Abstract
Aim
To assess the clinical utility of novel anthropometric indices and other traditional anthropometric indices in identifying the risk of type 2 diabetes mellitus (T2D) among South African adult females.
Methods
In the first South African National Health and Nutrition Examination Survey (SANHANES-1), traditional [body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR)] and novel [a-body shape index (ABSI), abdominal volume index (AVI), body adiposity index (BAI), body roundness index (BRI), conicity index (CI), and Clínica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE)] anthropometric indices were assessed. T2D was diagnosed using glycated haemoglobin (HbA1c) ≥ 6.5% among participants without known T2D. Basic statistics and multiple regression analyses were explored the association between anthropometric indices and newly diagnosed T2D. Receiver operating characteristic (ROC) curve analysis was used to measure the predictive ability of both traditional and novel indices.
Results
Among 2 623 participants, 384 (14.6%) had newly diagnosed T2D. All anthropometric indices mean values were significantly higher among participants with T2D (most p < 0.001). Higher mean values increased T2D odds e.g., in the model adjusted for age, employment, residence, and population group, odds ratio (OR) and 95% confidence interval (CI) for T2D with some of anthropometric indices were: 1.86 (1.60–2.15) for WC, 1.84 (1.59–2.13) for WHtR, 1.73 (1.51–1.99) for AVI, 1.71 (1.49–1.96) for BRI and 1.86 (1.57–2.20) for CUN-BAE. The top quartile for all indices had the highest T2D odds (p < 0.05). These outcomes were the highest for WC, AVI, and CUN-BAE and remained so even after removing the confounding effects of age, employment, population group, and residence. Based on the ROC analysis, none of the anthropometrical indices performed excellently (i.e., had an area under the curve [AUC] > 0.80). The WC, WHtR, AVI, BRI, and CUN-BAE, however, performed acceptably (AUCs 0.70–0.79), while also exhibiting corresponding cutoff values of 86.65 cm, 0.57, 15.52, 3.83, and 38.35, respectively.
Conclusions
The data shows that traditional and novel anthropometric indices similarly identifying newly diagnosed T2D among adult South African females. We recommend the continuing the use of traditional indices, as they are affordable and easy to use in our setting.
Similar content being viewed by others
Introduction
South Africa, like many other countries undergoing rapid urbanization and lifestyle transitions, has witnessed a significant increase in type 2 diabetes mellitus (T2D) with a recent systematic review estimating a prevalence of 15.3% among adults aged 15 years and older [1]. Given the high prevalence of T2D, early diagnosis and treatment are imperative to reduce the associated morbidity and mortality. However, biochemical diagnoses using oral glucose tolerance tests (OGTT) are time consuming and cumbersome deterring the widespread and frequent use of these biochemical tests in asymptomatic individuals. In the era where glycated haemoglobin (HbA1c) has become a common and reliable test for diabetes diagnosis, the role of anthropometric indices should not be overlooked. These indices provide additional insights into body fat distribution, visceral adiposity, and overall obesity, which are important factors in the development of insulin resistance and T2D [2]. For instance, while HbA1c reflects long-term glycemic control, anthropometric indices can help identify individuals at risk of developing diabetes before significant changes in blood glucose levels are detectable by HbA1c. Therefore, there is a need to identify individuals at high risk for T2D before undertaking biochemical testing. This is particularly true in resource constrained settings like South Africa.
Obesity is a major risk factor for T2D and easily determined using cost-effective tools such as a non-elastic tape measure to determine waist circumference (WC) and heights, and a calibrated scale to measure weights; these can be used to calculate body mass index (BMI), etc. Considering that > 80% of individuals with T2D are generally living with obesity [3], measuring adiposity is a practical tool to identify individuals at high risk for the condition. Together with WC and BMI, other traditional measures of adiposity include waist-to-hip ratio (WHR). More recently, due to the shortcomings of these traditional adiposity measures, BMI, for instance, does not differentiate between fat mass and lean body mass, leading to potential misclassification of individuals with high muscle mass as overweight or obese [4]. WC and WHR provide a more direct assessment of central adiposity but still fall short in distinguishing between subcutaneous and visceral fat, which have different metabolic risks. Furthermore, these measures do not account for fat distribution or the potential variability in adiposity related to age, sex, and ethnicity [5]. Therefore, researchers have explored novel anthropometric indices to enhance the predictive accuracy at identifying individuals at high risk for T2D [6, 7].
