Abstract
Background
The identification of NTRK fusions in tumours has become critically important due to the actionable events predictive of response to TRK inhibitor. It is not clear whether the NTRK breakpoint location is different for response to targeted therapy and NTRK fusions affects the efficacy of immunotherapy.
Case presentation
Here we reported a 60-year-old female diagnosed with advanced lung adenocarcinoma. NGS-based molecular profiling identified a novel NCOR2-NTRK1 fusion and high tumor mutational burden (TMB) (58.58 mutations/Mb) in this case. Additionally, program death-ligand 1 (PD-L1) expression was detected in 20–30% of the tumor cells by immunohistochemical (IHC) staining. The patient received treatment with anti-PD-1 immune checkpoint inhibitor of camrelizumab. After two cycles of treatment, the CT scan showed some tumor nodules were still enlarged, indicating disease progression. She was then changed to TRK inhibitor larotrectinib. One month later, the CT scan showed the volume of some lesions started to decrease, and no metastasis lesions were found. The patient then continued the administration of larotrectinib, and some lesion sizes were significantly reduced or even disappeared in the next few months. Currently, this patient is still alive.
Conclusions
Altogether, this report provided a new driver of lung adenocarcinoma expanded the mutational spectrum of NTRK1 fusion variants and suggested using larotrectinib as the targeted therapy is more effective than anti-PD-1 inhibitor in lung adenocarcinoma harboring with NTRK fusion, positive PD-L1 expression, and high TMB simultaneously.
Similar content being viewed by others
Background
Lung is a type of molecular-based cancer treatment in personalized medicine. These therapies involve drugs or substances which could interfere with the specific molecules such as fusion genes or mutated genes to exert functions in the cell growth, progression, and spreading of cancer cells, which do not affect the normal cells [1]. Recently, NTRK fusions have been identified as new targets of cancer therapy for TRK inhibitors, such as larotrectinib and entrectinib. Immunotherapy, as a research hotspot in recent years, has been approved in many cancer species, including non-small cell lung cancer. However, it is not clear whether the presence of NTRK fusion affects the efficacy of immunotherapy.
In this report, we presented a case of advanced lung adenocarcinoma for whom tumor tissue samples underwent NGS and IHC, and several druggable alterations were identified. Among them, a novel NCOR2-NTRK1 fusion, positive PD-L1 expression, and high TMB were found. By administration of the TRK inhibitor larotrectinib, the tumor had a partial response after treatment for 5 months and still survives currently.
Case presentation
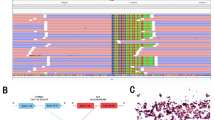
In October 2015, a 60-year-old non-smoker and non-alcoholic woman was admitted to our hospital due to the tumor found at the upper lobe of the left lung, who then underwent left upper lobe resection and left mediastinal hilar lymphadenectomy, and was diagnosed with lung adenocarcinoma with stage IIIA (T2aN2M0). After the operation, the patient refused all adjuvant treatments and did not turn up to the clinical examination on time. In June 2019, the patient showed symptoms of dyspnea. A chest computed tomography (CT) scan showed atelectasis in the left lower lobe of the lung and a massive pleural effusion in the left chest, but no metastatic lesions were found in other organs (Fig. 1a). After the pleural effusion was drawn, a further CT scan found that the metastatic nodules existed in the lower lobe and pleura of the left lung (Fig. 1b). Therefore, the tumor was stage IV. The patient subsequently underwent molecular tests, including NGS and IHC analyses to guide subsequent treatment. The results showed that the PD-L1 expression of the tumor cells was 20–30% (Fig. 2), TMB was 58.58 mutations/Mb, and intriguingly, a novel NCOR2-NTRK1 fusion was detected (Table 1, Fig. 3). This novel NTRK1 fusion has not been reported thus far. Since larotrectinib was not available at that time, and the patient refused chemotherapy. This patient started the treatment of PD-1 inhibitor, camrelizumab (Jiangsu Hengrui Medicine Co., Ltd, China) (200 mg/time, twice per week) alone in a clinical trial at other hospitals from July 2019. However, in September 2019, a chest CT scan revealed that the metastatic nodules still enlarged, indicating disease progression (Fig. 1c). We then switched to TRK inhibitor larotrectinib (200 mg/day). After 1 month, the chest CT scan showed that the volume of some lesions was reduced by 30%, indicating larotrectinib was a useful therapeutic option to this patient (Fig. 1d). We then continued to treat the case with larotrectinib. After another 2 months, the CT examination showed that some lesion sizes was reduced continuously by 50%, and no metastatic lesions in other organs were found (Fig. 1e). In March 2020, the further CT scan showed that some lesions were remarkably reduced or even disappeared (Fig. 1f). To December 2020, the patient continues to take larotrectinib and is still alive.
