cells and natural killer (NK) cells mediate anti-tumor responses, showing better overall survival, disease-free survival, and progression-free survival [8, 9]. Conversely, regulatory T cells (Tregs) can secrete inhibitory cytokines such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10). By inhibiting anti-tumor responses of helper T cells (Th1) and attracting activated Th2 cells, Tregs promote the progression of lung cancer through angiogenesis and immune suppression [10,11,12]. Observational research revealed that increased proportions of regulatory T cells and M2 macrophage indicated poor survival in advanced NSCLC patients [13]. Gaudreau et al. found that neoadjuvant chemotherapy is associated with increased infiltration of cytotoxic CD8+ T cells and CD20+ B cells, promoting anti-tumor immunity through changes in the phenotype of cytotoxic and memory CD8+ and CD4+ T cells [14]. McGrail et al. suggested that the count of CD8+ T cells in lung cancer is positively correlated with neoantigen load, with a significantly higher objective response rate in tumors with high tumor mutation burden (TMB) compared to those with low TMB [15]. Devi-Marulkar et al. observed high expression of TIGIT and CTLA-4 in TIL-Tregs within tertiary lymphoid structures (TLS) and non-TLS areas, indicating their involvement in the immune inhibitory mechanisms of lung tumors [16]. Moreover, chronic inflammation is also closely associated with the development of lung cancer, manifested by the infiltration and accumulation of inflammatory cells and the buildup of pro-inflammatory factors [17]. Despite extensive research on the genomic landscape of lung cancer, new factors that contribute to lung cancer are still being discovered [18, 19].
Mendelian randomization (MR) is an analytical method used for epidemiological causal inference, based on Mendel’s law of independent assortment [20]. A recent MR analysis demonstrated that C-C motif chemokine 27 (CCL27) levels in circulation are positively associated with the risk of lung cancer, whereas interleukin-18 levels are inversely associated with such risk [21].
While many pieces of evidence suggest that immune cells play a role in lung cancer risk and outcome, the systemic analysis of immune cells in this context remains undefined, making further comprehensive investigations necessary. In this study, we aimed to comprehensively explore the causal effects of 731 immune cells on lung cancer through a genome-wide association study (GWAS) pooled data using two-sample Mendelian randomization approach. To enhance persuasion, we also performed replication and meta-analysis on another lung cancer cohort. This is the most comprehensive study of the causal link of immune cells with lung cancer, and we hope that our results will provide a more informed assessment of lung cancer risk regarding immune cells.
Materials and methods
Study design
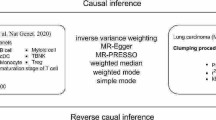
We utilized MR methodology to investigate the potential causal relationship between 731 immune cells and LUSC and LUAD. In our research, MR uses SNPs as “instrumental variables”(IV). For MR effect estimation to be robust, and for SNPs to serve as IVs. These assumptions must be as follows: (1) Assumption of Association: Genetic variants are strongly correlated with the exposure factor. (2) Assumption of Independence: Genetic variants are independent of confounding factors. (3) Assumption of Exclusivity: Genetic variants can only affect the outcome through the exposure factor [22]. Based on this, we make three assumptions:
Assumption 1
Gene SNPs are closely associated with immune cells.
Assumption 2
Gene SNPs are not associated with the outcome variable lung cancer or other confounding factors.
Assumption 3
Gene SNPs only affect lung cancer through their influence on immune cells and cannot affect lung cancer through other pathways (Fig. 1).
Overview of this Mendelian randomization (MR) analysis. Assumption 1, genetic instruments are strongly associated with the exposures of interest; Assumption 2, genetic instruments are independent of confounding factors; Assumption 3, genetic instruments are not associated with outcome and affect outcome only via exposures. LUSC lung squamous cell carcinoma; LUAD lung adenocarcinoma; IVW inverse variance weighted; LD linkage disequilibrium; LOO analysis leave-one-out analysis; MR-PRESSO MR‐Pleiotropy RESidual sum and outlier; SNPs single nucleotide polymorphisms; WM weighted median
STROBE-MR (strengthening the reporting of observational studies in epidemiology using mendelian randomisation) checklist
This study was guided by the STROBE-MR guidelines. This article adheres to the STROBE-MR checklist for reporting. (Supplementary STROBE-MR-checklist)
Genome-wide association study (GWAS) data sources for LUSC and LUAD
GWAS summary data for lung cancer (including LUSC and LUAD) were download from GWAS Catalog (https://www.ebi.ac.uk/gwas/) and the GWAS Catalog accession number is GCST004750 (LUSC) and GCST004744 (LUAD). The study performed a GWAS on 129,809 European individuals (Ncase=18,699, Ncontrol=111,110), with approximately 156,688,129 variants analyzed after quality control and imputation. Genetic information for lung cancer subtypes (LUSC: Ncase=7,426 and Ncontrol=55,627; LUAD: Ncase=11,273 and Ncontrol=55,483) were utilized for subgroup analysis. More detailed information about the GWAS data can be obtained from the study of James D McKay et al. [23]. The GWAS data for LUSC and LUAD mentioned above were used for preliminary analysis.