Several novel anthropometric indices, such as the body roundness index (BRI) [7], a body shape index (ABSI) [8], body adiposity index (BAI) [9], Clínica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE) [10], abdominal volume index (AVI) [11], and conicity index (CI) [12], have been proposed as alternatives to traditional measures for assessing diabetes risk. These indices combine multiple anthropometric variables, including waist and hip circumferences, height, and weight, to offer a more comprehensive evaluation of body composition and fat distribution compared to traditional methods.
In recent years, novel anthropometric indices have been developed to improve the assessment of obesity-related health risks, each offering unique characteristics that enhance their predictive power in specific contexts [13, 14]. ABSI, proposed by Krakauer et al. (2012) [8] and Biolo et al. (2015) [15], is based on WC, height, and BMI and can serve as an indicator of abdominal obesity. Similarly, the AVI focuses on estimating the volume of the abdomen by incorporating both WC and height, making it a more precise tool for assessing central obesity and its associated risks, such as diabetes, metabolic syndrome and cardiovascular disease [11]. Thomas et al. (2013) [16], proposed the BRI as a predictor of visceral adiposity tissue and body fat percentage. BRI has demonstrated efficacy as a reliable predictor of metabolic syndrome, of which T2D is a component, across various nationalities and ethnic groups [17]. The BAI offers another alternative by estimating body fat percentage using hip circumference and height. Unlike BMI, BAI is less influenced by muscle mass, making it a potentially more accurate indicator of adiposity [9]. Additionally, CI evaluates central adiposity using WC, weight, and height, effectively identifying those at higher cardiovascular risk [12]. Lastly, the CUN-BAE estimates body fat percentage based on BMI, age, and sex, proving accurate across various age groups and genders, making it a valuable tool for risk stratification in clinical settings [10].
Although these traditional and novel anthropometric indices have been shown to associate with T2D, most studies have focused on high-income countries [18, 19], rendering findings less generalizable, including among adult South African females [20, 21]. In South Africa, the clinical utility of the association of traditional and novel anthropometric indices with T2D has not been widely studied in national studies. Therefore, this study aims to investigate the associations of novel anthropometric indices (ABSI, AVI, BAI, BRI, CI, and CUN-BAE) with newly diagnosed T2D and to compare their performance with the traditional indices of BMI, WC, WHR and waist-to-height ratio (WHtR) in identifying T2D among South African adult females.
Methods
Study population and design
This study used data from the first South African National Health and Nutrition Examination Survey (SANHANES-1) conducted in 2012. SANHANES-1 was a cross-sectional survey aimed at assessing the nutritional status and health conditions of the South African populace as described in detail elsewhere [20, 22]. The survey used a stratified cluster sampling approach with multiple stages. A total of 1000 census enumeration areas (EAs) mapped using aerial photography in 2007 were used to produce the Master Sample. The EAs were chosen based on their province and locality type. From the Master Sample, 500 EAs were chosen to represent the socio-demographic profile of South Africa. Random samples of 20 visiting points (VPs) were chosen at random from each EA, providing a total sample of 10,000 homes. The multistage cluster sampling design of SANHANES-1 meant that some individuals had different probabilities of being selected, potentially leading to biased estimates. To address this, sample weights were applied to correct for biases at the EA, household, and individual levels, and adjusted for non-response. The survey was designed to be generalisable to the entire population of South Africans living in households. Sampling weights were thus benchmarked to the 2012 midyear population estimates [23]. The weighting process described was conducted using SAS version 9.3 in conjunction with CALMAR macro for benchmarking. As depicted in Fig. 1, the final sample comprised 8 166 households that were both valid and occupied, encompassing of 27 580 eligible individuals of all age groups. Among these eligible individuals, 92.6% (25 532) participated in the interviews, 43.6% (12 025) volunteered for physical examinations, including anthropometric measurements, and 29.3% (8 078) consented to provide blood samples for biomarker analysis.