The chest computed tomography (CT) scan images showing the locations of tumor lesions found in a left pleural effusion of lung when the patient presented with dyspnea in June 2019; b after thoracentesis, two metastatic nodules in the left lower lobe; c after accepted PD-1 inhibitor Camrelizumab, 200 mg/time, twice per week, for a total of 4 injections (2 cycles), the tumor nodules were still increased, indicating the disease progressed; d After taking larotectinib, 200 mg/day, orally for 1 month, some lesions showed decreased in size; e oral administration of larotectinib for 3 months indicated some cancerous lesions were continuously reduced in size; f Oral administration of larotectinib for 6 months showed that some cancerous lesions either are reducing or even disappeared
Discussion and conclusions
Advances in genomic research have promoted the development of personalized medicine for the treatment of lung cancer [2]. In this study, we studied the genetic profiling and treatment options for a 60-year-old female patient with advanced lung adenocarcinoma. By NGS-based molecular profiling and IHC analyses, we identified several potentially targetable genetic alterations, including NCOR2-NTRK1 fusion, ERBB2, and NF1mutations, as well as high levels of TMB and PD-L1 expression, which provided us some therapeutic options for this patient.
Based on the analysis of NGS, tumors could be defined as high and low TMB determined by the prevalence of somatic mutations in their genome [3]. In this case, the genomic profiling revealed the TMB was 58.58 mutations/Mb, and regarded as high TMB (Table 1), suggesting that this case is a genomically unstable tumor type. Previous studies reported that NSCLC patients with high TMB was associated with better survival outcomes of immune checkpoint inhibitors (ICI) therapy [4]. Recent evidence has suggested the use of programmed death-1 (PD-1) and PD-L1 inhibitors remarkably improves response rate of advanced metastatic NSCLC compared to the traditional chemotherapy [5]. On June 2020, the Food and Drug Administration granted accelerated approval to pembrolizumab (KEYTRUDA, Merck & Co., Inc.) for the treatment of adult and pediatric patients with unresectable or metastatic TMB-H solid tumors, which further indicated the important role of TMB in ICI therapy. Additionally, this patient also had 20–30% tumor cells expressing PD-L1. Thus, the patient was treated by camrelizumab (PD-1 inhibitor) which showed high efficacy and good response in breast cancer [6]. However, 2 months after the patient received treatment, the CT showed an enlarged tumor. Although we cannot rule out the possibility that the initial "lack" of response to camrelizumab could have been that effect was not seen yet after 2 months of therapy, we tend to think that tumor suppression effect of camrelizumab was failed. In addtion, based on clinical trial design, the patient was removed from the clinical trial.Gainor et al. reported that patients with EGFR mutations and ALK fusions had low response rates to PD-1/PD-L1 inhibitors compared than those in EGFR wild-type/ALK-negative patients in Non-Small Cell Lung Cancer (NSCLC) [7]. Hong et al. demonstrated that upregulation of PD-L1 by ALK fusion protein mediates the immune escape by inducing the apoptosis of T cells [8]. Based on the finding, a possible explanation for this unexpected finding could be driver gene of NCOR2-NTRK1 fusion could be causing resistance to PD-1/PD-L1 blockade immunotherapy, which needs to be further explored.