Immunity-wide GWAS data sources
We collected statistical data for each immune feature from the GWAS catalog, referencing accession numbers ranging from GCST0001391 to GCST0002121 [24] (Supplementary Table S1). This study utilized a comprehensive dataset containing 731 immune phenotypes, which were classified based on absolute cell counts (AC, n = 118), median fluorescence intensity reflecting surface antigen levels (MFI, n = 389), morphological parameters (MP, n = 32), and relative cell counts (RC, n = 192). The MFI, AC, and RC features included B cells, conventional dendritic cells (cDCs), mature T cells, monocytes, bone marrow cells, TBNK (T cells, B cells, NK cells), and Treg panel. The MP parameters (FSC-A and SSC-A) differentiated the size and intracellular complexity of immune cells for classification, including cDCs and TBNK panel (Appendix 1). This research conducted original GWAS studies on immune features using data from 3,757 European individuals, without overlapping cohorts. Approximately 22 million SNPs genotyped using high-density chips were analyzed for association using a reference panel based on the Sardinian sequence, adjusting for covariates such as gender and age [25].
Selection of instrumental variables
In our study, we identified instrumental variables (IVs) based on three primary criteria. Firstly, we selected SNPs with a P-value of less than 1 × 10–5 from the GWAS data related to each immune trait [26, 27]. To ensure the independence of the chosen SNPs, we employed the PLINK tool (version v1.90) to filter out SNPs exhibiting linkage disequilibrium (LD) r2 values greater than 0.1 within a 500 kb range [28]. Following this step, we assessed the strength of the selected SNPs as instrumental variables by calculating the F-statistic for each immune trait. Generally. When the F-value surpasses 10, the SNP is deemed suitable for subsequent MR analysis [29]. Ultimately, we performed MR analysis on immune traits with more than two SNPs and ultimately included 729 immune cells.
MR analysis
In this MR analysis, inverse variance weighted (IVW) method was primarily used to evaluate the causal relationship between immune traits and LUSC as well as LUAD. The IVW estimates are derived from a meta-analysis of the Wald ratios for all genetic variations [30]. IVW is based on the assumption that there is no horizontal pleiotropy across all SNPs, under which IVW provides the most accurate assessment of causal effects [31]. Therefore, we initially used IVW-based estimates to screen for immune cells that have causal impacts on LUSC and LUAD. The weighted median (WM) and MR-Egger methods were defined as the complementary analysis [32]. These two methods can provide more robust estimates under relaxed conditions. WM allows for less than 50% of SNPs to be ineffective [33], whereas MR-Egger provides detection of horizontal pleiotropy and heterogeneity when horizontal pleiotropy is present in all SNPs [30, 33]. However, inaccuracies may arise in the analysis outcomes when certain instrumental variables deviate from these assumptions. To tackle this problem, we conducted a series of sensitivity analyses. Initially, the Q-test method was utilized to evaluate potential heterogeneity among individual IVs, and p-value less than 0.05 from the Cochran Q test is considered indicative of heterogeneity in the results [34]. Subsequently, the MR-Egger intercept test was applied to estimate horizontal pleiotropy, guaranteeing that genetic variation has an independent relationship with both the exposure and outcome [35]. We used MR-PRESSO to re-examine the presence of heterogeneous SNPs [36]. Additionally, we conducted a leave-one-out (LOO) analysis, assessing whether the results were significantly influenced by individual SNPs by sequentially dropping each SNP and then performing MR analysis [33]. In summary, we rigorously screened for immune traits with potential causal effects on LUSC as well as LUAD through various criteria: (1) significant p-values in preliminary analysis (p < 0.05 from IVW) that remained significant after FDR correction; (2) Consistency in direction and magnitude across three MR methods; (3) MR results showed no heterogeneity or horizontal pleiotropy; (4) MR estimates were not severely disrupted by individual SNPs [28].
Replication and meta-analysis
To comprehensively assess the stability of the candidate immune traits that were selected based on the criteria mentioned earlier, we conducted a replication of the IVW analysis in an alternative LUSC and LUAD cohort for validation [37]. This cohort is derived from the Transdisciplinary Research Into Cancer of the Lung (TRICL) study, including 18,946 European ancestry lung cancer cases and 109,382 European ancestry controls, with specific subgroups for LUSC (Ncase=7,704 and Ncontrol= 54,763) and LUAD (Ncase=11,245 and Ncontrol= 54,619). In this replication analysis, the GWAS data for lung cancer were sourced from the GWAS catalog, with accession numbers ieu-a-989 and ieu-a-984. To summarize, the initial analysis was conducted using GWAS data with accession numbers GCST004750 and GCST004744, while the replication analysis utilized GWAS data with accession numbers ieu-a-989 and ieu-a-984 (Fig. 2). Ultimately, we merged the outcomes of the two MR analyses through a meta-analysis to discern the immune traits with a causal effect on LUSC and LUAD (Supplementary Table S2). The meta-analysis was executed using the Generic Effect IVW model in Review Manager 5.4 software.