Among these individuals, 14 283 had missing data for glycated haemoglobin (HbA1c), which was used to diagnose T2D and were consequently excluded. This means that HbA1c was not measured for these individuals, which were due to various factors such as non-participation in certain study components or exclusions based on defined study criteria. Is it worth mentioning that we further conducted a comparative analysis of background characteristics (e.g., age, gender) between participants who had their HbA1c measured and those who did not. The results indicated that, there were no significant differences between the two groups (p > 0.05). This analysis suggests that while missing data are present, they are unlikely to bias the overall findings. However, in this study, which focused on females, the 8 158 males were excluded, leaving a sample of 3 696 females. We further excluded females under the age of 20 years; hence, the realized sample was 2 623 eligible females aged 20 years and older. Figure 1 illustrates a summarized flowchart depicting participant selection for this study. For further details regarding the SANHANES-1 methodology, content, and laboratory procedures, additional information can be found elsewhere [20, 22].
Data collection and measurements
Sociodemographic variables
Sociodemographic data included for these analyses were age, population groups, employment status and residence location (rural vs. urban settings). These data were captured using a validated survey questionnaire.
Assessments of traditional anthropometric parameters, blood pressure and body composition
Anthropometric measurements including height, weight, WC were taken using standard methods based on the International Society for the Advancement of Kinanthropometry [24]. Height was measured in centimetres using a stadiometer (Seca Model 213; Medical Scales and Measuring Systems), and weight in kilograms using a digital scale (Model A1ZE, East Rand; electronic scales). Two measurements were taken and recorded in the appropriate section of the clinical examination form. If the two measurements differed by more than 0.1 cm, a third measurement was taken. The average of two measurements that were nearest to each other were selected for further analysis. BMI was then calculated as weight (kg)/height squared (m2). The recommended World Health Organization (WHO) cut-off points were used to determine underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5 kg/m2– 24.9 kg/m2), overweight (BMI = 25.0 kg/m2–29.9 kg/m2) and obesity (BMI ≥ 30 kg/m2) [25]. WC was measured in the horizontal plane midway between lowest rib and the iliac crest during normal breathing, while hip circumference was measured at the widest part of the buttocks while standing; both measurements used a non-stretch measuring tape for accuracy [26]. WHR and WHtR were measured by dividing the WC by the hip circumference and by the height, respectively.
Assessment of novel anthropometric indices AVI, BRI, and CUN-BAE
ABSI was calculated as \(\:=\frac{WC}{{BMI}^{2/3}\times\:{height}^{1/2}}\) [27].
BAI was calculated as (hip circumference/(height)^1.5) – 18 [9]. Where: hip circumference is in centimetres and height is in metres.
AVI was calculated as [2 cm (waist)2 + 0.7 cm (waist–hip)2]/1,000, in which both waist and hip measurements are in centimetres (cm) [1].
CI was calculated using the formula below [28].
CUN-BAE was calculated as − 44.988+(0.503 × age) + (10.689 × sex) + (3.172 × BMI) − (0.026 × BMI2) + (0.181 × BMI × sex) − (0.02 × BMI × age) – (0.005 × BMI2 × sex) + (0.00021 × BMI2 × age) where female = 1 for sex, and age in years [10].
Body roundness index was calculated using the following formula:
Blood pressure
Blood pressure (BP) was measured on the right arm twice following the American Heart Association (AHA) guidelines [29], with participants seated and using an automated monitor (Omron Health Care Co., Ltd., Kyoto, Japan). The average of the two measurements was considered for data analysis. Hypertension was defined as mean systolic BP > = 140 mmHg and/or diastolic BP > = 90 mmHg and/or use of antihypertensive medication [29].
Assessment of glycated hemoglobin and lipid profile
Blood samples, approximately 15–20 mL, were collected from consenting females by a registered nurse after they had fasted for at least 8 h and were transported to accredited laboratories (Pathcare and Lancet Laboratories) within 24 h for analyses. HbA1c was measured using high-performance liquid chromatography (HPLC). A 6.5% threshold for HbA1c was used to diagnose T2D [30]. The concentration of triglycerides was assessed using the phosphoglycerides oxidase peroxidase method while low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were obtained using the colorimetric non precipitation method. The cut-off point for abnormal triglycerides was > 1.5 mmol/L, HDL-C was < 1.2 mmol/L and that of LDL-C was > 3 mmol/L [31]. Quality control ensured accuracy (coefficient of variation: 0.5–3.75%). No deviations from established standards were reported. Ethical approval was obtained from the Research Ethics Committee (REC) of the South African Human Sciences Research Council (HSRC) (REC number: 6/16/11/11), which is in accordance with the Declaration of Helsinki. Informed written consent was obtained from all the survey participants.