The TRK family of receptor tyrosine kinases are encoded by NTRK1, NTRK2, and NTRK3 genes, and maintain normal functions and development of the nervous system [9]. The NTRK fusions in cancers have attracted the attention of many investigators because these NTRK fusions-associated cancers are susceptible to NTRK-TKIs [10]. Importantly, these TKIs either tested in phase III clinical trials or are commercially available [10]. NTRK1 gene rearrangements were firstly found in a small population of lung cancer patients, and this gene is frequently fused with several fusion partners in NSCLC (Table 2) [11]. Compared to NTRK1, NTRK2, and NTRK3 rearrangements are relatively rare but are still found in lung cancers (Table 2). In this study, we found that the exon 9 of NCOR2 fused to the exon 9 of NTRK1 gene to generate a novel NTRK1 fusion protein (NCOR2-NTRK1). NCOR2 (Nuclear Receptor Corepressor 2) gene functions as a co-repressor for mediating transcriptional silencing of specific target genes [12]. Loss or mutation in NCOR2 gene is involved in chemoresistance and tumor growth [13]. However, to our knowledge, there have been no reports so far on the identification of NCOR2-NTRK1 gene rearrangement in human cancers. According to the guidelines of The National Comprehensive Cancer Network (NCCN), there are several TKIs commercially available to target fusions of NTRK1, NTRK2, and NTRK3 with varying degrees of activity [14]. Among these TKIs, larotrectinib, and entrectinib are the most common targeted agents tested in clinical trials and showed a significant impact on solid tumors [14]. NCCN guidelines and other studies have suggested that larotrectinib is a relatively potent and selective inhibitor against all three NTRK fusions [15]. Consistently, our case study confirmed that larotrctinib could effectively inhibit the tumor progression and development of this lung cancer patient with NCOR2-NTRK1 fusion.
ERBB2 mutations were identified in this patient. ERBB2 gene is a member of the epidermal growth factor (EGF) receptor family of receptor tyrosine kinases, which is commonly amplified and overexpressed in aggressive human cancers [16]. Currently, many tyrosine kinase inhibitors (TKIs) such as trastuzumab, pertuzumab, lapatinib, pyrotinib, and trastuzumab emtansine (T-DM1) have developed for targeting ERBB2 amplification or overexpression in human tumors. However, the efficacy and sensitivity of these tyrosine kinase inhibitors for ERBB2 mutations, excluding amplification and overexpression, are still waiting for further investigation. Indeed, although ERBB2 mutation is a targetable driver in lung cancer, numerous studies reported the limited response rate and duration by using ERBB2 targeted therapies [17]. The potential resistance mechanisms may include PI3KCA mutation, SRC activation, MET overexpression, or other genomic alterations [17]. Due to the uncertainties of using ERBB2 targeted therapies, we decided not to use these inhibitors as the first targeted therapeutic option.
In this study, we used immunotherapy with PD-1 inhibitor of Camrelizumab and the targeted therapy with larotrectinib in this lung cancer patient. Regarding the treatment outcomes, larotrectinib had a marked response rate to target the tumor of this patient, indicating that novel NCOR2-NTRK1 fusion plays a critical oncogenic driver in this tumor. However, targeted therapies using these inhibitors have limited duration of responses due to the acquired resistance formed eventually [18]. To solve this limitation, one of the effective approaches might be the combination of TRK and PD-1 inhibitors applied in the subsequent cancer treatment. Clinical evidence has indicated this type of therapeutic approach could significantly improve the long-term outcomes of ALK fusion-positive NSCLC [19]. Similarly, recent evidence has shown that NSCLC with NTRK fusion also has increased PD-L1 expression [11]. This raises the possibility of using the combined TRK and PD-1 inhibitors in this case with NCOR2-NTRK1 fusion, positive PD-L1 expression, and high TMB simultaneously in the future.
Availability of data and materials
The data that support this case report are available from the corresponding author on reasonable request, since respecting the Ethics Committee to protect patient confidentiality.