Confounding analysis
In order to assess the potential horizontal pleiotropy of our MR results, we conducted several sensitivity tests to identify SNPs that may violate the MR assumptions. Nevertheless, it remains possible that some pleiotropic SNPs, which are difficult to detect, might still exist. To further evaluate whether each SNP is associated with established risk factors of LUSC and LUAD, including smoking [38], rheumatoid arthritis [39], body mass index [40], pollution [41] and genetic factors [42], we utilized the PhenoScanner V2 [43] website (http://www.phenoscanner.medschl.cam.ac.uk/) to scrutinize immune trait-related instrumental variables (IVs). If we identify any SNPs are significantly linked to the aforementioned confounding factors (p-value < 1 × 10− 5), we will exclude these SNPs and re-conduct the MR analysis. This crucial step aims to ensure the robustness and reliability of our analysis results.
Statistical analysis
All statistical analyses were performed using R version 4.3.0. Specifically, for MR analysis, we employed the MendelianRandomization package 0.9.0 [44]. and TwoSampleMR package 0.5.7, and for MR-PRESSO analysis, we utilized the MRPRESSO package 1.0 [45]. In instances where multiple testing was involved, we applied the False Discovery Rate (FDR) method for correction, thereby effectively mitigating the risk of false-positive findings. A statistically significant association with lung cancer was deemed present when the FDR value for the estimated causal effect of a particular immune trait was less than 0.05.
Data availability
All data used in this study are publicly available. No human subject approvals were necessary to conduct these analyses. All the data can be found from the GWAS directory (https://gwas.mrcieu.ac). The serial numbers of the immune cells are from GCST0001391 to GCST0002121, respectively. For the LUSC and LUAD data used for the primary analysis, the serial number is GCST004750 and GCST004744, and the LUSC and LUAD serial numbers used for the replication and meta-analysis are ieu-a-989 and ieu-a-984.
Results
Selection of IVs
For LUSC, the number of IVs for the 729 selected immune phenotypes ranged from 3 to 1182, with a median of 27. The minimum F-statistic values for validity testing consistently exceeded 10, with a range of 20 to 63 (Supplementary Table S3). Similarly, for LUAD, the number of IVs ranged from 3 to 1196. The minimum F-statistic values for their validity testing surpassed 10, spanning from 20 to 60 (Supplementary Table S4). These results indicate that the potential bias from weak instruments has been adequately resolved.
Preliminary analysis of lung cancer risk on immunophenotypes
To assess the causal effects of immunophenotypes on LUSC or LUAD, our primary analytical approach was the IVW method. Utilizing the FDR method for multiple tests, we identified seven suggestive immunophenotypes with a significance level of 0.05 in LUSC (Fig. 3). In contrast, no suggestive immunophenotypes were detected at this significance level in LUAD (Supplementary Table S5).
Consistency was then observed in the direction and magnitude of the IVW, MR-Egger, and WM estimates across these seven immune cells (Supplementary Fig. S1). Our subsequent analysis involved a further evaluation of these seven immune cell traits. We excluded four immune cells - SSC-A on lymphocyte, HLA DR on CD33- HLA, CD20 on IgD- CD24- B cell, and HLA DR on Dendritic Cell - based on not meeting the criteria of Q-test method p < 0.05, or MR-Egger intercept test p < 0.05, or MR-PRESSO method p < 0.05(Supplementary Table S5).
The refined analysis focused on the remained three types of immune cells, comprising two from the Treg panel and one from the cDC panel. Our analysis revealed two subtypes associated with increased risks of LUSC: CD28 on resting CD4 regulatory T cells (OR = 1.11, 95% CI = 1.06–1.16, p = 1.70E-05, FDR = 1.2E-02) and CD45RA + CD28- CD8 + T cell %T cell (OR = 1.00, 95% CI = 1.00–1.00, p = 2.60E-04, FDR = 3.10E-02). Additionally, we identified an immunophenotype, CCR2 on monocytes (OR = 0.93, 95% CI: 0.90–0.97, p = 8.10E-5, FDR = 1.6E-02), exhibiting protective effects against LUSC susceptibility.To address potential biases in MR estimation for these three immune cell types, we conducted a leave-one-out (LOO) analysis, confirming that no single SNP caused significant bias.
In summury, The IVW estimates for the three selected immune cells were significant, maintained significance after FDR correction, and were consistent in direction and magnitude (Fig. 4). The Cochran Q Test (p > 0.05) and MR-Egger intercept test (p > 0.05) indicated no heterogeneity or pleiotropy for these immune cells. Similarly, MR-PRESSO results, after outlier removal, suggest the absence of heterogeneous SNPs (Table 1). The LOO analysis further supported the reliability of our MR estimation, as shown in Supplementary Figure S1. These findings led us to consider these three immune cells as prime candidates for further analysis (Supplementary Fig. S2).
