Abstract
Background
Healthcare-Associated Infections (HAI) are most frequently associated with patients in the Intensive Care Unit (ICU). Coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), led to ICU hospitalization for some patients.
Methods
The study was conducted in 2020 and 2021 at a hospital in southern Poland. The Healthcare-Associated Infections Surveillance Network (HAI-Net) of the European Centre for Disease Prevention and Control (ECDC) was used for HAI diagnosis. The aim of this case-control study was to retrospectively assess the epidemiology of HAIs in ICU patients, distinguishing between COVID-19 and non-COVID-19 cases.
Results
The study included 416 ICU patients: 125 (30%) with COVID-19 and 291 (70%) without COVID-19, p < 0.05. The mortality rate was 80 (64%) for COVID-19 patients and 45 (16%) for non-COVID-19 patients, p < 0.001. Ventilator-Associated Pneumonia (VAP) occurred in 40 cases, with an incidence rate density of 6.3/1000 patient-days (pds): 14.1/1000 pds for COVID-19 patients vs. 3.6/1000 pds for non-COVID-19 patients. Odds Ratio (OR) was 2.297, p < 0.01. Acinetobacter baumannii was the most often isolated microorganism in VAP, with 25 cases (incidence rate 8.5%): 16 (18.2%) in COVID-19 patients vs. 9 (4.4%) in non-COVID-19 patients. OR was 4.814 (1.084–4.806), p < 0.001.
Conclusions
Patients treated in the ICU for COVID-19 faced twice the risk of VAP compared to non-COVID-19 patients. The predominant microorganism in VAP cases was Acinetobacter baumannii.
Similar content being viewed by others
Background
Coronaviruses, members of the Coronaviridae family, have been recognized in contemporary medicine since the 1960s [1]. In the realm of infectious diseases, coronaviruses were previously responsible for approximately 20% of upper respiratory tract infections in both children and adults. However, a significant paradigm shift occurred at the beginning of 2019 when cases of acute, unexplained lung inflammation emerged in China. This novel threat was identified as a new type of coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [2], leading to the nomenclature of Coronavirus Disease 2019 (COVID-19) by the World Health Organization (WHO).
For some patients, SARS-CoV-2 infection manifested as Acute Respiratory Distress Syndrome (ARDS), necessitating treatment in Intensive Care Units (ICUs). Patients in ICUs are at risk of invasive procedures, including mechanical ventilation (MV), which may result in nosocomial pneumonia (NP), specifically Ventilator-Associated Pneumonia (VAP).
A study conducted by Guan et al. [3] in China at the early stages of the pandemic (January 2020) revealed that 5% of COVID-19 patients required ICU admission, with 2.3% undergoing mechanical ventilation. It is estimated that approximately 20% of patients experience a severe or very severe course of the disease, primarily characterized by gas exchange disorders, notably hypoxemia [4].
The experience gained from hospitals during the COVID-19 pandemic underscores the frequent occurrence of nosocomial pneumonias as Healthcare-Associated Infections (HAI), often associated with high mortality rates [5,6,7,8]. The incidence rate of NP in ICUs typically ranges from 8.0 to 10.0% [8, 9]. Surveillance data from European ICUs indicate VAP incidence rates between 1.3% and 6.3% in various European countries [10].
The aim of this case-control study is to retrospectively analyze the epidemiology of VAP in patients treated in 2020–2021, categorizing them into COVID-19 and non-COVID-19 groups.
Methods
This analysis is based on the results of a two-year surveillance conducted in the ICU of St. Luke Regional Hospital in Tarnów in 2020 and 2021.
Patients diagnosed with COVID-19 were accommodated in the ICU in a dedicated nine-person room with specialized medical staff, sanitary, and hygienic facilities. Non-COVID-19 patients in the ICU were treated in a five-person room with their own specialized personnel and facilities. These two groups of patients and their respective medical personnel did not interact.
Active, continuous, and targeted surveillance of Healthcare-Associated Infections (HAI) was conducted. Approximately 50% of nurses in the ward treating COVID-19 patients were transferred from other non-ICU hospital wards. Data on patients and hospital infections were collected as part of an active and targeted surveillance process following the standardized protocol established by the European Centre for Disease Prevention and Control (ECDC), version 4.3 [11]. The definition of a hospital-acquired infection, as per the implementing decision of the European Commission in 2018, was applied [12]. Patients with an ICU stay of fewer than 48 h were excluded from the analysis.