Statistical analyses
All analyses were performed using the SPSS 28.0 statistical software (SPSS 28.0 for Windows; SPSS, Inc., Chicago, IL). We used Kolmogorov–Smirnov test to assess the normality of the data. Continuous variables are presented as means and standard deviations (M ± SD) for normal distribution and median with 25th -75th percentiles for skewed variables. Comparisons were conducted between groups using the Student’s t test and Mann–Whitney U test as appropriate. Prevalence data, stratified by type 2 diabetes (T2D) status, were compared using the chi-square test (χ²). To assess relationship between anthropometric indices and T2D, each anthropometric index was categorized into quartiles. Logistic regression analysis was employed to calculate odds ratios (ORs) and 95% confidence intervals (CIs), showing the association between increasing quartiles of each index and the presence of T2D. The lowest quartile (Q1) served as the reference group.
The adjusted models were developed through a systematic process that involved selecting relevant covariates based on both prior research and theoretical considerations. The covariates were chosen to account for potential confounders that could influence the relationship between anthropometric indices and T2D. Initially, age was selected as a primary covariate, given its well-established association with T2D risk (Model 2) [6]. Further adjustments were made to include employment status, residential area, and population group (Model 3), as these factors are known to impact both anthropometric measures and T2D prevalence based on the literature and theoretical frameworks related to social determinants of health [32].
The fit and appropriateness of the logistic regression models were evaluated using several methods. The likelihood ratio test was employed to compare the goodness of fit between nested models, specifically evaluating whether the inclusion of additional covariates significantly improved the model’s explanatory power. This test allowed us to assess whether the more complex models (e.g., Model 3) provided a better fit to the data than simpler models (e.g., Model 1). The data was weighted to produce estimates that represent the country’s socio-demographic profile (e.g. age, population group, and residence), based on the 2001 population census. The area under the curve (AUC) from the receiver operating characteristics (ROC) analysis was calculated, along with 95% CIs, to evaluate the discriminatory power of the anthropometric indices for T2D. The AUC is a measure of discrimination, and the AUC = 0.5; 0.6 ≤ AUC < 0.7; 0.7 ≤ AUC < 0.8, 0.8 ≤ AUC < 0.9, and ≥ 0.9 corresponded to no discrimination, poor, acceptable, excellent, and outstanding discrimination, respectively [31]. The optimal novel anthropometric indices cut-off points were determined through ROC analysis using the Youden index [maximum (sensitivity + specificity − 1). P-values < 0.05 were used to characterize statistically significant results.
Results
Table 1 summarizes the sociodemographic characteristics and cardiovascular risk factors of 2 623 females with and without T2D. Overall, 14.6% were newly diagnosed with T2D. The mean age of the participants was 44.5 years with T2D participants significantly older than their counterparts. In fact, all attributes considered (sociodemographic characteristics and cardiovascular risk factors) were significantly different between participants with and without T2D (all p < 0.001), except for HDL-C. The prevalence of other cardiometabolic disorders that commonly co-exist with T2D were as follows in women with and without newly diagnosed T2D: hypertension 64.94% vs. 34.61%, p < 0.001, elevated triglycerides 61.31% vs. 25.33%, p < 0.001, high LDL-C 54.43% vs. 33.78%, p < 0.001and low HDL-C 1.20% vs. 3.08%, p = 0.068 (Results are included in Table 1).
Table 2 presents the anthropometrical indices of South African women with and without T2D; always showing significantly higher values in participant with newly diagnosed T2D compared with their counterparts (all p < 0.001).
Table 3 shows the consistent increasing likelihood of T2D per standard deviation higher level of anthropometric measures in crude models (model 1); after adjustment for age (model 2), and sociodemographic factors (model 3). Notably, both WHtR and CUN-BAE showed a twofold increase in the likelihood of T2D, as indicated by the crude odds ratios. However, this estimate attenuated after adjustment for sociodemographic factors for WHtR, while the estimates increased for CUN-BAE. Equivalent estimates across increasing quartiles of anthropometric indices are shown in Table 4. The odds ratios were consistently higher across high quartiles.
