Abstract
Background
Numerous predictive formulas based on different ethnics have been developed to determine continuous positive airway pressure (CPAP) for patients with obstructive sleep apnea (OSA) without laboratory-based manual titrations. However, few studies have focused on patients with OSA in China. Therefore, this study aimed to develop a predictive equation for determining the optimal value of CPAP for patients with OSA in China.
Methods
526 pure moderate to severe OSA patients with attended CPAP titrations during overnight polysomnogram were spited into either formula derivation (419 patients) or validation (107 patients) group according to the treatment time. Predictive model was created in the derivation group, and the accuracy of the model was tested in the validation group.
Results
Apnea hypopnea index (AHI), body mass index (BMI), longest apnea time (LAT), and minimum percutaneous oxygen saturation (minSpO2) were considered as independent predictors of optimal CPAP through correlation analysis and multiple stepwise regression analysis. The best equation to predict the optimal value of CPAP was: CPAPpred = 7.581 + 0.020*AHI + 0.101*BMI + 0.015*LAT-0.028*minSpO2 (R2 = 27.2%, p < 0.05).The correlation between predictive CPAP and laboratory-determined manual optimal CPAP was significant in the validation group (r = 0.706, p = 0.000). And the pressure determined by the predictive formula did not significantly differ from the manually titrated pressure in the validation cohort (10 ± 1 cmH2O vs. 11 ± 3 cmH2O, p = 0.766).
Conclusions
The predictive formula based on AHI, BMI, LAT, and minSpO2 is useful in calculating the effective CPAP for patients with pure moderate to severe OSA in China to some extent.
Similar content being viewed by others
Background
Obstructive sleep apnea (OSA) is a kind of common form of sleep-disordered breathing, characterized by cessation or significant attenuation of oronasal airflow during sleep, accompanied by daytime drowsiness and fatigue. It has been reported that nearly one billion adults 30 to 69 years of age worldwide have OSA, and the number of affected individuals is highest in China, followed by the United States, Brazil, and India [1]. OSA has been shown to be associated with multiple-system damage due to intermittent hypoxia [2, 3].
Previous studies have shown that automatic continuous positive airway pressure (auto-CPAP) therapy plays a beneficial role in the treatment of OSA patients [4,5,6]. However, auto-CPAP titration followed to fixed continuous positive airway pressure (CPAP) under the help of a web platform are not commonly used in China, due to the economic factors and medical insurance policy, as well as the fact that telemedicine services are only at the primary stage with lack of relevant policies and regulations that govern its use at the national level [7]. At present, the main “gold standard” for the diagnosis and treatment of OSA in China includes an initial laboratory diagnostic polysomnogram (PSG), followed by a second PSG to titrate the appropriate value of CPAP manually, if moderate to severe OSA is detected in the baseline PSG [8, 9]. However, the procedure is time-consuming and labor-intensive. Furthermore, the duration of titration may not be sufficient to attain the appropriate pressure because of the patient’s poor ability to sleep in such an unknown environment [10]. In particular, the COVID-19 pandemic has had a major effect on the sleep medicine practices. Laboratory-based sleep diagnosis and treatment of OSA are forced to be postponed or cancelled worldwide, family-based telemedicine is more and more advocated in the current and post-pandemic era [11]. Therefore, it is necessary to find a simple method to determine the CPAP for patients with OSA. Miljeteig and Hoffstein et al. were the first to develop a predictive algorithm to facilitate the selection of initial pressure during an overnight titration study [12]. However, this algorithm may be not suitable for patients with OSA in other countries or regions because of ethnic and regional disparities [13]. Thus, the purpose of this study was to propose a predictive model for determining the optimal value of CPAP for patients with OSA in China.
Methods
Study population and design
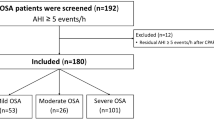
This study was approved by the Clinical Research Ethics Committee of Tianjin Medical University General Hospital, and all procedures were performed in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from all the participants. We retrospectively evaluated the clinical data of 419 consecutive pure moderate-to-severe OSA patients undergoing CPAP titration at the sleep laboratory of our hospital between March 2016 and August 2019 to determine the predictive model. Thereafter, we validated this predictive formula in a validation cohort of another 107 patients with pure moderate-to-severe OSA between September 2019 and December 2019. We excluded patients who still had high residual AHI (AHI ≥ 5 events per hour) after undergoing CPAP titration, and patients with central sleep apnea/mixed sleep apnea, and patients with severe chronic obstructive pulmonary disease or other severe medical conditions complicated with hypoxia, and patients on sedative/hypnotic medications, and patients with incomplete data.