The following types of HAIs were monitored: hospital-acquired pneumonia (Pneumonia NP), hospital-acquired bloodstream infection (Bloodstream Infections BSI), Urinary Tract Infections (UTI), Surgical Site Infections (SSI), Gastrointestinal Infections (GI), Skin and Soft Tissue Infections (SST), Lower Respiratory Tract Infections (LRI), and Systemic Infections (SYS).
Statistical analysis
A retrospective statistical analysis was performed using IBM SPSS (SPSS – Statistical Package for the Social Sciences, STATISTICS 24, Armonk, NY, USA) and Microsoft Excel (Microsoft Office 2016 Redmond, WA, USA). Statistical calculations included frequencies (n), percentages (%), medians (Me), standard deviations (SD), significance levels (p), where p < 0.05 indicated statistical significance. The analysis involved calculating odds ratios (OR) and 95% confidence intervals (95% CI) for both groups, classified by the presence or absence of HAI. Fisher’s exact probability test was used due to sample size considerations.
Incidence rates were calculated for VAP, indicating the number of new cases per 100 admissions in the ICU, as well as incidence density rates, reflecting the number of new VAP cases per 1000 patient-days with mechanical ventilation. Additionally, utilization rates (UR) for patients with mechanical ventilation (MV) were calculated as the number of days with the procedure per 100. A minimum sample size of 399 hospitalized patients was required for this study.
The data used for analysis were anonymized. The study was based on routinely collected hospitalization data, obviating the need for additional consent for usage.
The study was conducted with the approval of the Bioethics Commission of the Jagiellonian University in Krakow (no KBET 1075.6120.12.2023) and adhered to the principles of the Helsinki Declaration [13].
Results
From January 1, 2020, to December 31, 2021, a total of 416 patients who met the study criteria were admitted to the ICUs. Of these, 125 patients (30.0%) were diagnosed with COVID-19, while 291 patients (70.0%) were non-COVID-19 cases (p < 0.05). Notably, the patients admitted with SARS-CoV-2 were not vaccinated against COVID-19. COVID-19 patients were generally older (median age 68 years) compared to non-COVID-19 patients (median age 62), which was statistically significant (p < 0.001). Among patients with COVID-19, males were predominant. The duration of ICU stay was shorter for COVID-19 patients (median 17 days) compared to non-COVID-19 patients (median 22 days), with a statistically significant difference (p < 0.05). The death rate was significantly higher among COVID-19 patients (64.0%) compared to non-COVID-19 patients (15.5%) (p < 0.001). The incidence rate of HAI was comparable in both groups (32.8% in COVID-19 patients vs. 30.2% in non-COVID-19 patients; p = 0.332). Notably, broncho-alveolar lavage (BAL) tests were performed in a lower percentage of COVID-19 patients (5%) compared to non-COVID-19 patients (20%) (see Table 1).
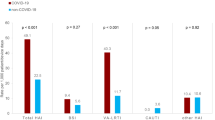
Various forms of HAI were identified, i.e. Pneumonia (PN) 40 (9.6%) cases including: 18 (14.4%) with COVID-19 vs. 22 (7.6%) non-COVID-19, p < 0.05, Bloodstream Infection (BSI) 37 (8.9%) cases, including: 10 (8.0%) with COVID-19 vs. 27 (9.3%) non-COVID-19, p = 0.791; Urinary Tract Infection (UTI) 26 (6.3%) cases, including: 7 (5.6%) with COVID-19 vs. 19 (6.5%) non-COVID-19, p = 0.806; Gastrointestinal system infection – Clostridioides difficile (GI-CDI) 8 (1.9%) cases, including: 2 (1.6%) with COVID-19 vs. 6 (2.1%) non-COVID-19, p = 0.578; Systemic infection (SYS) 9 (2.2%) cases, including: 1 (0.8%) with COVID-19 vs. 8 (2.7%) non-COVID-19, p = 0.294; Skin and Soft Tissue infection (SST) 6 (1.4%) cases, including: 1 (0.8%) with COVID-19 vs. 5 (1.7%) non-COVID-19, p = 0.677; Surgical Site Infection (SSI) 1 (0.2%) case, including 0 (0.0%) with COVID-19 vs. 1 (0.3%) non-COVID-19; Lower Respiratory Tract Infection (LRI) 2 (0.5%) cases, including: 2 (1.6%) with COVID-19 vs. 0 (0.0%) non-COVID-19.