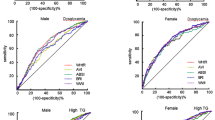
Finally, based on the ROC analysis, we observed that none of the anthropometrical indices (both traditional and novel) exhibited the AUC values above 0.8 (Table 5 and Fig. 2). The only indices that performed acceptably to predict T2D were WC and WHtR (traditional indices, yielding the AUC above 0.7 and cutoff points of > 86.65 cm and 0.57, respectively); and the AVI, BRI, and CUN-BAE (novel indices, yielding the AUC above 0.7 and the cutoff points of 15.52, 93.83 and 38.35, respectively).
The receiver operating characteristic (ROC) curve depicting accuracy of traditional and novel anthropometric indices in predicting diabetes mellitus (T2D). BMI: body mass index (AUC 0.67); WHR: waist-to-hip ratio (AUC 0.69); WHtR: waist-to-height ratio (AUC 0.72); WC: waist circumference (AUC 0.71); BRI: body roundness index (AUC 0.72); ABSI: a body shape index (AUC 0.61); CUN-BAE: clínica universidad de navarra-body adiposity estimator (AUC 0.70); CI: conicity index (0.69); AVI: abdominal volume index (AUC 0.71); BAI: body adiposity index (AUC 0.63).
Discussion
This study demonstrates that the ability of novel anthropometric indices such as AVI, BRI, and CUN-BAE to predict newly diagnosed T2D using HbA1c is acceptable; but without appreciably outperforming traditional anthropometric indices such as BMI, WC or WHtR. That AVI, BRI and CUN-BAE did not outperform WC and WHtR in predicting T2D is in line with studies from Spain [33] and Pakistan [34].
The current study identified WC and WHtR as two traditional anthropometric indices with satisfactory screening performances for identifying females at risk for T2D. This accords with the literature where WC and WHtR are recognized as robust indicators of central obesity and the development of diabetes [35, 36]. Our findings align with previous research which emphasise the importance of central adiposity in diabetes risk assessment [37].
Another finding of note is that individuals in the highest quartiles of AVI, BRI, and CUN-BAE exhibited the highest odds ratios for the occurrence of T2D. This suggests that individuals with elevated abdominal adiposity, and BRI are at a greater risk for T2D. These findings are in keeping with other studies that have reported the associations of increased CUN-BAE [38], BRI [39], and AVI [40] with a greater risk for T2D.
The current study has some notable strengths that range from using a large population-based cohort and considering various sociodemographic confounders including age, population group, residence and employments status. This study’s limitations include its cross-sectional design, which prevents the establishment of causal relationships. The dataset did not have information regarding the childbirth status of women, which is a limitation since recent childbirth alters the body fat distribution in women and may affect our findings [41]. It is important to acknowledge the limitation related to the narrow range of BMI observed in our sample (28.00 [23.49–33.53]). This limited variability may reduce the external validity of our findings, particularly when generalizing to populations with a wider or different distribution of BMI. As a result, the associations observed in this study may not fully capture the relationships between BMI and T2D in populations with lower or higher BMI ranges. Future research should aim to include a more diverse range of BMI values to ensure that findings are more broadly applicable across different populations. While the current WC cut-off points are primarily based on the homogeneous population labelled as “Asian population”, our study highlights the need for further research to establish appropriate cut-off values particularly in a region as ethnically diverse as South Africa. Additionally, HbA1c and not oral glucose tolerance tests were used to diagnose T2D; HbA1c may not have adequately identified all individuals with T2D [42].
In conclusion, traditional measures like WC and WHtR, as well as novel indices such as AVI, BRI, and CUN-BAE, performed similarly in identifying T2D among adult South African females. However, the challenge of computing these novel anthropometric indices argue against their promotion as alternatives to the use of traditional indices in the local clinical setting.
Data availability
The data presented in this study are available on request from the corresponding author. The SANHANES data are available on request from http://datacuration.hsrc.ac.za/ (accessed on 15 September 2023).
References
Pheiffer C, Pillay-van Wyk V, Turawa E, Levitt N, Kengne AP, Bradshaw D. Prevalence of type 2 diabetes in South Africa: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(11):5868.
Nayak VKR, Nayak KR, Vidyasagar S, Rekha P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab Syndr : Clin Res Rev. 2020;14(5):1265–72.
World Health Organization. Obesity and overweight fact sheet. 2017. Available on: http://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf. Accessed 10 June 2024.
Burkhauser RV, Cawley J, Beyond BMI. The value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27(2):519–29.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines and the obesity society. Circulation. 2014;129(25suppl2):S102–38.