Demographic information, anthropometric measurements, and medical histories were collected. The neck circumference (NC) was measured at the level of the cricothyroid membrane. The waist circumference (WC) was measured midway between the lower rib margin andanterior superior iliac spine [14].Subjective daytime drowsiness was assessed using the Epworth Sleepiness Scale (ESS), and patients with scores higher than 10 were considered drowsy.
PSG and CPAP titration for optimal pressure
All patients underwent overnight PSG using a sleep analysis system (Alice 5 diagnostic Sleep System, Philips, Respironics, Bend, OR, USA). Sixteen channels were used simultaneously to perform the following tests: electroencephalogram, electrooculogram, submental and leg electromyogram, electrocardiogram, airflow in the mouth and nose (thermistor, nasal pressure transducer), chest and abdominal respiratory efforts, blood oxygen saturation (pulse oximetry), snoring, and body position parameters. A sleep technician observed the behavior of patients and confirmed their sleep positions through an infrared camera placed in the room [15].
Laboratory-based manual titration was performed throughout the night to determine the optimal pressure for CPAP. Optimal pressure was defined as the lowest effective pressure that could control most respiratory disturbances, including apnea and hypopnea events and snoring in all body positions and stages, especially in the supine position during rapid-eye movement (REM) sleep [15].The specific manifestations were residual AHI < 5 events per hour, and supine REM at least 15 min after treatment [16].
The polysomnographic data were automatically analyzed by the sleep analysis system and were assessed by a polysomnographic technologist. An apnea event was defined as an airflow amplitude reduction of more than 90% from pre-event baseline for at least 10 s, and the hypopnea event was defined as a reduction of airflow by ≥ 30% of pre-event baseline for at least 10 s, and accompanied with ≥ 3% oxygen desaturation from pre-event baseline and/or arousal on electroencephalogram, according to the 2012 American Academy of Sleep Medicine recommendations [17].
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of distribution. All continuous variables are presented as the mean ± standard deviation (SD) or median with interquartile range (IQR).The Spearman correlation analysis was used to evaluate the relationship between demographic, anthropometric, polysomnographic variables and the observed optimal value of CPAP in derivation cohort. Variables with a significant correlation were considered as potential predictors. Then multiple stepwise linear regression analysis was utilized to select the independent predictive variables and to develop a predictive equation for determining the optimal value of CPAP. In the validation cohort, Spearman correlation analysis was utilized to evaluate the association between optimal CPAP (CPAPopt) and predictive CPAP (CPAPpred) obtained from the predictive formula, and the pressure between the CPAPopt and CPAPpred was compared using the Wilcoxon signed-rank test. Agreement between the CPAPpred and CPAPopt was assessed by the Bland–Altman plot. All statistical analyses were performed using the SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at a two-sided p < 0.05.
Results
Clinical and polysomnographic characteristics of the study population
The baseline characteristics of patients in the derivation and validation groups were shown in Table 1. The derivation cohort included 419 adult patients with OSA, 73.3% men, age 50.0 (40.0, 59.0) years, BMI 29.4 (27.1, 32.0) kg/m2; and a total of 107 adult patients with OSA were included in the validation cohort: 72.9% men, age 49.0 (38.0, 56.0) years, BMI 28.9 (26.9, 30.8) kg/m2.
The correlations between CPAPopt and demographic, anthropometric, and polysomnographic variables in the derivation cohort
The results showed significantly positive correlations between CPAPopt and variables including NC, WC, body mass index (BMI), ESS, apnea hypopnea index (AHI), oxygen desaturation index, arousal index, apnea index, longest apnea time (LAT), mean apnea time, proportion of cumulative sleep time with oxygen saturation below 90% in total sleep time, and N1%. However, significantly negative correlations were noted between CPAPopt and variables including age, rapid-eye movement (REM %), N3%, minimum percutaneous oxygen saturation (minSpO2), and mean percutaneous oxygen saturation. These variables were considered as potential independent predictors for CPAP (Table 2).