Among the etiological factors, particularly noteworthy were non-fermenters, with Acinetobacter baumannii being the dominant pathogen, accounting for 47 (36.4%) of the cases (see Table 3).
Patients diagnosed with COVID-19 who required intensive care experienced a shorter duration of invasive mechanical ventilation compared to patients treated for other medical conditions. The utilization rates (UR) were notably lower in COVID-19 patients (0.36) compared to non-COVID-19 patients (0.94). The incidence rate of Ventilator-Associated Pneumonia (VAP) was significantly higher in COVID-19 patients, with an incidence rate of 14.1 per 1000 patient-days with a ventilator, in contrast to the lower rate of 3.6 per 1000 patient-days in non-COVID-19 patients (as detailed in Table 4). The basis for microbiological diagnosis of VAP in patients with COVID-19 was the material from lower airways, 18 (100%) (see Tables 3 and 4).
The mortality rate among patients treated for COVID-19 with HAIs was nearly twofold higher compared to non-COVID-19 patients, OR = 2.624 (95% confidence interval (CI) 1.221–5.644, p < 0.05). The death rate for hospital-acquired PN was twofold higher, OR = 2.325 (95% CI 1.199–7.205), p < 0.05 (see Table 2, Figs. 1 and 2). The incidence rate of HAIs attributed to Acinetobacter baumannii was three times higher among COVID-19 patients compared to non-COVID-19 patients, OR = 3.342 (95% CI 1.799–6.208, p < 0.001, Table 3). The VAP incidence rate was found to be two times higher among COVID-19 patients than in the case of non-COVID-19 patients, reflected in an Odds Ratio (OR) of 2.297 (95% CI 1.236–4.267, p < 0.01, Table 4). Notably, the incidence rate of HAIs linked to Acinetobacter baumannii was four times higher among COVID-19 patients compared to non-COVID-19 patients, OR = 4.814 (95% CI 2.037–11.380, p < 0.001, Table 4).
Discussion
From our study population, which consisted of patients admitted to the ICU with SARS-CoV-2 infection, none had been vaccinated against COVID-19. Vaccination against COVID-19 significantly lowers the risk of severe disease. Consequently, the studied COVID-19 patients were more exposed to a severe course of SARS-CoV-2 infection and the risk of being treated in the ICU. Poland’s COVID-19 vaccination coverage is relatively low compared to other countries. According to the European Centre for Disease Prevention and Control (ECDC), only 61% of the Polish population has received at least one dose [14]. In the city of Tarnów, where this study took place, vaccination coverage was 49%, while outside the city, it was no more than 41% [15]. These low vaccination rates are likely a consequence of the increasing influence of anti-vaccination movements. These movements have undermined public trust in vaccinations and led to a rise in refusals of mandatory immunizations [16, 17]. This situation has significantly burdened the Polish healthcare system and has become a significant public health issue.
The HAI incidence rate per 100 hospitalized patients in our study was 31%, and it was similar in both the group of COVID-19 patients (33%) and the group of non-COVID-19 patients (30%). Another Polish study conducted in two ICUs among COVID-19 patients reported a considerably higher HAI incidence rate at 56% [18]. Similarly, studies from other European countries have also shown high HAI incidence rates during the COVID-19 pandemic among patients hospitalized in the ICU [19, 20]. For instance, Grasselli et al. [19], in a multicenter study across 8 Italian hospitals, reported an ICU incidence rate of 46% among COVID-19 patients. In a single-center study in Spain, patients treated in the ICU due to COVID-19 had a 41% HAI incidence rate. Several factors may explain the increased rate of healthcare-related infections in the population of ICU patients with COVID-19, including structural factors such as the introduction of new ICU beds, organizational factors such as the inclusion of new teams of physicians and nurses without prior intensive care experience, and functional factors like changes in patient care standards [21]. All of these structural, organizational, and functional changes were present in the dedicated ICU ward for COVID-19 that we investigated.