Abbreviations
- TMB:
-
Tumor mutational burden
- PD-L1:
-
Program death-ligand 1
- IHC:
-
Immunohistochemical
- CT:
-
Computed tomography
- ICI:
-
Immune checkpoint inhibitors
- PD-1:
-
Programmed death-1
- NSCLC:
-
Non-small cell lung cancer
- NCCN:
-
National Comprehensive Cancer Network
- EGF:
-
Epidermal growth factor
- TKIs:
-
Tyrosine kinase inhibitors
References
Gambardella V, Tarazona N, Cejalvo JM, Lombardi P, Huerta M, Rosello S, et al. Personalized medicine: recent progress in cancer therapy. Cancers (Basel). 2020;12(4):1009.
Kruglyak KM, Lin E, Ong FS. Next-generation sequencing and applications to the diagnosis and treatment of lung cancer. Adv Exp Med Biol. 2016;890:123–36.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–6.
Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–41.
Tanvetyanon T, Gray JE, Antonia SJ. PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer? Expert Opin Biol Ther. 2017;17(3):305–12.
Liu J, Liu Q, Li Y, Li Q, Su F, Yao H, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000696.
Gainor J, Shaw A, Sequist L, Fu X, Azzoli C, Piotrowska Z, et al. EGFR Mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–93.
Hong S, Chen N, Fang W, Zhan J, Liu Q, Kang S, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2016;5(3):e1094598.
Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169(2):107–14.
Sartore-Bianchi A, Pizzutilo EG, Marrapese G, Tosi F, Cerea G, Siena S. Entrectinib for the treatment of metastatic NSCLC: safety and efficacy. Expert Rev Anticancer Ther. 2020;20(5):333–41.
Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147–53.
Jiang Q, Galiegue-Zouitina S, Roumier C, Hildebrand MP, Thomas S, Coignet LJ. Genomic organization and refined mapping of the human nuclear corepressor 2 (NCOR2)/silencing mediator of retinoid and thyroid hormone receptor (SMRT) gene on chromosome 12q24.3. Cytogenet Cell Genet. 2001;92(3–4):217–20.
Leung MH, Tsoi H, Gong C, Man EP, Zona S, Yao S, et al. A splice variant of NCOR2, BQ323636.1, confers chemoresistance in breast cancer by altering the activity of NRF2. Cancers (Basel). 2020;12(3):533.
Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47.
Doebele RC, Davis LE, Vaishnavi A, Le AT, Estrada-Bernal A, Keysar S, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 2015;5(10):1049–57.
Zhou BP, Hung MC. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin Oncol. 2003;30(5 Suppl 16):38–48.
Chuang JC, Stehr H, Liang Y, Das M, Huang J, Diehn M, et al. ERBB2-Mutated metastatic non-small cell lung cancer: response and resistance to targeted therapies. J Thorac Oncol. 2017;12(5):833–42.
Santarpia M, Gil N, Rosell R. Strategies to overcome resistance to tyrosine kinase inhibitors in non-small-cell lung cancer. Expert Rev Clin Pharmacol. 2015;8(4):461–77.
Spigel DR, Reynolds C, Waterhouse D, Garon EB, Chandler J, Babu S, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation—positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682–8.
Acknowledgements
We thank the patients who contributed to this study, as well as the staff of each department.
Funding
This study was not supported by grants.
Author information
Authors and Affiliations
Contributions
The paper was conceived, designed and written by LZ, HW, XY, and HL. Disease diagnosis was achieved by LZ. Treatment was completed YT and XY. Data were analyzed and interpreted by YT and LZ. The paper was revised by HL and HW. All authors reviewed and provided feedback on the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of The Fourth Affiliated Hospital of China Medical University.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying clinical data. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
All authors have completed the ICMJE uniform disclosure form. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Liu, H., Tian, Y. et al. A novel NCOR2-NTRK1 fusion detected in a patient of lung adenocarcinoma and response to larotrectinib: a case report. BMC Pulm Med 21, 125 (2021). https://doi.org/10.1186/s12890-021-01490-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01490-x