Derivation of prediction formula for optimal CPAP level
The potential independent predictors, as shown in Table 2, were included in multiple stepwise linear regression analysis. Finally, AHI, BMI, LAT, and minSpO2, were identified as independent predictors in the final predictive model. The final predictive formula for CPAP was:
This equation accounted for 27.2% of the total variance (R2 = 27.2%, p < 0.05) (Table 3).
Validation of the predictive formula in the validation cohort
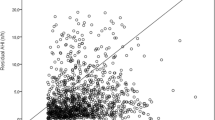
CPAPpred was validated in a separate group of 107 patients with OSA to evaluate the performance of the predictive formula. Just as Fig. 1 showed, a significant correlation was observed between CPAPpred obtained from the predictive formula and CPAPopt obtained by laboratory-based manual titration (r = 0.706, p = 0.000).
In addition to, there was no significant difference between the CPAPopt and the CPAPpred (10 ± 1cmH2O vs. 11 ± 3cmH2O, p = 0.766) (Fig. 2).
The Bland–Altman plot showed that 5.6% of CPAPpred were without 95% confidence interval (CI) of the calculated mean CPAP difference. In 95% CI, the average value of differential pressure was 0.32cmH2O, and the maximum value of that was 4.13 cmH2O (Fig. 3).
Discussion
There is an increasing evidence to support OSA as an independent risk factor for a variety of diseases, such as cardiovascular, cerebrovascular, metabolic, and digestive disorders [2, 3]. The group of disorders have tendency to worsen over time if no or inappropriate treatment is provided. Therefore, early diagnosis and effective treatment in patients with OSA are important [18]. Although various treatment options, including weight control, position therapy, oral appliances, and surgical modifications of the upper airway, have been suggested for the management of OSA, CPAP has been the first-choice of treatment since its introduction in 1981 [19]. However, high level of non-adherence to CPAP treatment is a major limitation of this mode of therapy. Optimal value of CPAP is one of the most important factors influencing adherence, because a lower value of CPAP may result in insufficient treatment and/or unintentional mask removal, whereas a higher value of CPAP may induce pressure intolerance and/or mouth dryness [20]. Thus, achieving the optimal value of CPAP is necessary in patients with OSA. The conventional method of laboratory-based manual titration is time-consuming, labor-intensive, expensive, and delays prescription. Some patients even have to repeat the titration multiple times to get the optimal value of CPAP. Especially, since the outbreak of the COVID-19 epidemic, laboratory-based sleep medicine activities were forced to be postponed or stopped worldwide, and more and more family-based telemedicine have been advocated, which poses a greater challenge to determine the optimal value of CPAP for medical staff. Hence, we proposed a predictive formula for determining the optimal value of CPAP for patients with pure moderate to severe OSA.
The results in this study showed that BMI was one of the important influencing factors of the optimal CPAP. Obesity is widely recognized as a risk factor for OSA, and a pattern of fat distribution around the neck, torso, and abdominal viscera is strongly linked to the pathophysiological mechanism of OSA [21]. Camacho et al. systematically reviewed the international literatures for studying the mathematical equations used to determine the values of effective pressures for CPAP devices. They concluded that BMI were the most important independent predictors of optimal value of CPAP [22]. Extensive clinical researches have shown that BMI was one of the independent factors affecting the optimal CPAP [16, 23,24,25] (Table 4).
AHI is the gold standard for the diagnosis and severity classification of OSA. We declared that AHI was significantly correlated with optimal value of CPAP. Consistently, previous studies have demonstrated AHI was the independent factor for determining the optimal value of CPAP [8, 23, 25,26,27]. Tsuiki et al. reported that patients with higher AHI required higher CPAPs to manage OSA [28] (Table 4).