Causal effects of lung squamous cell carcinoma on immune cells concentration. SNP single nucleotide polymorphisms. a: Scatter plot between CD28 on resting CD4 regulatory T cells and LUSC risk. b: Scatter plot between CD45RA + CD28- CD8 + T cell %T cell and LUSC risk. c: Scatter plot between CCR2 on monocytes and LUSC risk
Replication and meta-analysis
To enhance the persuasiveness of our estimates, we conducted a replication of the MR analysis using additional GWAS data related to LUSC and LUAD. Similarly, we also found same three suggestive immunophenotypes at a significance level of 0.05 (Supplementary Table S6), while we did not identify any suggestive immunophenotypes with a significance level of 0.05 in LUAD (Supplementary Table S7). As anticipated, we observed similar trends in candidate immunophenotypes in this new LUSC dataset. It’s important to note that, despite these consistent trends, the results did not achieve P FDR < 0.05 statistical significance in replication analysis. This lack of significance can be attributed primarily to the minor differences in experimental conditions or study designs.
To strengthen our findings, we combined the outcomes of the GWAS data and conducted a meta-analysis that yielded further insights. As shown in Supplement Table S8, the analysis validated the causal relationship between three specific immunophenotypes and LUSC: CD28 expression on resting CD4 regulatory T cells (OR 1.10, 95% CI: 1.06–1.13, p < 0.01) and CD45RA + CD28- CD8 + T cell %T cell (OR 1.00, 95% CI: 1.00–1.00, p < 0.01), suggesting increased risk of LUSC; the expression of CCR2 on monocytes (OR 0.94, 95% CI: 0.92–0.96, p < 0.01) was associated with a reduced risk of LUSC (Fig. 5). Notably, these findings exhibited consistent directions in both MR analyses, and the meta-analysis yielded statistically significant estimations (Supplementary Table S8).
Meta-analysis of significantly associated (IVW derived p < 0.05) between immune cells and lung Squamous Cell Carcinoma. 95% CI, 95% confidence interval; OR, odds ratio. a: Meta plot between CD28 on resting CD4 regulatory T cells and LUSC. b: Meta plot between CD45RA + CD28- CD8 + T cell %T cell and LUSC. c: Meta plot between CCR2 on monocytes and LUSC
Confounding analysis
To ensure that our instrumental variables (IVs) are independent of confounding factors, we examined our selected SNPs for their autonomy from common risk factors for LUSC. Specifically, we assessed their associations with recognized risk factors such as smoking, RA, BMI, pollution and genetic predispositions. In the CD45RA + CD28-CD8 + T cell percentage, three SNPs were identified as being associated with the risk factors for LUSC (Supplementary Table S9). The remaining immune cells did not identify the suspected SNP. Re-analysis with exclusion of three SNPs demonstrated that our estimates remain significant: CD45RA + CD28- CD8 + T cell percentage: (OR 1.00 95% CI: 1.00–1.00, p < 0.01).
Discussion
In this study, we integrated two extensive GWAS datasets to investigate the causal relationships between 731 genetically proxied immune cell traits and LUSC or LUAD through a Mendelian randomization design. Our study suggests that CD28 on resting CD4 regulatory T cells and CD45RA + CD28- CD8 + T cell %T cell increases the risk of LUSC, while a phenotype of CCR2 on monocytes is associated with a reduced risk of LUSC. However, we did not find any immune cells with a causal relationship in LUAD. Our conclusions were further reinforced by replication and meta-analysis, and subsequently, our estimates remained significant after excluding SNPs associated with confounding factors. To our knowledges, this study represents the first MR investigation to utilize comprehensive GWAS data on immune cell traits to explore their causal links with LUSC and LUAD.
Our study revealed that the expression of CD28 on resting CD4 regulatory T cells correlates with an increased risk of LUSC. CD28 is a transmembrane protein located on the surface of T cells, which is crucial for signal transduction during T cell activation [46]. Experimental studies indicate that CD28 conditional knockout results in severe autoimmunity and inappropriate resolution of allergy, suggesting that CD28 plays post-maturational roles in Tregs [47]. The expression of CD28 on CD4 + Treg is essential for Treg homeostasis, and its interaction with B7 is required for their immunosuppressive function [48, 49]. This phenotype suggests an immunosuppressive milieu that may raise the vulnerability of LUSC, corroborating our MR analysis findings.