Marcadenti A, Fuchs SC, Moreira LB, Wiehe M, Gus M, Fuchs FD. Accuracy of anthropometric indexes of obesity to predict diabetes mellitus type 2 among men and women with hypertension. Am J Hypertens. 2011;24(2):175–80.
Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, et al. A body shape index and body roundness index: two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health. 2015;15:1–8.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504.
de Oliveira CM, Pavani J, Krieger JE, de Oliveira Alvim R, Mourão-Junior CA, da Costa Pereira A. Body adiposity index in assessing the risk of type 2 diabetes mellitus development: the Baependi Heart Study. Diabetol Metab Syndr. 2019;11:1–4.
Gómez-Ambrosi J, Silva C, Catalán V, Rodríguez A, Galofré JC, Escalada J, et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35(2):383–8.
Guerrero-Romero F, Rodrı́guez-Morán M. Abdominal volume index. An anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. 2003;34(5):428–32.
Tonding SF, Silva FM, Antonio JP, Azevedo MJ, Canani LHS, Almeida JC. Adiposity markers and risk of coronary heart disease in patients with type 2 diabetes mellitus. Nutr J. 2014;13:1–7.
Wang K, Li Y, Ye W, Chen B, Zeng J, Xu S. Predictive ability of traditional and novel anthropometric measurement indices for cardio-metabolic diseases in Chinese adults: China Health and Nutrition Survey (CHNS) cohort study. Nutr Metab Cardiovasc Dis. 2023;33(4):737–48.
Saqlain M, Akhtar Z, Karamat R, Munawar S, Iqbal M, Muhammad F, et al. Body mass index versus other adiposity traits: best predictor of cardiometabolic risk. Iran J Public Health. 2019;48(12):2224.
Biolo G, Di Girolamo FG, Breglia A, Chiuc M, Baglio V, Vinci P, et al. Inverse relationship between a body shape index(ABSI) and fat-free mass in women and men: insights into mechanisms of sarcopenic obesity. Clin Nutr. 2015;34(2):323–7.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–71.
Rico-Martín S, Calderón‐García JF, Sánchez‐Rey P, Franco‐Antonio C, Martinez Alvarez M, Sánchez Muñoz‐Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta‐analysis. Obes Rev. 2020;21(7):e13023.
Nazari M, Mirzaei K, Keshavarz S. Association between lifelines Diet score (LLDS) and some novel anthropometric indices, including BRI, ABSI, VAI, and BAI, in Iranian women: a cross-sectional study. 2023.
Endukuru CK, Gaur GS, Dhanalakshmi Y, Sahoo J, Vairappan B. Cut-off values and clinical efficacy of body roundness index and other novel anthropometric indices in identifying metabolic syndrome and its components among southern-indian adults. Diabetol Int. 2022;13:188–200.
Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: the health and nutritional status of the nation. 2014. Available on: http://www.hsrc.ac.za/en/research-outputs/view/6493 (Accessed 19 June 2024).
Mchiza ZJ-R, Parker W-A, Hossin MZ, Heshmati A, Labadarios D, Falkstedt D, et al. Social and psychological predictors of body mass index among south africans 15 years and older: SANHANES-1. Int j Environ res Public Health. 2019;16(20):3919.
Stokes A, Berry KM, Mchiza Z, Parker W-a, Labadarios D, Chola L, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: evidence from the SANHANES-1, 2011–2012. PLoS ONE. 2017;12(10):e0184264.
Stats S. General Household Survey: http://www.statssa.gov.za/publications/P0318. P03182013 pdf (Accessed: 18 March 2015). 2013.
Norton K, Olds T. Anthropometrica: a textbook of body measurement for sports and health courses. UNSW; 1996.
Organization Health Organisation. Obesity: preventing and managing the global epidemic: report of a WHO consultation. 2000. Available on: https://apps.who.int/iris/handle/10665/42330. Accessed 15 September 2023.
Consultation W. Waist circumference and waist-hip ratio. Report of a WHO Expert Consultation Geneva. World Health Organ. 2008;2008:8–11.
Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44(9):955–6.
Gomez-Peralta F, Abreu C, Cruz-Bravo M, Alcarria E, Gutierrez-Buey G, Krakauer NY, et al. Relationship between a body shape index (ABSI) and body composition in obese patients with type 2 diabetes. Diabetol Metab Syndr. 2018;10:1–8.