Our results declared that minSpO2 was also an independent predictor for optimal value of CPAP. This was similar to several previous studies [16, 23, 24, 29]. Oxyhemoglobin desaturation during sleep is directly related to the duration of apnea and indirectly to end-tidal lung volume at the beginning of apnea, without considering other causes such as lung diseases and/or hypoventilation. Oxyhemoglobin saturation during sleep can be considered as a marker of the severity of OSA, and lower the saturation, greater the CPAP required for its correction [29] (Table 4).
LAT was shown to be another significant contributor to CPAP, which was consistent with those from previous reports. Two studies based on a small sample of patients with OSA in China found that LAT was an independent risk factor for CPAP [26, 27] (Table 4).
In order to verify the accuracy of the formula, we validated the equation in another 107 patients with moderate to severe OSA. We found a significant strong correlation between CPAPopt and CPAPpred. Previous research has found similar correlations. Lee et al. found that predictive pressure was positively correlated with titrated pressure (r = 0.490, p < 0.001) [24]. Choil et al. also reported that pressure determined using their predictive equation was strongly correlated with the full-night titrated pressure (r = 0.883, p < 0.001) [19]. In another study conducted in 250 Turkish patients with OSA, the manually measured optimal pressure was significantly correlated with the pressure determined using predictive equation (r = 0.651, p < 0.001) [18]. Additionally, the results declared that the pressure of CPAPpred did not significantly differ from that of CPAPopt. Similarly, Choil et al. demonstrated that the pressure determined using full-night manual titration was not different from the pressure determined using the predictive formula (9.0 ± 3.6 cmH2O vs.8.1 ± 1.6 cmH2O, p = 0.080) [19]. Schiza et al. also found that CPAPpred was not statistically different from CPAPopt (7.26 ± 0.5 cmH2O vs. 6.44 ± 1.3 cmH2O, p > 0.05) [25]. The predictive formula proposed by us was helpful to obtain the optimal value of CPAP for patients with pure moderate to severe OSA to some extent, which could help sleep clinicians to have a certain understanding of the approximate range of pressure before the titration, and may further contribute to improving the success rate of lab-based manual titration and auto-CPAP titration through a web platform. Fitzpatrick et al. have demonstrated that home self-titration of CPAP using a predictive formula was as effective as laboratory manual titration through a full-night polysomnogram, and showed similar compliance and subjective and objective outcomes [30]. Masa et al. found that titration using a predictive formula was as effective as manual titration in patients with severe OSA, and could lower costs and significantly shorten the waiting list [31]. Rowley et al. reported that the use of a predictive formula increased the success rate of manual CPAP titration from 50 to 68% [32].
There were several limitations in this study. Firstly, our study did not consider the effect of gender. Schiza et al. found that gender was a statistically significant factor affecting CPAP [25]. However, gender was not significant predictor for our CPAP prediction, which may be related to the small sample size of female patients. We will further expand the sample size, especially female patients in the future research. Secondly, cephalometric data have been considered as significant predictive factors of optimal value of CPAP [33]. Craniofacial structures were not considered in the derivation of our predictive formula, because the additional cost of craniofacial examination may diminish the clinical utility of this equation. Moreover, other factors such as life style habits, and the type of mask (oronasal mask/nasal mask) may influence predictive value of CPAP. We will continue this research in the future, and incorporate as more relevant factors as possible to further improve the accuracy of the predictive formula. Thirdly, our validation cohort was relatively small, which was related to the significant reduction in the number of patients undergoing lab-based manual titration since the COVID-2019 outbreak. In the future, a prospective cohort study is also needed to determine its accuracy in clinical practice.
Conclusions
In this study, we found that the predictive formula based on AHI, BMI, LAT, and minSpO2 was useful in calculating the effective CPAP in patients with pure moderate to severe OSA in China to some extent. Larger, fully powered studies are needed to determine its efficacy in the future.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available because other study involving this data are in the progress, but are available from the corresponding author on reasonable request.
