Abstract
Anaplastic lymphoma kinase (ALK) gene fusion is a classic driver mutation in non-small cell lung cancer (NSCLC); however, ALK double-fusion variants in NSCLC have rarely been reported. In this study, we reported a case with extremely uncommon ALK double-fusion variants. A 32-year-old female diagnosed with lung adenocarcinoma, who had developed multiple intrapulmonary and brain metastases, experienced worsening of her condition despite undergoing prior chemotherapy. Subsequent testing using next-generation sequencing (NGS) detected the presence of PLEKHA7-ALK and INPP5D-ALK double-fusion. The prescription of alectinib revealed potent efficacy and resulted in an increase in the survival rate. This case presented two uncommon and concomitant ALK fusion partners in NSCLC; more importantly, the INPP5D-ALK subtype has not been reported, therefore this study broadens the spectrum of ALK double-fusion variants and provides insight into the use of ALK inhibitors for the treatment of NSCLC in patients with double ALK fusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ALK gene fusion is a well-known driver mutation present in 3–7% of cases diagnosed with non-small cell lung cancer (NSCLC) [1]. Echinoderm microtubule-associated protein-like 4 (EML4) is the most common ALK fusion partner, accounting for over 80% of fusion subtypes. The deep coverage of next-generation sequencing (NGS) for ALK fusion partners (over 90 types) increased between 2007 and 2020, and ALK tyrosine kinase inhibitors (TKIs) exhibited potent activities [2]. In addition, several ALK double-fusion variants have been occasionally discovered and reported in cases with NSCLC [3,4,5]. Of note, these rare double-fusion partners might result in discrepant responses to ALK inhibitors, and further evidence would be urgently needed in this field. Here, we reported a case with concomitant ALK rare double-fusion, namely PLEKHA7-ALK and INPP5D-ALK in a patient diagnosed with lung adenocarcinoma who showed favorable response to alectinib following chemotherapy failure.
2 Case presentation
A 32-year-old female with no smoking history was diagnosed with a mass in the upper lobe of the right lung with bilateral multiple intrapulmonary and brain metastases in July 2020 (Fig. 1A). Subsequent pulmonary puncture biopsy demonstrated lung adenocarcinoma, showing strongly positive expression of TTF-1 and Napsin A according to immunohistochemical analysis. Polymerase chain reaction (PCR) assay via tumor tissue found no alteration in EGFR, ALK and ROS-1 genes when assayed in the resected lung tumor. Since July 2020, she underwent whole brain radiotherapy (WBRT) at a dose of 36 Gy/1.8 Gy/20f, along with a simultaneous integrated boost (SIB) of up to 56 Gy/20f using the intensity modulated radiation therapy (IMRT) technique. Also, she underwent chemotherapy with pemetrexed plus carboplatin, combined with an antiangiogenic agent, bevacizumab. Following two cycles of chemotherapy, she developed a partial response (PR), as shown in Fig. 1B. She experienced PR after maintenance therapy with pemetrexed and bevacizumab started after six cycles of treatment.
Multiple intrapulmonary and brain metastases at baseline before treatment (A); partial response to chemotherapy plus bevacizumab after two cycles of treatment (B); progression of intrapulmonary metastasis and emergence of lymphangitis carcinomatosis (C); partial response to alectinib with significant decrease of intrapulmonary metastasis and lymphangitis carcinomatosis (D)
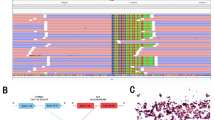
In November 2022, her intrapulmonary metastasis significantly increased, and she developed lymphangitis carcinomatosis, while the brain metastasis remained stable (Fig. 1C). She underwent clinical examinations to monitor disease progression and achieved a progression-free survival (PFS) of 27 months without any further advancement. Owing to the outbreak of COVID-19 in China in December 2022, the patient discontinued her treatment regimen. In February 2023, two months later, the patient developed bilateral pleural metastasis, which resulted in a worsening of her dyspnea. Subsequently, she underwent pleural space drainage, and the presence of malignant adenocarcinoma cells was confirmed. The NGS analysis was carried out in Geneseeq Technology Inc. using a 6-gene targeted panel (EGFR, ALK, ROS-1, BRAF, KRAS, and HER2), which identified the simultaneous presence of two fusion genes PLEKHA7-ALK (P18:A20, allelic frequency: 16.60%) and INPP5D-ALK (I2:A19, allelic frequency: 13.92%) in the pleural effusion sample (Fig. 2). Finally, alectinib was administered since April 2023. Notably, both the multiple intrapulmonary metastases and lymphangitis carcinomatosis significantly decreased after only one month of targeted therapy (Fig. 1D). She continued alectinib until December 2023, with a favorable PR and a significant 8-month PFS benefit..
3 Discussion
This is the first case presenting a rare concomitant ALK double-fusion, namely PLEKHA7-ALK and INPP5D-ALK, in a patient with lung adenocarcinoma, who exhibited favorable sensitivity to alectinib. Due to the low incidence, research devoted to double ALK fusions is extremely lacking. It is critical to determine the activity of different ALK fusion partners in response to different ALK TKIs in order to make personalized therapeutic decisions. According to the previous evidence, only several cases with distinct ALK double-fusions have been reported (Table 1), and crizotinib and alectinib showed exceptional activities [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].