Meanwhile, the study found that the CD45RA + CD28- CD8 + T cell correlated with increased risk of LUSC. According to Orrù et al., we categorized CD8 + T cells into naïve T cells (CD45RA + CCR7+), central memory T cells (CD45RA − CCR7+), effector memory T cells (CD45RA − CCR7−), and terminally differentiated T cells (CD45RA + CCR7−) [24]. There are reports suggesting that the CD45RA + CD28 − CD8 + T cell subset appears to be similar to terminally differentiated CD45RA + CD8 + T cells with negative staining for the chemokine receptor CCR7 [50,51,52,53,54]. Terminally differentiated T cells exhibit lower proliferative capacity, reduced differentiation plasticity, but have strong effector activity (such as cytotoxicity and cytokine release), even without T cell receptor cross-linking through antigen exposure (via bystander activation through cytokine receptor) [55]. Terminally differentiated T cells are frequently linked to chronic inflammatory conditions in the setting of aging and chronic infections [56, 57]. Recent studies have confirmed that the terminally differentiated T cells and its CD28- subsets have the potential to serve as a biomarker of immunosenescence in the context of cytomegalovirus infection [58]. The absence of CD28 expression on CD8 + TEMRA possibly indicates weak T cell receptor engagement, suggesting weaker protective effects against infection and cancer development. These findings are in harmony with our MR analysis, indicating that this phenotype was associated with an increased risk of LUSC. Additionally, research has found that terminally differentiated CD8 + T cells originate from TCF-1 + stem-like T cells in the tumor microenvironment [59]. Activation of STAT3 signaling by IL-10 and IL-21 promotes the development and survival of terminally differentiated T cells [60]. Activated terminally differentiated T cells are producers of inflammatory cytokines, and the inflammatory cytokines found in tumors are more likely to promote tumor growth, progression, and immune suppression rather than eliciting effective host anti-tumor responses [61, 62]. Therefore, further research and analysis are needed to elucidate the exact role of CD45RA + CD28- CD8 + T cells in LUSC.
Notably, our study revealed one immune cell trait, CCR2 on monocytes, as a protective factor against LUSC. CCR2 is generally considered to have a detrimental role in various cancers, particularly in prostate cancer [63]. But in our MR analysis, the expression of CCR2 on monocytes seems to be a protective factor in LUSC. This finding challenges the existing research on the function of CCR2 in cancer.
The role of CCR2 in cancer is complex and diverse. CCR2 is primarily expressed by monocytes/macrophages with strong pro-inflammatory functions [63], which is expected to prevent carcinogenesis. Paradoxically, increased expression of CCL2, a molecule that interacts with CCR2, has been linked to the accumulation of tumor-associated macrophages in esophageal squamous cell carcinoma (ESCC). These macrophages exhibit potent immunosuppressive activities within the tumor microenvironment, which could potentially facilitate cancer progression [64, 65].
Moreover, it has been observed that CCR2 can play a dual role in cancer. On one hand, it may boost immune responses against tumors by encouraging the migration of immune cells to the tumor site. On the other hand, once these CCR2-positive immune cells are within the tumor microenvironment, they might contribute to tumor growth, metastasis, and immune evasion through their interaction with CCL2 [66, 67]. This dual role presents a paradox: while CCR2 is expected to aid in cancer prevention through its pro-inflammatory action, it can also inadvertently support tumor progression in certain contexts. Our MR analysis, which suggests a protective role of CCR2 in LUSC, adds to this complexity. These conflicting perspectives highlight the need for more in-depth investigations to fully understand the multifaceted role of CCR2 in cancer biology.
These findings suggest a dualistic and context-dependent role of CCR2 in cancer, which our study contributes to by highlighting its potential protective role in LUSC. This underscores the need for a more detailed understanding of the diverse functions of immune cell traits in cancer, particularly in lung squamous cell carcinoma. Our research adds to the growing body of evidence that the role of immune cells in cancer is not straightforward and warrants further investigation.
Cancer cells exhibit less invasiveness in never-smoking LUAD, while the immune environment shows more immunosuppressive effects, indicating vulnerability in the treatment of LUAD [68]. Studies have found that smoking-induced dysfunction of alveolar cells contributes more to the aggressiveness of LUAD in smokers, while the immunosuppressive microenvironment has a greater impact on the aggressiveness of LUAD in never-smokers [69]. Never-smoking LUAD patients have unique subtypes of cancer cells expressing high levels of MHC-II molecules involved in antigen presentation and activation of anti-tumor immunity [70]. However, we did not find a relationship between immune cell characteristics and LUAD in our study, possibly because of the close association between LUAD and smoking, which we did not differentiate between smokers and non-smokers. Additionally, pulmonary involvement is a common extra-articular manifestation of RA [71]. Immune dysregulation is considered a key factor in RA patients developing LUAD. Bioinformatics studies have shown that CD8A, GZMA, and PRF1 are associated with CD8 T cells in RA and positively correlated with 33 types of tumors [72]. Shi et al. also found common physiological and pathological processes and molecular spectra between RA and LUAD [73]. Therefore, we finally excluded confounding factor-related SNPs, and the obtained SNPs were only related to immune cells, thereby influencing lung cancer.