Abbreviations
- NC:
-
Neck circumference
- WC:
-
Waist circumference
- BMI:
-
Body mass index
- ESS:
-
Epworth sleepiness score
- AHI:
-
Apnea hypopnea index
- ODI:
-
Oxygen desaturation index
- ArI:
-
Arousal index
- AI:
-
Apnea index
- HI:
-
Hypopnea index
- LAT:
-
Longest apnea time
- MAT:
-
Mean apnea time
- LHT:
-
Longest hypopnea time
- MHT:
-
Mean hypopnea time
- minSpO2 :
-
Minimum percutaneous oxygen saturation
- meanSpO2 :
-
Mean percutaneous oxygen saturation
- T90:
-
Proportion of cumulative sleep time with SpO2 below 90% in total sleep time
- CPAPopt:
-
Optimal CPAP
- CPAPpred:
-
Predictive CPAP
References
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98. https://doi.org/10.1016/s2213-2600(19)30198-5.
Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202(261):e1-5. https://doi.org/10.1016/j.ajog.2009.10.867.
Cao J, Wang Y, Chen B. Systemic damage and coping strategies of sleep apnea hypopnea syndrome. Chin J Lung Dis (Electronic Edition). 2014;7:611–3.
McArdle N, Singh B, Murphy M, Gain KR, Maguire C, Mutch S, Hillman DR. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax. 2010;65:606–11. https://doi.org/10.1136/thx.2009.116756.
Hui DS, Ng SS, Tam WWS. Home-based approach noninferior to hospital-based approach in managing patients with suspected obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2018;197:1233–4. https://doi.org/10.1164/rccm.201711-2185LE.
Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Kapur V, Rueschman M, Zee P, Redline S. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–67. https://doi.org/10.5665/sleep.1870.
Zhang XL, Wang W, Xiao Y. Sleep disordered breathing diagnosis and treatment during the COVID-19 pandemic: a nationwide survey in China. Nat Sci Sleep. 2021;13:21–30. https://doi.org/10.2147/nss.S292373.
Ebben MR, Narizhnaya M, Krieger AC. A new predictive model for continuous positive airway pressure in the treatment of obstructive sleep apnea. Sleep Breath. 2017;21:435–42. https://doi.org/10.1007/s11325-016-1436-7.
Sleep disordered breathing group, respiratory branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of obstructive sleep apnea hypopnea syndrome (2011 Revision). Chin J Tuberc Respir Dis. 2012;35:9–12. https://doi.org/10.3760/cma.j.issn.1001-0939.2012.01.007.
El Solh AA, Aldik Z, Alnabhan M, Grant B. Predicting effective continuous positive airway pressure in sleep apnea using an artificial neural network. Sleep Med. 2007;8:471–7. https://doi.org/10.1016/j.sleep.2006.09.005.
Miller MA, Cappuccio FP. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev. 2021;55: 101382. https://doi.org/10.1016/j.smrv.2020.101382.
Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1526–30. https://doi.org/10.1164/ajrccm/147.6_Pt_1.1526.
Zeng Y, Zhong N. Prediction of the level of continuous optimal level of continuous positive airway pressure in the mamagement of obstructive sleep apnes syndrome via nasal mask. Chin J Tuberc Respir Dis. 1996;19:269–72.
Bouloukaki I, Kapsimalis F, Mermigkis C, Kryger M, Tzanakis N, Panagou P, Moniaki V, Vlachaki EM, Varouchakis G, Siafakas NM, Schiza SE. Prediction of obstructive sleep apnea syndrome in a large Greek population. Sleep Breath. 2011;15:657–64. https://doi.org/10.1007/s11325-010-0416-6.
Choi JH, Kim EJ, Kim KW, Choi J, Kwon SY, Lee HM, Kim TH, Lee SH, Shin C, Lee SH. Optimal continuous positive airway pressure level in korean patients with obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2010;3:207–11. https://doi.org/10.3342/ceo.2010.3.4.207.
Saiphoklang N, Leelasittikul K, Pugongchai A. Prediction of optimal continuous positive airway pressure in Thai patients with obstructive sleep apnea. Sci Rep. 2021;11:13935. https://doi.org/10.1038/s41598-021-93554-5.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med. 2012;8:597–619. https://doi.org/10.5664/jcsm.2172.
Basoglu OK, Tasbakan MS. Determination of new prediction formula for nasal continuous positive airway pressure in Turkish patients with obstructive sleep apnea syndrome. Sleep Breath. 2012;16:1121–7. https://doi.org/10.1007/s11325-011-0612-z.