One of the most common clinical forms of infections in Polish ICUs is nosocomial pneumonia (NP) [22, 23]. In studies conducted before the COVID-19 pandemic in southern Poland, the frequency of nosocomial pneumonia ranged from 4 to 10% [8, 9], [24, 25]. However, a considerably higher incidence rate (17%) of hospital-acquired pneumonia was reported in the period before the COVID-19 pandemic (2017–2018) by Dubiel et al. [23] in a study that involved 11 Polish ICU wards located in the northern region of Poland. According to the ECDC report [26] from studies conducted before the COVID-19 pandemic in European countries from 2008 to 2012, the average incidence rate of NP in ICUs was 6%. Other ECDC reports [10, 22] from studies conducted in European ICUs also indicated a 6% incidence rate of NP.
During the COVID-19 pandemic, significantly higher incidence rates of nosocomial pneumonia were reported among patients hospitalized in the ICU due to COVID-19. In our study, the incidence rate of NP in this group of patients was 14% and it was almost twofold higher compared to the group of non-COVID-19 patients (8%). Kozłowski et al. [18], in their study involving two ICU wards in northern Poland, reported a frequency of nosocomial pneumonia in patients treated for COVID-19 as 30%. Conversely, in a single-center ICU study conducted by Bardi et al. [20] in Spain, the incidence rate of nosocomial pneumonia in COVID-19 patients was 23%.
In our study, we calculated the VAP incidence rate per 100 patients treated in the ICU, which was 8% for both non-COVID-19 and COVID-19 patients. The VAP incidence rate obtained in our study aligns with results from other studies. Chinese studies from Wuhan reported a VAP incidence rate per 100 patients treated in the ICU due to COVID-19 at 31% [27]. Italian researchers observed an even higher VAP incidence rate of 50% [18]. A systematic review and meta-analysis conducted by Ippolito et al. [28] estimated the overall VAP frequency in patients treated in the ICU due to COVID-19 to be 45%. The elevated incidence of hospital-acquired ICU infections among patients with COVID-19 may be attributed to their increased susceptibility to lung tissue infections by bacteria present in the ICU environment, owing to the initial damage caused by SARS-CoV-2 [29]. Patients admitted to the ICU often had acute pneumonia due to SARS-CoV-2, accompanied by respiratory distress syndrome, comorbidities, and advanced age [20]. In many cases, a majority of patients (96%) [20], and even the entire cohort in some investigations [18], required invasive mechanical ventilation, a significant risk factor for VAP. It has been demonstrated that intubation and mechanical ventilation can increase the risk of pneumonia by 6 to 21 times [30].
In our study, 292 (70%) of the patients required mechanical ventilation of lungs. Interestingly, we observed a higher incidence of hospital-acquired pneumonia related to ventilation (VAP) in the group of COVID-19 patients, with an incidence rate density of 14/1000 days of ventilation, compared to the group of non-COVID-19 patients where it was 4/1000 days of ventilation. This increased VAP incidence rate in the COVID-19 patient group aligns with findings from Maes et al. in Cambridge, Great Britain [31], who reported rates of 28/1000 days of ventilation for COVID-19 patients compared to 13/1000 days of ventilation for non-COVID-19 patients.
COVID-19 patients frequently require prolonged invasive mechanical ventilation (MV), involving prone positioning, heavy sedation, and muscle blockers for several weeks. Furthermore, there is substantial evidence of prolonged immunosuppression, including deep lymphopenia [32]. This accounts for a high risk of secondary hospital-acquired infections, primarily ventilator-associated pneumonia (VAP) [33]. Diagnosing ventilator-associated infections remains a challenge, primarily due to the significant heterogeneity in clinical presentations. There is currently no consensus on appropriate diagnostic strategies for VAP. Regardless of the definition, a precise diagnosis of VAP necessitates clinical signs of infection, microbiological evidence, and chest X-ray findings. However, the interpretation of the latter can be complicated by pre-existing parenchymal injuries [34].