Here, we identified novel and concomitant ALK double-fusion partners, namely PLEKHA7-ALK and INPP5D-ALK, in a patient with NSCLC. The PLEKHA7-ALK fusion was first reported as an acquired drug resistance in a patient with lung cancer who had failed to adequately respond to osimertinib. When osimertinib and alectinib were subsequently administered in combination, it achieved a dramatic and confirmed PR with a 6-month duration of response [18]. In addition, the PLEKHA7-ALK fusion was also reported in renal cell carcinoma [19]. The pleckstrin homology domaincontaining A7 gene (PLEKHA7) encodes an adherens junction protein which is highly preserved in epithelial cells [20]. The PLEKHA7- ALK fusion protein remained the pleckstrin homology domain of PLEKHA7 and the kinase domain of ALK. Notably, the pleckstrin homology domain might further facilitate the dimerization and activation of the ALK kinase [21]. To the best of our knowledge, the INPP5D-ALK fusion has not been reported in lung cancer to date. The inositol polyphosphate-5-phosphatase D gene (INPP5D) is a risk-conferring gene for Alzheimer’s disease (AD) specifically expressed in microglia in the brain, and negatively regulates the phosphoinositide 3-kinase pathway controlling cell migration, proliferation, and survival [22, 23]. The present case demonstrated the rearrangement in exon 18 of the PLEKHA7 gene and exon 20 of the ALK gene, as well as the fusion of INPP5D (exon 2) and ALK (exon 19), forming a double-fusion variant PLEKHA7-ALK and INPP5D-ALK. Although there is no direct evidence to support INPP5D-ALK as a driver mutation, it is worth considering that INPP5D-ABL1 fusion has exhibited sensitivity to imatinib in a patient with acute lymphoblastic leukemia [24]. Therefore, it is possible that INPP5D-ALK fusion could be a driver mutation in our case of NSCLC. The patient in our case showed partial response to alectinib when her disease progressed following previous chemotherapy. Despite the presence of a rare ALK double-fusion, this patient demonstrated a significant partial response and has benefited from alectinib for the past eight months in terms of PFS. Our findings, in this case, lack explicit research support; therefore, we can only speculate that the rare ALK double-fusion PLEKHA7-ALK and INPP5D-ALK might provide a promising response to the ALK inhibitor alectinib.
The findings of the worldwide phase III ALEX trial indicate that alectinib and crizotinib provide comparable benefits in terms of PFS, objective response rate (ORR), and duration of response (DOR) for patients with EML4-ALK variants 1 and 3a/b when used as the first-line treatement [25]. Although the available evidence on the association between variant types and treatment outcomes in the ALEX clinical trial is limited, patients treated with alectinib demonstrate superior response and improved survival compared to those treated with crizotinib in treatment-naive patients with ALK-positive NSCLC, irrespective of the EML4-ALK variant. However, the ALK double-fusion remains rare, with only a few case reports being published owing to its low incidence and detection rate. Over the past five years, we conducted a thorough search and identified 16 distinct types of ALK double-fusion variants. The characteristics of these variants, as reported in these case reports, are summarized in Table 1. Among them, more than half (9/16) are EML4-ALK together with another rare ALK fusion type. Crizotinib and alectinib demonstrated a positive response and significant PFS improvement for patients carrying these heterogeneous ALK double-fusion variants. In addition, the ALK double-fusion partners, namely MRPL13-ALK and PPP1CB-ALK, were reported as acquired resistant variants to EGFR-TKI treatment. According to these 16 case reports examined, we could not find valuable information regarding differences in patient background, treatment efficacy, and prognosis between double-fusion and single-fusion variants. However, both crizotinib and alectinib showed potent activity and a favorable survival rate for these rare ALK double-fusion variants.
In conclusion, this case report described a novel ALK double-fusion involving PLEKHA7-ALK and INPP5D-ALK in lung adenocarcinoma. Also, alectinib demonstrated potent activity and improved survival outcomes for this rather rare variant. In addition, the NGS testing provides a reliable insight for the identification of novel fusion partners for ALK-rearranged NSCLC.
Data availability
All data generated or analysed are included in this published article.
References
Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–81.
Ou SI, Zhu VW, Nagasaka M. Catalog of 5′ fusion partners in ALK-positive NSCLC Circa 2020. JTO Clin Res Rep. 2020;1(1): 100015.
Wu X, Zhou H, He Z, et al. Coexistence of a novel CCNY-ALK and ATIC-ALK double-fusion in one patient with ALK-positive NSCLC and response to crizotinib: a case report. Transl Lung Cancer Res. 2020;9(6):2494–9.
Wu X, Wang W, Zou B, et al. Novel NLRC4-ALK and EML4-ALK double fusion mutations in a lung adenocarcinoma patient: a case report. Thorac Cancer. 2020;11(6):1695–8.