Organization WH, Group ISoHW. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of hypertension. 2003;21(11):1983-92.
Organization WH. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. World Health Organization; 2011. Available on: https://iris.who.int/bitstream/handle/10665/70523/WHO_NMH_CHP_CPM_11.1_eng.pdf. Accessed 03 April 2024.
Klug E, Raal F, Marais A, Smuts C, Schamroth C, Jankelow D, et al. South African dyslipidaemia guideline consensus statement: 2018 update a joint statement from the South African Heart Association (SA Heart) and the lipid and Atherosclerosis Society of Southern Africa (LASSA). S Afr Med J. 2018;108(11b):973–1000.
Sifunda S, Mbewu AD, Mabaso M, Manyaapelo T, Sewpaul R, Morgan JW, et al. Prevalence and psychosocial correlates of diabetes mellitus in South Africa: results from the South African National Health and Nutrition Examination Survey (SANHANES-1). Int J Environ Res. 2023;20(10):5798.
Perona JS, Schmidt Rio-Valle J, Ramírez‐Vélez R, Correa‐Rodríguez M, Fernández‐Aparicio Á, González‐Jiménez E. Waist circumference and abdominal volume index are the strongest anthropometric discriminators of metabolic syndrome in Spanish adolescents. Eur J Clin Investig. 2019;49(3):e13060.
Khan SH, Shahid R, Fazal N, Ijaz A. Comparison of various abdominal obesity measures for predicting metabolic syndrome, diabetes, nephropathy, and dyslipidemia. J Coll Phys Surg Pak. 2019;29(12):1159–64.
Hsieh S, Yoshinaga H, Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J Obes. 2003;27(5):610–6.
Ashwell M, Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: simpler and more predictive than using a ‘matrix’based on BMI and waist circumference. BMJ open. 2016;6(3):e010159.
Wildman RP, Janssen I, Khan UI, Thurston R, Barinas-Mitchell E, El Khoudary SR, et al. Subcutaneous adipose tissue in relation to subclinical atherosclerosis and cardiometabolic risk factors in midlife women. Am J Clin Nutr. 2011;93(4):719–26.
Geng S, Chen X, Bai K, Ding J, Li H, Shi S. Association of the Clínica Universidad De Navarra-Body Adiposity Estimator with type 2 diabetes: a retrospective cohort study. Int J Public Health. 2023;68:1606063.
Zhao W, Tong J, Li J, Cao Y. Relationship between body roundness index and risk of type 2 diabetes in Japanese men and women: a reanalysis of a cohort study. Int J Endocrinol. 2021;2021(1):4535983.
Hu X, Appleton AA, Ou Y, Zhang Y, Cai A, Zhou Y, et al. Abdominal volume index trajectories and risk of diabetes mellitus: results from the China Health and Nutrition Survey. J Diabetes Investig. 2022;13(5):868–77.
Nglazi MD, Ataguba JE-O. Overweight and obesity in non-pregnant women of childbearing age in South Africa: subgroup regression analyses of survey data from 1998 to 2017. BMC Public Health. 2022;22(1):395.
Global variation in. Diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c. Nat Med. 2023;29(11):2885–901.
Acknowledgements
We thank the community leaders and the South Africans who participated inthe national SANHANES.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.D.S. and R.S. conceptualized the study and handled validation. M.D.S. developed the methodology and performed formal analysis. R.S. and Z.M. also took care of resources, while M.D.S. carried out the investigation and data curation.The software was developed by Z.D. and M.D.S. wrote the initial draft, with review and editing done by all authors. Visualization was done by N.P. and A.P. Project supervision and administration were managed by N.P., A.P. and Z.M., with funding acquisition handled by M.D.S. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Research Ethics Committee (REC) of the South African Human Sciences Research Council (HSRC) (REC number: 6/16/11/11), which is in accordance with the Declaration of Helsinki. Informed written consent was obtained from all the survey participants before the interview. The interviews were conducted by trained interviewers in the respondents’ homes and the respondents’ preferred language.
Consent for publication
Not application.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sekgala, M.D., Sewpaul, R., Kengne, A.P. et al. Clinical utility of novel anthropometric indices in identifying type 2 diabetes mellitus among South African adult females. BMC Public Health 24, 2676 (2024). https://doi.org/10.1186/s12889-024-20168-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-20168-7