In our study, bronchoscopy was performed in only 5% of COVID-19 patients and 20% of non-COVID-19 patients. The basis for microbiological VAP diagnosis in COVID-19 patients was derived from material obtained from the lower airways in all 18 cases, using a diagnostic approach known as non-protected sample with quantitative culture (PN2). A study conducted before the COVID-19 pandemic, involving seven Polish ICU wards, observed that the duration of treatment for VAP patients who were correctly diagnosed using PN1 was shorter [34]. There was also a notable shift over time in the microbiological diagnostic methods employed for VAP patients. Notably, A. baumannii was predominantly observed in VAP cases diagnosed using substandard methods (non-PN1) [35]. The clinical presentation of COVID-19 pneumonia tends to be relatively uniform, commonly featuring high fever, hyperleukocytosis, severe hypoxemia, extensive bilateral radiologic infiltrates, and biological inflammatory syndrome. Given the similarity in presentation between COVID-19 pneumonia and VAP, the traditional diagnostic criteria for VAP are not applicable to the critically ill COVID-19 population [33]. Performing fiberoptic bronchoalveolar lavage in severely hypoxemic COVID-19 patients is often impractical due to the inherent risk of exacerbating hypoxemia. As a result, many ICUs resort to less invasive endotracheal aspirate (ETA) sampling with quantitative or semi-quantitative cultures, even though these methods may be less reliable for determining the necessity of antibiotic treatment. It is exceedingly challenging to distinguish between COVID-19-associated ARDS with asymptomatic bacterial colonization and a true VAP based solely on traditional threshold values, such as the 105 CFU/ml for ETA samples [33]. These microbiological diagnostic challenges contribute to distinct differences in VAP classification and diagnosis in patients with COVID-19.
The precise identification of COVID-19 patients in need of new antibiotics for clinically relevant bacterial superinfections is a challenging task, which often results in the overuse of broad-spectrum antibiotics, even in the absence of supporting data in the literature [36]. Consequently, the majority of ventilated COVID-19 patients with ARDS receive prophylactic antibiotics as a preventive measure against undocumented VAP. This strategy carries a substantial risk of selecting multi-drug-resistant bacteria or even fungi, particularly in patients expected to remain on invasive MV for a long period [33].
The predominant causative agent of infections in our study was Acinetobacter baumannii, accounting for 36% of cases. However, in the group of patients with COVID-19, this microorganism was responsible for 63% of infections, whereas in the non-COVID-19 group, it accounted for 24% of infections. Previous Polish studies have consistently reported the frequent isolation of Acinetobacter baumannii in ICUs [9, 25, 37]. In a study by Kozłowski et al. [17], Klebsiella pneumoniae and Acinetobacter baumannii were identified as the most common pathogens responsible for VAP. Another study conducted by seven Polish ICUs from 2013 to 2015 found that Acinetobacter baumannii was primarily associated with VAP cases diagnosed using suboptimal methods (non-PN1) [35]. The concerning observation in our study is the increasing trend in the incidence rate of Acinetobacter baumannii. In 2020, it accounted for 10% of cases, rising to 13% in 2021 (OR = 3.342, 95% CI 1.799–6.208, p < 0.001). It is noteworthy that the incidence rate of Acinetobacter baumannii in patients admitted to our investigated ICU between 2012 and 2019 was 4%. An important characteristic of Acinetobacter baumannii is its ability to survive in dry conditions for extended periods, making the hospital environment a significant reservoir for this microorganism. It is suggested that Acinetobacter is more likely to cause infections in facilities with older infrastructure [23].
In our study, the mortality rate among COVID-19 patients was 64%, which was more than four times higher compared to non-COVID-19 patients (16%). Furthermore, significant disparities in mortality were noted among patients with HAI: in the COVID-19 group, a nearly twofold higher mortality rate of 21% was observed compared to 12% in the non-COVID-19 group. This pattern aligns with the findings of Kozłowski et al. [18], who reported a 72% mortality rate in COVID-19 patients with HAI versus 65% in those without HAI. Notably, a multicenter Italian study reported a 30% mortality rate among COVID-19 patients [19]. Bardi et al. [20] reported a 36% mortality rate in a university clinic in Madrid and highlighted a significant association between HAI and patient mortality. Specifically, the death rate was 54% in the group of patients with HAI compared to 24% in the group without HAI.
Hospital-acquired infections are a common complication in patients with COVID-19 treated in the ICU, which may contribute to the elevated mortality observed in this patient population [20].
In our study, it was also observed that among patients with NP, the mortality rate in the group of COVID-19 patients was almost twice as high compared to the non-COVID-19 group, at 10% versus 4%, respectively. This pattern is consistent with the findings of Maes et al. [31], where the mortality rate in the COVID-19 patient group with VAP was nearly twice as high as in non-COVID-19 patients, with rates of 38% versus 21%. According to a meta-analysis of 20 studies, the average mortality rate due to VAP in the group of COVID-19 patients was 43% [28]. It appears that critically ill COVID-19 patients, hospitalized in the ICU, grappling with acute viral infections, often necessitating mechanical ventilation and other invasive treatments, and exposed to multidrug-resistant strains that colonize the ICU, frequently face a challenging battle for survival.