This study also has some limitations. Firstly, MR analysis cannot replace clinical trials in the objective field, as it is only a method of analyzing causality between exposure and outcome. Therefore, further research is needed to confirm the potential association between immune cells and lung cancer risk. Secondly, this study is based on publicly available GWAS data, and our MR analysis was conducted only in European populations. Given the genetic specificity among different races, it may not be applicable to other populations. Future studies should conduct subgroup analysis including different populations to draw more comprehensive conclusions. Thirdly, our observations on CCR2 + monocytes differ from current literature viewpoints. Such discrepancies may arise due to variations in sample sources, experimental conditions, or statistical methods. Biological complexity in dynamic systems of the tumor microenvironment may also be a relevant factor. Thus, investigating the precise role of circulating CCR2 + monocytes in LUSC risk becomes necessary, considering the current controversy. Finally, our study primarily examines specific immune cell traits, but we cannot disregard the impacts of other cell types or factors that may impact the risk of LUSC. For instance, certain tumor-infiltrating myeloid cell subsets, m6A regulation, genomic alterations, and specific somatic mutations can play pivotal roles in regulating the interactions between various immune cells and influence tumor development [74,75,76,77]. Therefore, while laying the foundation in our study, it is also necessary to gain a more comprehensive understanding of these factors and their interactions. We will strive to improve in future research endeavors.
In summary, we performed a two-sample Mendelian randomization study to investigate the causal connections between different immune phenotypes and LUSC or LUAD. Our analysis demonstrated that the causal relationship is more pronounced in LUSC and three immune cell types correlated with LUSC susceptibility, while the association with LUAD is statistically insignificant. Although these findings are derived only from European populations, they still enhance our comprehension of the complex interplay between the immune system and lung cancers. This study yields new perspectives into managing cancer risk, potentially providing more precise therapeutic alternatives for individuals with lung cancer and furnishing valuable information for future scientific research.
Data availability
The GWAS datasets analyzed during this study are publicly accessible. The initial analysis was conducted using GWAS data with accession numbers GCST004750 and GCST004744, available from the GWAS Catalog. The replication analysis utilized GWAS data with accession numbers ieu-a-989 and ieu-a-984, which can be accessed through the IEU OpenGWAS project.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Zheng M. Classification and Pathology of Lung Cancer. Surg Oncol Clin N Am. 2016;25(3):447–68.
Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–45.
Wang BY, Huang JY, Chen HC, Lin CH, Lin SH, Hung WH, Cheng YF. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146(1):43–52.
Li J, Li X, Zhang C, Zhang C, Wang H. A signature of tumor immune microenvironment genes associated with the prognosis of non–small cell lung cancer. Oncol Rep. 2020;43(3):795–806.
Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, Xin Y, Wang Y, Yang C, Cheng Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964.
Peng H, Wu X, Zhong R, Yu T, Cai X, Liu J, Wen Y, Ao Y, Chen J, Li Y, et al. Profiling Tumor Immune Microenvironment of Non-small Cell Lung Cancer using multiplex immunofluorescence. Front Immunol. 2021;12:750046.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwe H, Pircher A, Van den Eynde K, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277–89.
Kim HR, Park HJ, Son J, Lee JG, Chung KY, Cho NH, Shim HS, Park S, Kim G et al. In Yoon H : Tumor microenvironment dictates regulatory T cell phenotype: Upregulated immune checkpoints reinforce suppressive function. J Immunother Cancer 2019, 7(1):339.
Miyake M, Tatsumi Y, Gotoh D, Ohnishi S, Owari T, Iida K, Ohnishi K, Hori S, Morizawa Y, Itami Y et al. Regulatory T cells and Tumor-Associated macrophages in the Tumor Microenvironment in Non-muscle invasive bladder Cancer treated with Intravesical Bacille Calmette-Guerin: a long-term Follow-Up study of a Japanese cohort. Int J Mol Sci 2017, 18(10).
Faget J, Groeneveld S, Boivin G, Sankar M, Zangger N, Garcia M, Guex N, Zlobec I, Steiner L, Piersigilli A, et al. Neutrophils and snail orchestrate the establishment of a Pro-tumor Microenvironment in Lung Cancer. Cell Rep. 2017;21(11):3190–204.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9.
Zhang X, Shi X, Zhao H, Jia X, Yang Y. Identification and Validation of a Tumor Microenvironment-Related Gene Signature for Prognostic Prediction in Advanced-Stage Non-Small-Cell Lung Cancer. Biomed Res Int 2021, 2021:8864436.
Gaudreau PO, Negrao MV, Mitchell KG, Reuben A, Corsini EM, Li J, Karpinets TV, Wang Q, Diao L, Wang J, et al. Neoadjuvant chemotherapy increases cytotoxic T cell, tissue Resident Memory T Cell, and B Cell Infiltration in Resectable NSCLC. J Thorac Oncol. 2021;16(1):127–39.
McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–72.
Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, Knockaert S, Olive D, Panouillot M, Validire P, et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoid structures and are associated with poor clinical outcome in NSCLC. Commun Biol. 2022;5(1):1416.
Palucka AK, Coussens LM. The basis of Oncoimmunology. Cell. 2016;164(6):1233–47.
Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48(6):607–16.
Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98.
Bouras E, Karhunen V, Gill D, Huang J, Haycock PC, Gunter MJ, Johansson M, Brennan P, Key T, Lewis SJ, et al. Circulating inflammatory cytokines and risk of five cancers: a mendelian randomization analysis. BMC Med. 2022;20(1):3.
Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511.
McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, Caporaso NE, Johansson M, Xiao X, Li Y, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017;49(7):1126–32.
Orru V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, Sole G, Lai S, Dei M, Mulas A, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–45.
Sidore C, Busonero F, Maschio A, Porcu E, Naitza S, Zoledziewska M, Mulas A, Pistis G, Steri M, Danjou F, et al. Genome sequencing elucidates sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet. 2015;47(11):1272–81.
Wang C, Zhu D, Zhang D, Zuo X, Yao L, Liu T, Ge X, He C, Zhou Y, Shen Z. Causal role of immune cells in schizophrenia: mendelian randomization (MR) study. BMC Psychiatry. 2023;23(1):590.
Gu Y, Jin Q, Hu J, Wang X, Yu W, Wang Z, Wang C, Liu Y, Chen Y, Yuan W. Causality of genetically determined metabolites and metabolic pathways on osteoarthritis: a two-sample mendelian randomization study. J Transl Med. 2023;21(1):357.
Yun Z, Guo Z, Li X, Shen Y, Nan M, Dong Q, Hou L. Genetically predicted 486 blood metabolites in relation to risk of colorectal cancer: a mendelian randomization study. Cancer Med. 2023;12(12):13784–99.
Burgess S, Thompson SG. Bias in causal estimates from mendelian randomization studies with weak instruments. Stat Med. 2011;30(11):1312–23.
Pierce BL, Burgess S. Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013;178(7):1177–84.
Baranova A, Song Y, Cao H, Yue W, Zhang F. Causal associations of tea intake with COVID-19 infection and severity. Front Nutr. 2022;9:1005466.
Zhang K, Li A, Zhou J, Zhang C, Chen M. Genetic association of circulating C-reactive protein levels with idiopathic pulmonary fibrosis: a two-sample mendelian randomization study. Respir Res. 2023;24(1):7.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PM. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol. 2015;68(3):299–306.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Verbanck M, Chen CY, Neale B, Do R. Publisher correction: detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(8):1196.
Battram T, Richmond RC, Baglietto L, Haycock PC, Perduca V, Bojesen SE, Gaunt TR, Hemani G, Guida F, Carreras-Torres R, et al. Appraising the causal relevance of DNA methylation for risk of lung cancer. Int J Epidemiol. 2019;48(5):1493–504.
Shehata SA, Toraih EA, Ismail EA, Hagras AM, Elmorsy E, Fawzy MS. Vaping, Environmental Toxicants Exposure, and Lung Cancer Risk. Cancers (Basel) 2023, 15(18).
Cho MH, Cho JH, Eun Y, Han K, Jung J, Cho IY, Yoo JE, Lee H, Kim H, Park SY, et al. Rheumatoid arthritis and risk of Lung Cancer: a Nationwide Cohort Study. J Thorac Oncol. 2024;19(2):216–26.
You D, Wang D, Wu Y, Chen X, Shao F, Wei Y, Zhang R, Lange T, Ma H, Xu H, et al. Associations of genetic risk, BMI trajectories, and the risk of non-small cell lung cancer: a population-based cohort study. BMC Med. 2022;20(1):203.
Huang Y, Zhu M, Ji M, Fan J, Xie J, Wei X, Jiang X, Xu J, Chen L, Yin R, et al. Air Pollution, genetic factors, and the risk of Lung Cancer: a prospective study in the UK Biobank. Am J Respir Crit Care Med. 2021;204(7):817–25.
Moes-Sosnowska J, Szpechcinski A, Chorostowska-Wynimko J. Clinical significance of TP53 alterations in advanced NSCLC patients treated with EGFR, ALK and ROS1 tyrosine kinase inhibitors: an update. Tumour Biol 2023.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, Butterworth AS, Staley JR. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261(5121):609–12.
Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123(2):580–93.
Liu Z, Geboes K, Hellings P, Maerten P, Heremans H, Vandenberghe P, Boon L, van Kooten P, Rutgeerts P, Ceuppens JL. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J Immunol. 2001;167(3):1830–8.
Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22(1):1–7.
Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6.
Strickland M, Lee S, Neo SY, Balachander A, Low I, Mustafah S, Goh WI, Wright GD, Larbi A, Pender SLF. Mitochondrial dysfunction in CD4 + T effector memory RA + cells. Biology (Basel) 2023, 12(4).
Hohn H, Julch M, Pilch H, Kortsik C, Tully G, Neukirch C, Freitag K, Maeurer M. Definition of the HLA-A2 restricted peptides recognized by human CD8 + effector T cells by flow-assisted sorting of the CD8 + CD45RA + CD28- T cell subpopulation. Clin Exp Immunol. 2003;131(1):102–10.
Jager E, Hohn H, Necker A, Forster R, Karbach J, Freitag K, Neukirch C, Castelli C, Salter RD, Knuth A, et al. Peptide-specific CD8 + T-cell evolution in vivo: response to peptide vaccination with Melan-A/MART-1. Int J Cancer. 2002;98(3):376–88.
Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410(6824):106–11.
Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, Dahne M, Hartwig T, Akyuz L, Meisel C, et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5(177):177ra136.
Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience. 2017;39(3):261–71.
Lanna A, Gomes DC, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW, Lee JH, Karin M, Akbar AN. A sestrin-dependent erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat Immunol. 2017;18(3):354–63.
Salumets A, Tserel L, Rumm AP, Turk L, Kingo K, Saks K, Oras A, Uibo R, Tamm R, Peterson H, et al. Epigenetic quantification of immunosenescent CD8(+) TEMRA cells in human blood. Aging Cell. 2022;21(5):e13607.
Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–70.
Sun Q, Zhao X, Li R, Liu D, Pan B, Xie B, Chi X, Cai D, Wei P, Xu W et al. STAT3 regulates CD8 + T cell differentiation and functions in cancer and acute infection. J Exp Med 2023, 220(4).
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45.
Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of inflammatory cytokines within the breast Tumor Microenvironment. Int J Mol Sci 2023, 24(4).
Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102(8):522–8.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5.
Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, Li Y, Zhang X, Cui W, Jia X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19(1):41.
Hartwig T, Montinaro A, von Karstedt S, Sevko A, Surinova S, Chakravarthy A, Taraborrelli L, Draber P, Lafont E, Arce Vargas F, et al. The TRAIL-Induced Cancer Secretome promotes a tumor-supportive Immune Microenvironment via CCR2. Mol Cell. 2017;65(4):730–e742735.
Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, Vipotnik N, Grimminger F, Seeger W, Pullamsetti SS, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med. 2015;191(4):437–47.
Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, Petralia F, Li Y, Liang WW, Reva B, et al. Proteogenomic characterization reveals therapeutic vulnerabilities in Lung Adenocarcinoma. Cell. 2020;182(1):200–e225235.
Luo W, Zeng Z, Jin Y, Yang L, Fan T, Wang Z, Pan Y, Yang Y, Yao M, Li Y, et al. Distinct immune microenvironment of lung adenocarcinoma in never-smokers from smokers. Cell Rep Med. 2023;4(6):101078.
Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–36.
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170(1):ITC1–16.
Zhao Z, Ren J, Xie S, Zou L, Zhao Q, Zeng S, Zha D. Identification of biomarkers associated with CD8 + T cells in rheumatoid arthritis and their pan-cancer analysis. Front Immunol. 2022;13:1044909.
Shi L, Zou H, Yi J. Construction of shared gene signature between rheumatoid arthritis and lung adenocarcinoma helps to predict the prognosis and tumor microenvironment of the LUAD patients. Front Mol Biosci. 2023;10:1314753.
Saab S, Zalzale H, Rahal Z, Khalifeh Y, Sinjab A, Kadara H. Insights into Lung Cancer Immune-Based Biology, Prevention, and treatment. Front Immunol. 2020;11:159.
Yao N, Zuo L, Yan X, Qian J, Sun J, Xu H, Zheng F, Efird JT, Kawagoe I, Wang Y, et al. Systematic analysis of ferroptosis-related long non-coding RNA predicting prognosis in patients with lung squamous cell carcinoma. Transl Lung Cancer Res. 2022;11(4):632–46.
Mangogna A, Varghese PM, Agostinis C, Alrokayan SH, Khan HA, Stover CM, Belmonte B, Martorana A, Ricci G, Bulla R, et al. Prognostic Value of Complement Properdin in Cancer. Front Immunol. 2020;11:614980.
Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495–509.
Acknowledgements
We extend our heartfelt gratitude to all contributors who have played a pivotal role in bringing this research to fruition.
Funding
This study was made possible through the generous support from the Natural Science Foundation of China (grant no. 82060078), the Guangxi Key Research and Development Program (Guike AB23026006), the Natural Science Youth Fund of Guangxi Zhuang Autonomous Region, China (2022GXNSFBA035497), the Guangxi Health Commission, and the Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (grant no. S2022001).
Author information
Authors and Affiliations
Contributions
ZJM, as the first author, was instrumental in the research design, experimental data collection, data analysis, and interpretation, and played a primary role in manuscript writing while providing significant supervision throughout the research process. LCX, also a first author, contributed significantly to data collection and was pivotal in data analysis and interpretation. HYL, as the second author, made substantial contributions to data collection and actively participated in research discussions. SWT, the third author, provided valuable insights for data analysis. LYC, as the fourth author, contributed importantly to the interpretation of results. MGY and TLY were key in the acquisition of data. CSW and LBJ, as corresponding authors, jointly provided comprehensive supervision and management of the entire research project.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All data used in this study were sourced from publicly accessible databases. Therefore, obtaining additional ethical approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zheng, JM., Lou, CX., Huang, YL. et al. Associations between immune cell phenotypes and lung cancer subtypes: insights from mendelian randomization analysis. BMC Pulm Med 24, 242 (2024). https://doi.org/10.1186/s12890-024-03059-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03059-w