Choi JH, Jun YJ, Oh JI, Jung JY, Hwang GH, Kwon SY, Lee HM, Kim TH, Lee SH, Lee SH. Optimal level of continuous positive airway pressure: auto-adjusting titration versus titration with a predictive equation. Ann Otol Rhinol Laryngol. 2013;122:339–43. https://doi.org/10.1177/000348941312200509.
Lai CC, Friedman M, Lin HC, Wang PC, Hwang MS, Hsu CM, Lin MC, Chin CH. Clinical predictors of effective continuous positive airway pressure in patients with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2015;125:1983–7. https://doi.org/10.1002/lary.25125.
Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. https://doi.org/10.1513/pats.200708-137MG.
Camacho M, Riaz M, Tahoori A, Certal V, Kushida CA. Mathematical equations to predict positive airway pressures for obstructive sleep apnea: a systematic review. Sleep Disord. 2015;2015: 293868. https://doi.org/10.1155/2015/293868.
Wu MF, Hsu JY, Huang WC, Shen GH, Wang JM, Wen CY, Chang KM. Should sleep laboratories have their own predictive formulas for continuous positive airway pressure for patients with obstructive sleep apnea syndrome? J Chin Med Assoc. 2014;77:283–9. https://doi.org/10.1016/j.jcma.2014.02.015.
Lee GH, Kim MJ, Lee EM, Kim CS, Lee SA. Prediction of optimal CPAP pressure and validation of an equation for Asian patients with obstructive sleep apnea. Respir Care. 2013;58:810–5. https://doi.org/10.4187/respcare.01860.
Schiza SE, Bouloukaki I, Mermigkis C, Panagou P, Tzanakis N, Moniaki V, Tzortzaki E, Siafakas NM. Utility of formulas predicting the optimal nasal continuous positive airway pressure in a Greek population. Sleep Breath. 2011;15:417–23. https://doi.org/10.1007/s11325-010-0352-5.
Liu JH, Liu H, Liang DH, Mo XY. Multivariate analysis of nCPAP levels in patients with obstructive sleep apnea hypopnea syndrome. Guangxi Med J. 2006;28:45–6. https://doi.org/10.3969/j.issn.0253-4304.2006.01.020.
Xia SY. Effect of non-mechanicall therapy to the obstructive sleep apnea hypopnea syndrome. Xinjiang medical university, 2011.
Tsuiki S, Kobayashi M, Namba K, Oka Y, Komada Y, Kagimura T, Inoue Y. Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J. 2010;35:1098–105. https://doi.org/10.1183/09031936.00121608.
Loredo JS, Berry C, Nelesen RA, Dimsdale JE. Prediction of continuous positive airway pressure in obstructive sleep apnea. Sleep Breath. 2007;11:45–51. https://doi.org/10.1007/s11325-006-0082-x.
Fitzpatrick MF, Alloway CE, Wakeford TM, MacLean AW, Munt PW, Day AG. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med. 2003;167:716–22. https://doi.org/10.1164/rccm.200204-360OC.
Masa JF, Jiménez A, Durán J, Capote F, Monasterio C, Mayos M, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. https://doi.org/10.1164/rccm.200312-1787OC.
Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. https://doi.org/10.1093/sleep/23.7.929.
Cunha TCA, Guimarães TM, Almeida FR, Haddad FLM, Godoy LBM, Cunha TM, Silva LO, Tufik S, Bittencourt L. Using craniofacial characteristics to predict optimum airway pressure in obstructive sleep apnea treatment. Braz J Otorhinolaryngol. 2020;86:174–9. https://doi.org/10.1016/j.bjorl.2018.10.012.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81970084).
Author information
Authors and Affiliations
Contributions
LW, XC, BC, JZ, and JC designed the trial, reviewed the medical literature, participated in the data analysis and interpretation, and drafted and wrote the manuscript. DW, ML, and YW participated in the data collection, analysis and interpretation. All authors reviewed and contributed to the manuscript during its development. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Tianjin Medical University General Hospital. Written informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Chen, X., Wei, Dh. et al. A predictive model for optimal continuous positive airway pressure in the treatment of pure moderate to severe obstructive sleep apnea in China. BMC Pulm Med 22, 232 (2022). https://doi.org/10.1186/s12890-022-02025-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02025-8