Limitations of the study
Our study has several limitations. The most significant of them include its single-setting nature, the relatively small sample size and the short-term duration of the study. Another notable limitation is the absence of data on comorbidities.
Conclusions
In patients treated in the ICU with COVID-19, the incidence of PN and VAP and the risk of Acinetobacter baumannii infection were much higher than in patients treated in the ICU for reasons other than COVID-19. Although high, the risk of infections in our study was similar to the results reported by other authors. However, the proportion of Acinetobacter baumannii correlated with sub-optimal sample type for microbiological diagnostics. This observation indicates important challenge for infection control which is improving microbiological diagnostics methods and cooperation with infection control team and microbiological laboratory.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- HAI:
-
Healthcare-Associated Infections
- ICUs:
-
Intensive Care Units
- PN:
-
Hospital-acquired pneumonia
- BSI:
-
Hospital-acquired bloodstream infection
- UTI:
-
Urinary Tract Infections
- SSI:
-
Surgical Site Infections
- GI:
-
Gastrointestinal Infections
- SST:
-
Skin and Soft Tissue Infections
- LRI:
-
Lower Respiratory Tract Infections
- SYS:
-
Systemic Infections
- ARDS:
-
Acute Respiratory Distress Syndrome
- MV:
-
Mmechanical ventilation
- VAP:
-
Ventilator-Associated Pneumonia
- ECDC:
-
European Centre for Disease Prevention and Control
References
Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005. https://doi.org/10.1097/01.inf.0000188166.17324.60.
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019. https://doi.org/10.1038/s41579-018-0118-9.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2002032.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. https://doi.org/10.1001/jamainternmed.2020.0994.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired Pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur Respir J. 2017. https://doi.org/10.1183/13993003.00582-2017.
European Centre for Disease Prevention and Control. European surveillance of healthcare- associated Infections in intensive care units – HAI-Net ICU protocol, version 1.02. Stockholm: ECDC; 2015.
Kózka M, Sega A, Wojnar-Gruszka K, Tarnawska A, Gniadek A. Risk factors of pneumonia associated with mechanical ventilation. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17020656.
Walaszek M, Rozanska A, Bulanda M, Wojkowska-Mach J, Team PSOHI. Epidemiology of healthcare-associated infections in Polish intensive care. A multicenter study based on active surveillance. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018. https://doi.org/10.5507/bp.2018.006.
Kołpa M, Wałaszek M, Gniadek A, Wolak Z, Dobroś W, Incidence. Incidence, microbiological profile and risk factors of healthcare-associated infections in intensive care units: a 10 year observation in a provincial hospital in Southern Poland. Int J Environ Res Public Health. 2018. https://doi.org/10.3390/ijerph15010112.
European Centre for Disease Prevention and Control. Healthcare-associated Infections acquired in intensive care units. ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019.
European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated Infections and antimicrobial use in European acute care hospitals – protocol version 4.3. Stockholm: ECDC; 2012.
Commission Implementing Decision (EU). 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=uriserv%3AOJ.L_.2018.170.01.0001.01.ENG. Accessed 2 Feb 2023.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013. https://doi.org/10.1001/jama.2013.281053.
ECDC. Covid-19 vaccine tracker. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab. Accesed 2 Feb 2023.
Serwis Rzeczypospolitej Polskiej. Szczepienia w gminach. https://www.gov.pl/web/szczepienia-gmin#/poziomwyszczepienia. Accesed 2 Feb 2023.
Żuk P, Żuk P, Lisiewicz-Jakubaszko J. The anti-vaccine movement in Poland: the socio-cultural conditions of the opposition to vaccination and threats to public health. Vaccine. 2019. https://doi.org/10.1016/j.vaccine.2019.01.073.
de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)31558-0.
Kozłowski B, Kubiak-Pulkowska J, Pałka J, Bożiłow D, Zając M, Deptuła A. Healthcare-associated Infections in COVID-19 ICU patients - two-centre study. Cent Eur J Public Health. 2022. https://doi.org/10.21101/cejph.a7135.
Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically Ill patients with COVID-19. Chest. 2021. https://doi.org/10.1016/j.chest.2021.04.002.