Tao H, Liu Z, Mu J, et al. Concomitant novel ALK-SSH2, EML4-ALK and ARID2-ALK, EML4-ALK double-fusion variants and confer sensitivity to crizotinib in two lung adenocarcinoma patients, respectively. Diagn Pathol. 2022;17(1):27.
Luo J, Gu D, Lu H, et al. Coexistence of a novel PRKCB-ALK, EML4- ALK double-fusion in a lung adenocarcinoma patient and response to crizotinib. J Thorac Oncol. 2019;14(12):e266–8.
Qin BD, Jiao XD, Liu K, et al. Identification of a novel EML4-ALK, BCL11A-ALK double-fusion variant in lung adenocarcinoma using NextGeneration sequencing and response to crizotinib. J Thorac Oncol. 2019;14(6):e115–7.
Lin H, Ren G, Liang X. A novel EML6-ALK FBXO11-ALK double fusion variant in lung adenocarcinoma and response to crizotinib. J Thorac Oncol. 2018;13(11):e234–6.
Yin J, Zhang Y, Zhang Y, et al. Reporting on two novel fusions, DYSF-ALK and ITGAV-ALK, coexisting in one patient with adenocarcinoma of lung, sensitive to crizotinib. J Thorac Oncol. 2018;13(3):e43–5.
Yan L, Zheng J, Pan Q, et al. Novel PPFIA1-ALK, ALK-C2orf91(intergenic) double-fusion responded well to alectinib in an advanced lung adenocarcinoma patient: a case report. Front Oncol. 2023;13:1264820.
Wu X, Wang Q, Xu X. Coexistence of a novel STRN-ALK, NBEA-ALK double-fusion in an ovarian malignant mesothelioma patient: a case report and review. Front Oncol. 2023;13:1156329.
Zhu L, Qin J. Coexistence of a novel SETD2-ALK, EML4-ALK double-fusion in an advanced lung adenocarcinoma patient after alectinib resistant and response to immunotherapy combined with chemotherapy: a case report. Discov Oncol. 2023;14(1):44.
Guo J, Shi J, Yao M, et al. A rare double ALK fusion variant EML4-ALK and CDK15-ALK in lung adenocarcinoma and response to crizotinib: a case report. Medicine (Baltimore). 2020;99(45): e22631.
Zhong JM, Zhang GF, Lin L, et al. A novel EML4-ALK BIRC6-ALK double fusion variant in lung adenocarcinoma confers sensitivity to alectinib. Lung Cancer. 2020;145:211–2.
Liang Q, Xu H, Liu Y, et al. Coexistence of a novel NBEA-ALK, EML4-ALK double-fusion in a lung adenocarcinoma patient and response to alectinib: a case report. Lung Cancer. 2021;162:86–9.
Zeng H, Li Y, Wang Y, et al. Case report: identification of two rare fusions, PDK1-ALK and STRN-ALK, that coexist in a lung adenocarcinoma patient and the response to alectinib. Front Oncol. 2021;11: 722843.
Jiao Y, Liu M, Luo N, et al. Novel MRPL13-ALK and PPP1CB-ALK double fusion as a potential mechanism of acquired resistance to first-line osimertinib in EGFR-mutant high-grade neuroendocrine tumor of the lung. JTO Clin Res Rep. 2020;1(4): 100079.
Schrock AB, Zhu VW, Hsieh WS, et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13:1312–23.
Hang JF, Chung HJ, Pan CC. ALK-rearranged renal cell carcinoma with a novel PLEKHA7-ALK translocation and metanephric adenoma-like morphology. Virchows Arch. 2020;476(6):921–9.
Pulimeno P, Bauer C, Stutz J, et al. PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-cadherin. PLoS ONE. 2010;5(8): e12207.
Chung JK, Nocka LM, Decker A, et al. Switch-like activation of Bruton’s tyrosine kinase by membrane-mediated dimerization. Proc Natl Acad Sci USA. 2019;116(22):10798–803.
Robins C, Liu Y, Fan W, et al. Genetic control of the human brain proteome. Am J Hum Genet. 2021;108:400–10.
Damen JE, Liu L, Rosten P, et al. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5- triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–93.
Poukka M, Lund-Aho T, Raittinen P, et al. Acute lymphoblastic leukemia with INPP5D-ABL1 fusion responds to imatinib treatment. J Pediatr Hematol Oncol. 2019;41(7):e481–3.
Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233–43.
Acknowledgements
We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.
Funding
The authors have no funding sources to report.
Author information
Authors and Affiliations
Contributions
PL: visualization, validation, methodology, writing—original draft preparation. XJ: data curation. GY: conceptualization, project administration, writing—reviewing and editing, supervision. Both authors have read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was granted an exemption from requiring ethics approval from the Ethics Committee of Shandong Cancer Hospital and Institute, as it was an observational report. All the human data was in accordance with the guidelines of the 1964 Declaration of Helsinki, and written informed consent from the study patient was obtained.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, P., Ju, X. & Yang, G. Concomitant double-fusion of PLEKHA7-ALK and INPP5D-ALK reveals favorable alectinib sensitivity in lung adenocarcinoma: a case report and literature review. Discov Onc 15, 43 (2024). https://doi.org/10.1007/s12672-024-00899-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-00899-0