Bardi T, Pintado V, Gomez-Rojo M, Escudero-Sanchez R, Azzam Lopez A, Diez-Remesal Y, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021. https://doi.org/10.1007/s10096-020-04142-w.
Marin-Corral J, Pascual-Guardia S, Muñoz-Bermúdez R, Salazar-Degracia A, Climent C, Vilà-Vilardell C, et al. Health care-associated Infections in patients with COVID-19 Pneumonia in COVID critical care areas. Med Intensiva. 2022. https://doi.org/10.1016/j.medin.2021.04.003.
European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018.
Dubiel G, Kozłowski B, Deptuła A, Hryniewicz W. Raport NPOA z programu czynnego monitorowania zakażeń w oddziałach anestezjologii i intensywnej terapii w 2018 roku w Polsce. http://antybiotyki.edu.pl/wp-content/uploads/2020/07/Raport-ICU-2018.pdf. Accesed 10 Oct 2022.
Wójkowska-Mach J, Bulanda M, Rózańska A, Kochan P, Heczko PB. Hospital-acquired pneumonia in the intensive care units of Polish hospitals. Infect Control Hosp Epidemiol. 2006. https://doi.org/10.1086/504447.
Rafa E, Wałaszek MZ, Wałaszek MJ, Domański A, Różańska A. The incidence of healthcare-associated infections, their clinical forms, and microbiological agents in intensive care units in Southern Poland in a multicentre study from 2016 to 2019. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18052238.
European Centre for Disease Prevention and Control. Incidence and attributable mortality of healthcare-associated infections in intensive care units in Europe, 2008–2012. Stockholm: ECDC; 2018.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)30566-3.
Ippolito M, Misseri G, Catalisano G, Marino C, Ingoglia G, Alessi M, et al. Ventilator-associated pneumonia in patients with COVID-19: a systematic review and Meta-analysis. Antibiot (Basel). 2021. https://doi.org/10.3390/antibiotics10050545.
Llitjos JF, Bredin S, Lascarrou JB, Soumagne T, Cojocaru M, Leclerc M, et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: a multicentre retrospective cohort study. Ann Intensive Care. 2021. https://doi.org/10.1186/s13613-021-00812-w.
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005. https://doi.org/10.1164/rccm.200405-644ST.
Maes M, Higginson E, Pereira-Dias J, Curran MD, Parmar S, Khokhar F, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021. https://doi.org/10.1186/s13054-021-03460-5.
Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020. https://doi.org/10.1038/s41392-020-0148-4.
François B, Laterre PF, Luyt CE, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020. https://doi.org/10.1186/s13054-020-03013-2.
Chastre J, Luyt CE. Does this patient have VAP? Intensive Care Med. 2016. https://doi.org/10.1007/s00134-016-4239-1.
Wałaszek M, Różańska A, Wałaszek MZ, Wójkowska-Mach J, Polish Society of Hospital Infections Team. Epidemiology of ventilator-associated pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish intensive care units in the years 2013–2015. BMC Infect Dis. 2018. https://doi.org/10.1186/s12879-018-3212-8.
Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa530.
Duszynska W, Rosenthal VD, Szczesny A, Zajaczkowska K, Fulek M, Tomaszewski J. Device associated -health care associated Infections monitoring, prevention and cost assessment at intensive care unit of University Hospital in Poland (2015–2017). BMC Infect Dis. 2020. https://doi.org/10.1186/s12879-020-05482-w.
Acknowledgements
Non applicable.
Funding
No external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization MW, MK; Methodology, MW, AR, J.W.-M; Formal Analysis, MW, RS, ER; Investigation PS, ZC, AK, WSK, KN; Resources MK, MW; Data Curation, MW Writing—Original Draft Preparation, MW, RS, KN, ER Writing—Review and Editing AR, JWM, PS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent for patient participation in the study was waived by the Bioethics Committee of the Jagiellonian University in Krakow that approved the study under approval number KBET 1072.6120.12.2023 (granted on 15.02.2023).
Consent for publication
Informal consent for publication was waived by the Bioethics Committee of the Jagiellonian University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wałaszek, M., Serwacki, P., Cholewa, Z. et al. Ventilator-associated pneumonia in Polish intensive care unit dedicated to COVID-19 patients. BMC Pulm Med 23, 443 (2023). https://doi.org/10.1186/s12890-023-02743-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02743-7